Ruthenium Complexes as NO Donors for Vascular Relaxation Induction
Abstract
:1. Introduction
2. Nitric Oxide and Its Main Vasodilating Mechanism
2.1. NO Release and Vascular Biological Target
2.2. Cellular Mechanisms for Vasodilation
3. Sodium Nitroprusside and Its Effects on Vascular Tone and Arterial Pressure Control
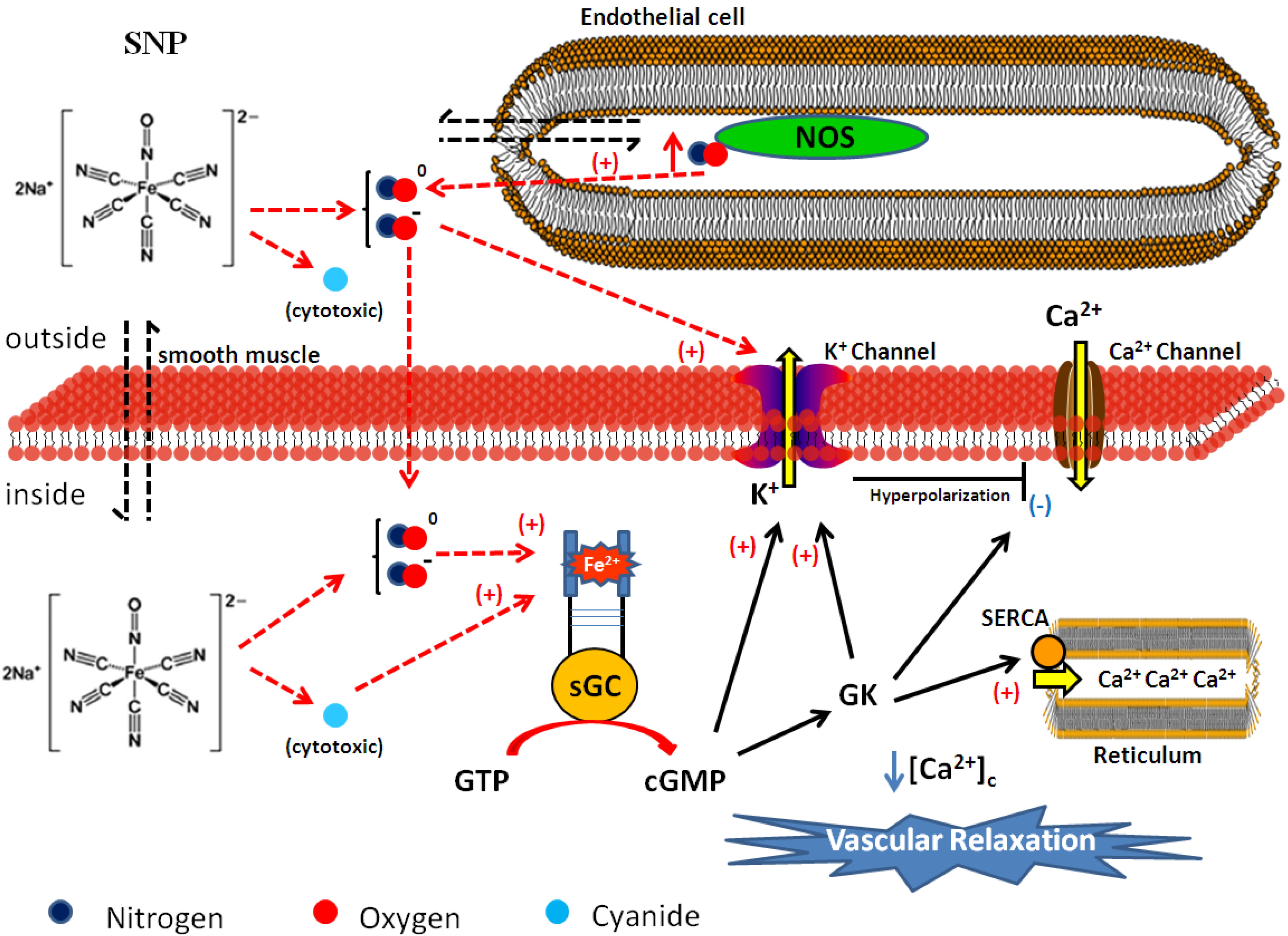
4. Development of Ruthenium-Derived NO Donor Complexes
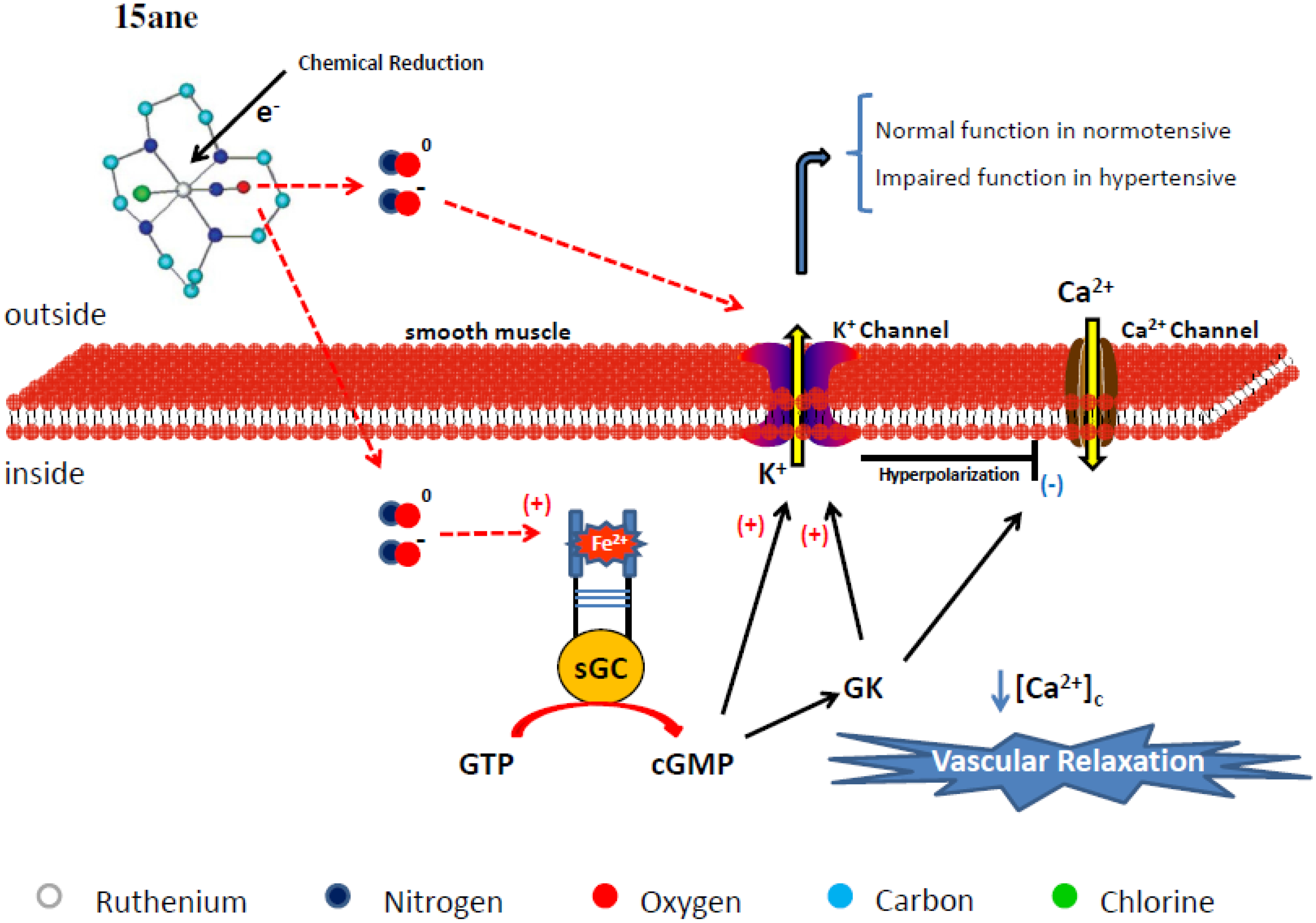
4.1. Tetraazacyclotetradecane Ruthenium Complexes



4.1.1. Vasodilating Effect in Normotensive and Hypertensive Rat Vessels
4.1.2. Effects of the Macrocyclic Ruthenium Complexes on Arterial Pressure Control
4.2. Polypyridine Ruthenium Complexes

| Compounds | ν(NO) cm−1 a | E1/2 NO+/NO0 (V) vs. Ferrocene b |
|---|---|---|
| cis-[RuII(bpy)2(NO)Cl]2+ | 1940 | –0.21 |
| cis-[RuII(bpy)2(py)(NO)]3+ | 1944 | 0.12 |
| cis-[RuII(bpy)2(4-pic)(NO]3+ | 1947 | 0.14 |
| cis-[RuII(bpy)2(4-acpy)(NO)]3+ | 1943 | 0.16 |
| [RuIICl2(tpy)(NO)]3+ | 1870 | –0.51c |
| [RuII(tpy)(bpy)(NO)]3+ | 1944 | +0.053 |
| [RuII(tpy)(NH.NHq)(NO)]3+ | 1888 | - |
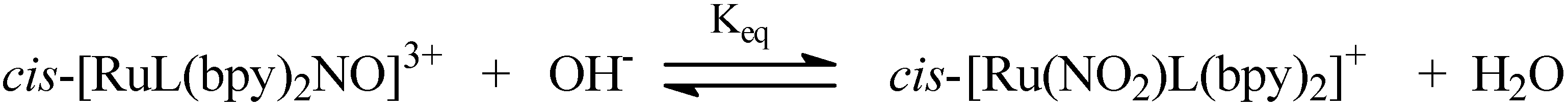
| Compounds | ϕNO mol einstein −1 |
|---|---|
| cis-[RuII(bpy)2(NO)Cl]2+ | 0.98 * |
| cis-[RuII(bpy)2(py)(NO)]3+ | 0.16 |
| cis-[RuII(bpy)2(4-pic)(NO]3+ | 0.17 |
| cis-[RuII(bpy)2(4-acpy)(NO)]3+ | 0.07 |
| [RuII(tpy)(bpy)(NO)]3+ | 0.14 |
| [RuII(tpy)(NH.NHq)(NO)]3+ | 0.47 |
| Compounds | ϕNO mol eistein−1 |
|---|---|
| cis-[RuII(NO2)(bpy)2(py)]+ | 0.007 |
| cis-[RuII(NO2)(bpy)2(4-pic)]+ | 0.009 |
| cis-[RuII(NO2)(bpy)2(pz)]+ | 0.037 |
| [RuII(NO2)(tpy)(bpy)]+ | 0.036 |
4.2.1. RUNOCL

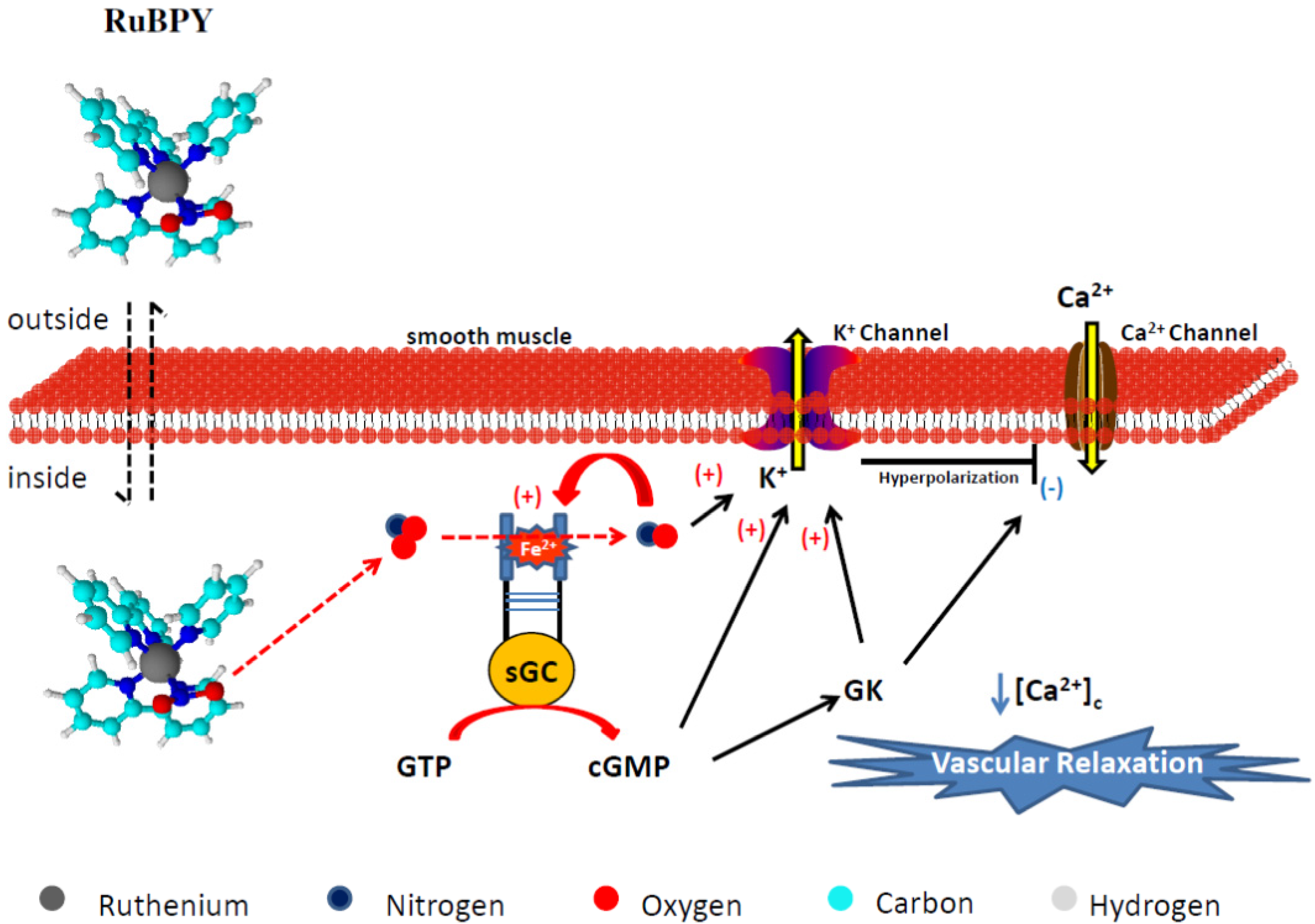
4.2.2. RuBPY
4.2.3. Terpy ([Ru(tpy)(NH.NHq)NO]3+)
4.2.3.1. Vasodilating Effect in Normotensive and Hypertensive Rat Vessels

4.2.3.2. Effects of Terpy on Arterial Pressure Control
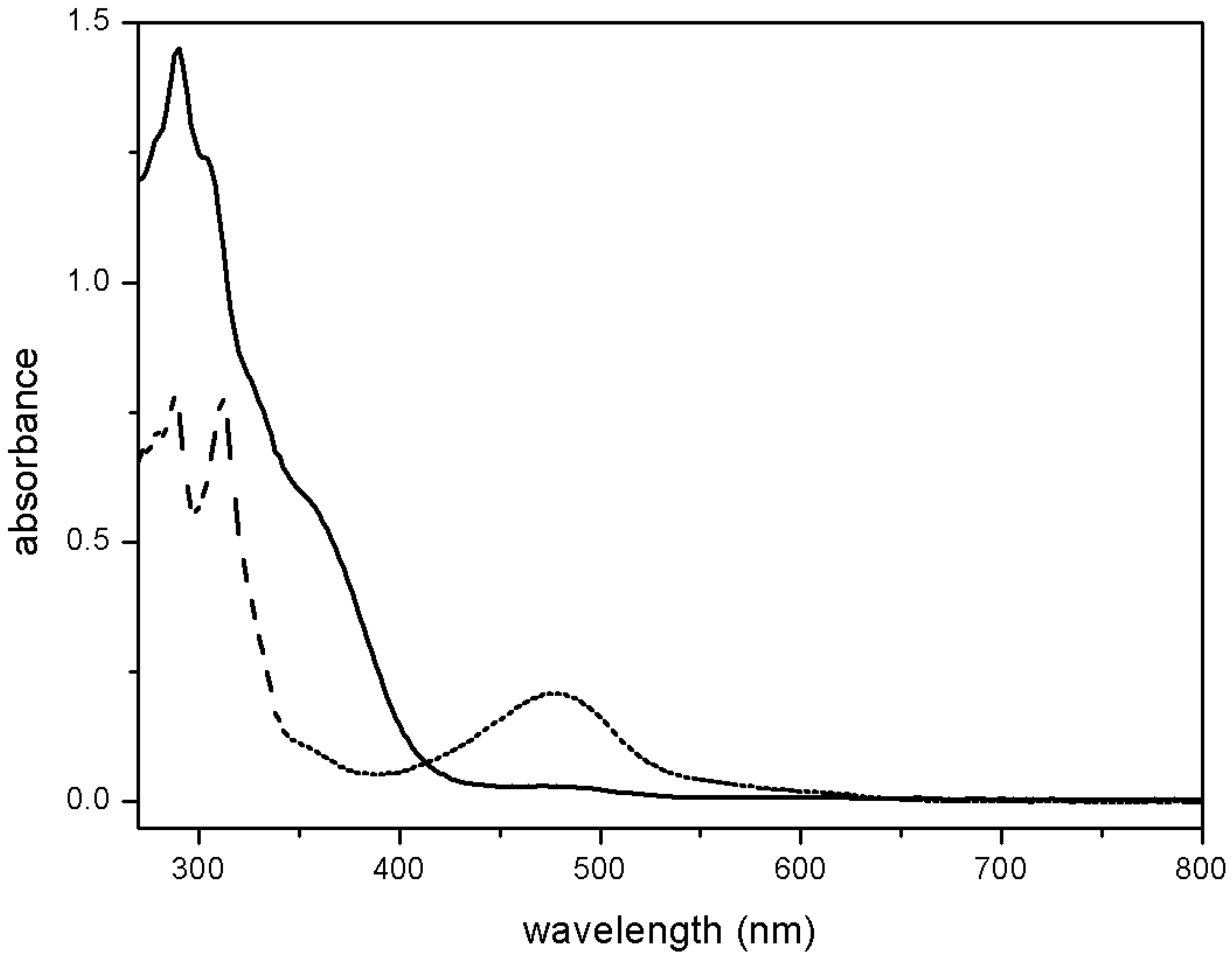
4.3. NO Production by Visible Light Irradiation of Nitrosyl Ruthenium Complex

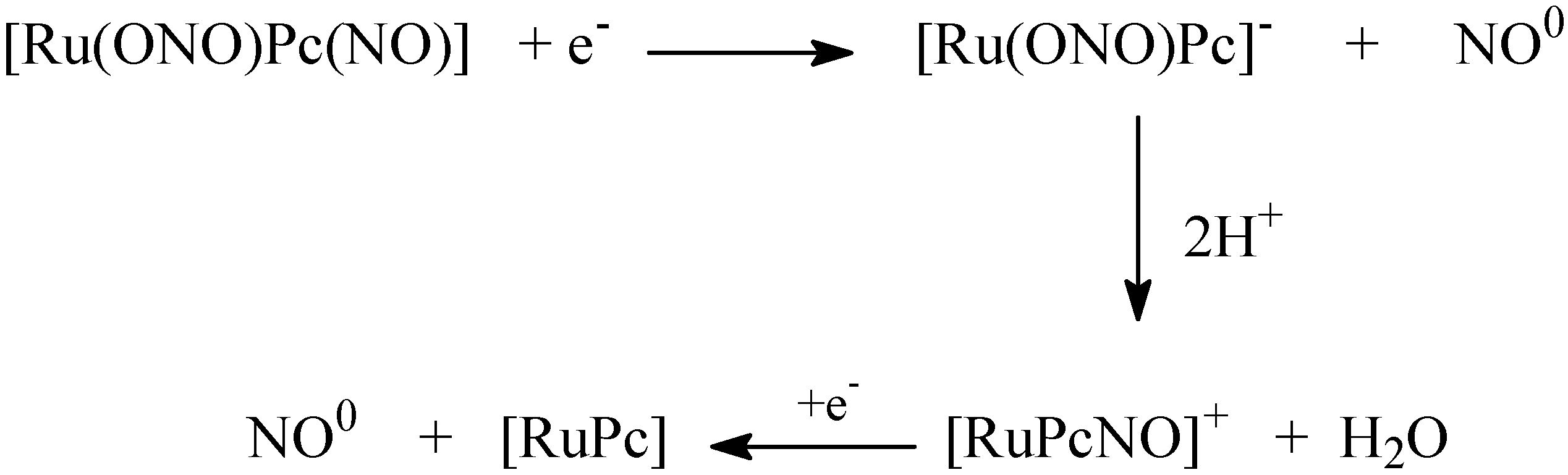
5. General Aspects and Conclusions
Acknowledgements
Author Contributions
Conflicts of interest
References
- Radomski, M.W.; Palmer, R.M.J.; Moncada, S. The antiaggregating properties of vascular endothelium: Interactions between prostacyclin and nitric oxide. Br. J. Pharmacol. 1987, 92, 639–646. [Google Scholar] [CrossRef]
- Kline, D.D.; Yang, T.N.; Huang, P.L.; Prabhakar, N.R. Altered respiratory responses to hypoxia in mutant mice deficient in neuronal nitric oxide synthase. J. Physiol. 1998, 511, 273–287. [Google Scholar] [CrossRef]
- Bredt, D.S.; Hwang, P.M.; Snyder, S.H. Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature 1990, 347, 768–770. [Google Scholar] [CrossRef]
- Garthwaite, J. Neural nitric oxide signaling. Trends Neurosci. 1995, 18, 51–52. [Google Scholar] [CrossRef]
- Akesson, B.; Lundquist, I. Influence of nitric oxide modulators on cholinergically stimulated hormone release from mouse islets. J. Physiol. 1999, 515, 463–473. [Google Scholar] [CrossRef]
- Triyoso, D.H.; Good, T.A. Pulsatile shear stress leads to DNA fragmentation in human SH-SU5Y neuroblastoma cell line. J. Physiol. 1999, 515, 355–365. [Google Scholar] [CrossRef]
- Moncada, S.; Palmer, R.M.J.; Higgs, E.A. Nitric oxide: Physiology, pathophysiology and pharmacology. Pharmacol. Rev. 1991, 43, 109–42. [Google Scholar]
- Furchgott, R.F.; Zawadzki, J.V. The obligatory role of endothelial cells and the relaxation of arterial smooth muscle by acetylcholine. Nature 1980, 288, 373–376. [Google Scholar] [CrossRef]
- Plane, F.; Wiley, K.E.; Jeremy, J.Y.; Cohen, R.A.; Garland, C.J. Evidence that different mechanisms underlie smooth muscle relaxation to nitric oxide and nitric oxide donors in the rabbit isolated carotid artery. Br. J. Pharmacol. 1998, 123, 1351–1358. [Google Scholar] [CrossRef]
- Van Hove, C.E.; van der Donckt, C.; Herman, A.G.; Bult, H.; Fransen, P. Vasodilator efficacy of nitric oxide depends on mechanisms of intracellular calcium mobilization in mouse aortic smooth muscle cells. Br. J. Pharmacol. 2009, 158, 920–930. [Google Scholar] [CrossRef]
- Palmer, R.M.; Ashton, D.S.; Moncada, S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature 1988, 333, 664–666. [Google Scholar] [CrossRef]
- Rees, D.D.; Palmer, R.M.; Moncada, S. Role of endothelium derived nitric oxide in the regulation of blood pressure. Proc. Nat. Acad. Sci. USA 1989, 86, 3375–3378. [Google Scholar] [CrossRef]
- Stuehr, D.J.; Santolini, J.; Wang, Z.Q.; Wei, C.C.; Adak, S. Update on mechanism and catalytic regulation in the NO synthases. J. Biol. Chem. 2004, 279, 36167–36170. [Google Scholar]
- Francis, S.H.; Busch, J.L.; Corbin, J.D. cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol Rev. 2010, 62, 525–563. [Google Scholar]
- Gao, Y. The multiple actions of NO. Pflug Arch. Eur. J Phy. 2010, 459, 829–839. [Google Scholar] [CrossRef]
- Hall, C.N.; Garthwaite, J. What is the real physiological NO concentration in vivo? Nitric Oxide 2009, 21, 92–103. [Google Scholar] [CrossRef]
- Silva, B.R.; Pernomian, L.; Bendhack, L.M. Contribution of oxidative stress to endothelial dysfunction in hypertension. Front. Physiol. 2012, 3, 441. [Google Scholar]
- Marsh, N.; Marsh, A. A short history of nitroglycerine and nitric oxide in pharmacology and physiology. Clin. Exp. Pharmacol. Physiol. 2000, 27, 313–319. [Google Scholar] [CrossRef]
- Gruetter, C.A.; Barry, B.K.; McNamara, D.B.; Gruetter, D.Y.; Kadowitz, P.J.; Ignarro, L. Relaxation of bovine coronary artery and activation of coronary arterial guanylate cyclase by nitric oxide, nitroprusside and a carcinogenic nitrosoamine. J. Cyclic. Nucleotide Res. 1979, 5, 211–224. [Google Scholar]
- Ignarro, L.J.; Cirino, G.; Casini, A.; Napoli, C. Nitric oxide as a signaling molecule in the vascular system: An overview. J. Cardiovasc. Pharmacol. 1999, 34, 879–886. [Google Scholar] [CrossRef]
- Friederich, J.A.; Butterworth, J.F. Sodium nitroprusside: Twenty years and counting. Anesth. Analg. 1995, 81, 152–162. [Google Scholar]
- Allardyce, C.S.; Dyson, P.J. Ruthenium in medicine: Current clinical uses and future prospects. Platin Met. Rev. 2001, 45, 62–69. [Google Scholar]
- Marchesi, M.S.P.; Cicillini, S.A.; Prazias, A.C.L.; Bendhack, L.M.; Batista, A.A.; da Silva, R.S. Chemical mechanism of controlled nitric oxide release from trans-[RuCl([15]aneN4)NO](PF6)2 as a vasorelaxant agent. Transit. Metal Chem. 2012, 37, 475–479. [Google Scholar] [CrossRef]
- Swayze, R.D.; Braun, A.P. Catalytically inactive mutant of type I cGMP-dependent protein kinase prevents enhancement of large conductance, calcium-sensitive K+ channels by sodium nitroprusside and cGMP. J. Biol. Chem. 2001, 276, 19729–19737. [Google Scholar] [CrossRef]
- Lee, M.R.; Li, L.; Kitazawa, T. Cyclic GMP causes Ca2+ desensitization in vascular smooth muscle by activating the myosin light chain phosphatase. J. Biol. Chem. 1997, 272, 5063–5068. [Google Scholar]
- Playfair, L. On the Nitroprussides, a new class of salts. Proc. R. Soc. London 1849, 139, 477–518. [Google Scholar]
- Johnson, C.C. The actions and toxicity of sodium nitroprusside. Arch. Int. Pharmacodyn. Ther. 1929, 35, 489–496. [Google Scholar]
- Moracca, P.P.; Bilte, E.M.; Hale, D.E.; Wasmuth, C.E.; Pontasse, E.F. Clinical evaluation of sodium nitroprusside as a hypotensive agent. Anesthesiology 1962, 23, 193–199. [Google Scholar]
- Butler, A.R.; Megson, I.L. Non-heme iron nitrosyls in Biology. Chem. Rev. 2002, 102, 1155–1166. [Google Scholar] [CrossRef]
- Olabe, J.A. The coordination chemistry of nitrosyl in cyanoferrates. An exhibit of bioinorganic relevant reactions. Dalton Trans. 2008, 28, 3633–3648. [Google Scholar] [CrossRef]
- Bates, J.N.; Baker, M.T.; Guerra, R., Jr.; Harrison, D.G. Nitric oxide generation from nitroprusside by vascular tissue. Evidence that reduction of the nitroprusside anion and cianide loss are required. Biochem. Pharmacol. 1991, 42, S157–S165. [Google Scholar]
- Harrison, D.G.; Bates, J.N. The nitrovasodilators. New ideas about old drugs. Circulation 1993, 87, 1461–1467. [Google Scholar]
- Clarke, M.J.; Gaul, J.B. Chemistry relevant to the biological effects of nitric oxide and metallonitrosyls. Struct. Bonding 1993, 81, 147–181. [Google Scholar] [CrossRef]
- Rodrigues, G.J.; Pereira, A.C.; Vercesi, J.A.; Lima, R.G.; da Silva, R.S.; Bendhack, L.M. Long-lasting hypotensive effect in renal hypertensive rats induced by nitric oxide released from a ruthenium complex. J. Cardiovasc. Pharm. 2012, 60, 193–198. [Google Scholar] [CrossRef]
- Bonaventura, D.; Oliveira, F.S.; Togniolo, V.; Tedesco, A.C.; da Silva, R.S.; Bendhack, L.M. A macrocyclic nitrosyl ruthenium complex is a NO donor that induces rat aorta relaxation. Nitric Oxide 2004, 10, 83–91. [Google Scholar]
- Pereira, A.C.; Lunardi, C.N.; Paulo, M.; da Silva, R.S.; Bendhack, L.M. Nitric oxide generated by the compound RuBPY promotes the vascular smooth cell membrane hyperpolarization. Eur. J. Pharm. Sci. 2013, 48, 604–610. [Google Scholar] [CrossRef]
- Pereira, A.C.; Ford, P.C.; da Silva, R.S.; Bendhack, L.M. Ruthenium-nitrite complex as pro-drug releases NO in a tissue and enzyme-dependent way. Nitric Oxide 2011, 24, 192–198. [Google Scholar]
- López-López, J.G.; Pérez-Viscaíno, F.; Cogolludo, A.L.; Ibarra, M.; Zaragozá-Arnáez, F.; Tamargo, J. Nitric oxide- and nitric oxide donors-induced relaxation and its modulation by oxidative stress in piglet pulmonary arteries. Br. J. Pharmacol. 2001, 133, 615–624. [Google Scholar] [CrossRef]
- Paulo, M.; Rodrigues, G.J.; da Silva, R.S.; Bendhack, L.M. A new NO donor failed to release NO and to induce relaxation in the rat basilar artery. Eur. J. Pharm. Sci. 2012, 45, 344–350. [Google Scholar]
- Irvine, J.C.; Ritchie, R.H.; Favaloro, J.L.; Andrews, K.L.; Widdop, R.; Kemp-Harper, B.K. Nitroxyl (HNO): The cinderella of the nitric oxide story. Trends Pharmacol. Sci. 2008, 29, 601–608. [Google Scholar] [CrossRef]
- Favaloro, J.L.; Kemp-Harper, B.K. Redox variants of NO (NO° and HNO) elicit vasorelaxation of resistance arteries via distinct mechanisms. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H1274–H1280. [Google Scholar] [CrossRef]
- Bonaventura, D.; Oliveira, F.S.; Lunardi, C.N.; Vercesi, J.A.; da Silva, R.S.; Bendhack, L.M. Characterization of the mechanisms of action and nitric oxide species involved in the relaxation induced by the ruthenium complex. Nitric Oxide 2006, 15, 387–394. [Google Scholar] [CrossRef]
- Lunardi, C.N.; Cacciari, A.L.; da Silva, R.S.; Bendhack, L.M. Cytosolic calcium concentration is reduced by photolysis of a nitrosyl ruthenium complex in vascular smooth muscle cells. Nitric Oxide 2006, 15, 252–258. [Google Scholar] [CrossRef]
- Bonaventura, D.; de Lima, R.G.; da Silva, R.S.; Bendhack, L.M. NO donors-relaxation is impaired in aorta from hypertensive rats due to a reduced involvement of K+ channels and sarcoplasmic reticulum Ca2+-ATPase. Life Sci. 2011, 89, 595–602. [Google Scholar] [CrossRef]
- Otsuka, Y.; DiPiero, A.; Hirt, E.; Brennaman, B.; Lockette, W. Vascular relaxation and cGMP in hypertension. Am. J. Physiol. 1988, 254, H163–H169. [Google Scholar]
- Paulo, M.; Araujo, A.V.; Bendhack, L.M. Sodium nitroprusside activates potassium channels in the vena cava in normotensive but not in hypertensive rats. Hypertens Res. 2013, 36, 765–769. [Google Scholar]
- Bonaventura, D.; Lunardi, C.N.; Rodrigues, G.J.; Neto, M.A.; Bendhack, L.M. A novel mechanism of vascular relaxation induced by sodium nitroprusside in the isolated rat aorta. Nitric Oxide 2008, 18, 287–295. [Google Scholar]
- Munhoz, F.C.; Potje, S.R.; Pereira, A.C.; Daruge, M.G.; da Silva, R.S.; Bendhack, L.M.; Antoniali, C. Hypotensive and vasorelaxing effects of the new NO-donor [Ru(terpy)(bdq)NO+]3+ in spontaneously hypertensive rats. Nitric Oxide 2012, 26, 111–117. [Google Scholar]
- Shaki, M.; Khanam, S.; Firdaus, F.; Latif, A.; Aatif, M.; Al-Resayes, S.I. Synthesis, spectroscopic characterization, DNA interaction and antibacterial study of metal complexes of tetraazamacrocyclic Schiff base. Spectrochim. Acta A 2012, 93, 354–362. [Google Scholar] [CrossRef]
- Tfouni, E.; Ferreira, K.Q.; Doro, F.G.; Takata, F.M.; da Silva, R.S.; da Rocha, Z.N. Ru(II) and Ru(III) complexes with cyclam and related species. Coord. Chem. Rev. 2005, 249, 405–418. [Google Scholar] [CrossRef]
- Ferreira, K.Q.; Lucchesi, A.M.; da Rocha, Z.N.; da Silva, R.S. Synthesis, characterization and kinetic studies of the cis-[RuCl2(cyclen)]+ complex. Inorg. Chim. Acta 2002, 30, 147–151. [Google Scholar]
- Togniolo, V.; da Silva, R.S.; Tedesco, A.C. Photo-induced nitric oxide release from chlorobis(2,2'-bipyridine)nitrosylruthenium(II) in aqueous solution. Inorg. Chim. Acta 2001, 316, 7–12. [Google Scholar]
- Sauaia, M.G.; da Silva, R.S. The reactivity of nitrosyl ruthenium complexes containing polypyridyl ligands. Transit. Metal Chem. 2003, 28, 254–259. [Google Scholar]
- Oliveira, F.S.; Togniolo, V.; Bonaventura, D.; Pupo, T.; Tedesco, A.C.; da Silva, R.S. Nitrosyl ruthenium complex as nitric oxide delivery agent: Synthesis, characterization and photochemical properties. Inorg. Chem. Comm. 2004, 7, 160–164. [Google Scholar]
- De Lima, R.G.; Sauaia, M.G.; Bonaventura, D.; Tedesco, A.C.; Bendhack, L.M.; da Silva, R.S. Influence of ancillary ligand in the nitric oxide photo-release from the [Ru(L)(terpy)NO]3+ and its vasodilator activity based on visible light irradiation. Inorg. Chim. Acta 2006, 359, 2543–2549. [Google Scholar] [CrossRef]
- Oliveira, F.S.; Ferreira, K.Q.; Bonaventura, D.; Bendhack, L.M.; Tedesco, A.C.; Machado, S.P.; Tfouni, E.; da Silva, R.S. The macrocyclic effect and vasodilation response based on the photoinduced nitric oxide release from trans-[RuCl(tetraazamacrocycle)NO]2+. J. Inorg. Biochem. 2007, 161, 313–320. [Google Scholar]
- Ferezin, C.Z.; Oliveira, F.S.; da Silva, R.S.; Simioni, A.R.; Tedesco, A.C.; Bendhack, L.M. The complex trans-[RuCl([15]aneN4)NO]2+ induces rat aorta relaxation by ultraviolet light irradiation. Nitric Oxide 2005, 13, 170–175. [Google Scholar] [CrossRef]
- Ceron, P.I.B.; Cremonez, D.C.; Bendhack, L.M.; Tedesco, A.C. The relaxation induced by S-nitroso-glutathione and S-nitroso-N-acetylcysteine in rat aorta is not related to nitric oxide production. J. Pharmacol. Exp. Ther. 2001, 298, 686–694. [Google Scholar]
- Fricker, S.P.; Slade, E.; Powell, N.A.; Vaughan, O.J.; Henderson, G.R.; Murrer, B.A.; Megson, I.L.; Bisland, S.K.; Flitney, S.W. Ruthenium complexes as nitric oxide scavengers: A potential therapeutic approach to nitric oxidemediated diseases. Br. J. Pharmacol. 1997, 122, 1441–1449. [Google Scholar] [CrossRef]
- Hutchings, S.R.; Song, D.; Fricker, S.P.; Pang, C.C. The ruthenium-based nitric oxide scavenger, AMD6221, augments cardiovascular responsiveness to noradrenaline in rats with streptozotocin-induced diabetes. Eur. J. Pharmacol. 2005, 528, 132–136. [Google Scholar] [CrossRef]
- Sguilla, F.S.; Tedesco, A.C.; Bendhack, L.M. A membrane potential-sensitive dye for vascular smooth muscle cells assays. Biochem. Biophys. Res. Commun. 2003, 301, 113–118. [Google Scholar] [CrossRef]
- Callera, G.E.; Varanda, W.A.; Bendhack, L.M. Impaired relaxation to acetylcholine in 2K-1C hypertensive rat aortas involves changes in membrane hyperpolarization instead of an abnormal contribution of endothelial factors. Gen. Pharmacol. 2000, 34, 379–389. [Google Scholar]
- Bonaventura, D.; Oliveira, F.S.; da Silva, R.S.; Bendhack, L.M. Decreased vasodilation induced by a new nitric oxide donor in two kidney, one clip hypertensive rats is due to impaired K+ channel activation. Clin. Exp. Pharmacol. Physiol. 2005, 32, 478–481. [Google Scholar] [CrossRef]
- Marcondes, F.G.; Ferro, A.A.; Souza-Torsoni, A.; Sumitani, M.; Clarke, M.J.; Franco, D.W.; Tfouni, E.; Krieger, M.H. In vivo effects of the controlled NO donor/scavenger ruthenium cyclam complexes on blood pressure. Life Sci. 2002, 70, 2735–2752. [Google Scholar] [CrossRef]
- De Gaitani, C.M.; de Melo, M.C.C.; Lunardi, C.N.; de Oliveira, F.S.; da Silva, R.S.; Bendhack, L.M. Hypotensive effect of the nitrosyl ruthenium complex nitric oxide donor in renal hypertensive rats. Nitric Oxide 2009, 20, 195–199. [Google Scholar] [CrossRef]
- Tfouni, E.; Krieger, M.H.; McGarvey, B.R.; Franco, D.W. Structure, chemical and photochemical reactivity and biological activity of some ruthenium amine nitrosyl complexes. Coord. Chem. Rev. 2003, 236, 57–69. [Google Scholar] [CrossRef]
- Suen, H.F.; Wilson, S.W.; Pomerantz, M.; Walsh, J.L. Photosubstitution reactions of terpyridine complexes of ruthenium(II). Inorg. Chem. 1989, 28, 786–791. [Google Scholar] [CrossRef]
- Caspar, A.; Meyer, T.J. Photochemistry of Ru(bpy)32+. Solvent effects. J. Am. Chem. Soc. 1983, 105, 5583–5590. [Google Scholar] [CrossRef]
- Johnson, E.C.; Sullivan, B.P.; Salmon, D.J.; Adeyemi, S.A.; Meyer, T.J. Synthesis and properties of the chloro-bridged dimer [(bpy)2RuCl]22+ and its transient 3+ mixed-valence ion. Inorg. Chem. 1978, 17, 2211–2215. [Google Scholar] [CrossRef]
- Durham, B.; Wilson, S.R.; Hodgson, D.J.; Meyer, T.J. Cis-trans photoisomerization in Ru(bpy)2(OH2)22+.Crystal structure of trans-[Ru(bpy)2(OH2)(OH)](ClO4)2. J. Am. Chem. Soc. 1980, 102, 600–607. [Google Scholar] [CrossRef]
- Dovletoglou, A.; Adeyemi, S.A.; Meyer, T.J. Coordination and redox chemistry of substituted-polypyridyl complexes of ruthenium. Org. Chem. 1996, 35, 4120–4127. [Google Scholar]
- Hirano, T.; Ueda, T.; Mukaiad, M.; Nagao, H.; Oi, T. Reactions of [RuCl2NOterpy]+ (terpy=2,2'-6',2''-terpyridine) whit mono anions such as NO2−, Br−, and N3− and structural studies in terpy diruthenium having a nitrosyl ligand. J. Chem. Soc. Dalton Trans. 2001, 234, 1–4. [Google Scholar]
- Nagao, H.; Nishimura, H.; Funato, H.; Ichikawa, Y.; Howell, F.S.; Mukaida, M.; Kakihana, H. Synthesis, properties, and molecular-structure of trans-chloronitrosylbis(2,2'-bipyridine)ruthenium(2+): Trans and cis isomer characteristics compared. Inorg. Chem. 1989, 28, 3955–3959. [Google Scholar] [CrossRef]
- Godwin, J.B.; Meyer, T.J. Nitrosyl-nitrite interconversion in ruthenium complexes. Inorg. Chem. 1971, 24, 2150–2153. [Google Scholar]
- Sauaia, M.G.; de Lima, R.G.; Tedesco, A.C.; da Silva, R.S. Photoinduced NO release by visible light irradiation from pyrazine-bridged nitrosyl ruthenium complexes. J. Am. Chem. Soc. 2003, 125, 14718–14719. [Google Scholar] [CrossRef]
- Sauaia, M.G.; Oliveira, F.S.; Tedesco, A.C.; da Silva, R.S. Control of NO release by light irradiation from nitrosyl-ruthenium complexes containing polypyridyl ligands. Inorg. Chim. Acta. 2003, 355, 191–196. [Google Scholar] [CrossRef]
- De Lima, R.G.; Sauaia, M.G.; Bonaventura, D.; Tedesco, A.C.; Lopez, R.F.V.; Bendhack, L.M.; da Silva, R.S. Controlled nitric oxide photo-release from nitro ruthenium complexes: The vasodilator response produced by UV light irradiation. Inorg. Chim. Acta 2005, 358, 2643–2650. [Google Scholar] [CrossRef]
- Da Rocha, Z.N; de Lima, R.G.; Doro, F.G.; Tfouni, E.; da Silva, R.S. Photochemical production of nitric oxide from a nitrosyl phthalocyanine ruthenium complex by irradiation with light in the phototherapeutic window. Inorg. Chem. Comm. 2008, 11, 737–740. [Google Scholar]
- Lunardi, C.N.; Vercesi, J.A.; da Silva, R.S.; Bendhack, L.M. Vasorelaxation induced by the new nitric oxide donor cis-[Ru(Cl)(bpy)2(NO)](PF6) is due to activation of KCa by a cGMP-dependent pathway. Vasc. Pharmacol. 2007, 47, 139–144. [Google Scholar] [CrossRef]
- Lunardi, C.N.; da Silva, R.S.; Bendhack, L.M. New nitric oxide donors based on ruthenium complexes. Braz. J. Med. Biol. Res. 2009, 42, 87–93. [Google Scholar] [CrossRef] [Green Version]
- Lundberg, J.O.; Weitzberg, E. Nitrite reduction to nitric oxide in the vasculature. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H477–H478. [Google Scholar] [CrossRef]
- Bryan, N.S.; Rassaf, T.; Maloney, R.E.; Rodriguez, C.M.; Saijo, F.; Rodriguez, J.R.; Feelisch, M. Cellular targets and mechanisms of nitros(yl)ation: An insight into their nature and kinetics in vivo. Proc. Natl. Acad. Sci. 2004, 101, 4308–4313. [Google Scholar] [CrossRef]
- Stamler, J.S.; Singel, D.J.; Loscalzo, J. Biochemistry of nitric oxide and its redox-activated forms. Science 1992, 258, 1898–1902. [Google Scholar]
- Thomas, D.D.; Ridnour, L.A.; Isenberg, J.S.; Flores-Santana, W.; Switzer, C.H.; Donzelli, S.; Hussain, P.; Vecoli, C.; Paolocci, N.; Ambs, S.; et al. The chemical biology of nitric oxide: Implications in cellular signaling. Free Rad. Biol. Med. 2008, 45, 18–31. [Google Scholar] [CrossRef]
- Heitzer, T.; Wenzel, U.; Hink, U.; Krollner, D.; Skatchkov, M.; Stahl, R.A.K.; Macharzina, R.; Brase, J.H.; Meinertz, T.; Münzel, T. Increased NAD(P)H oxidase-mediated superoxide production in renovascular hypertension: Evidence for na involvement of protein kinase C. Kidney Int. 1999, 55, 252–260. [Google Scholar] [CrossRef]
- Rodrigues, G.J.; Lunardi, C.N.; Lima, R.G.; Santos, C.X.; Laurindo, F.R.; da Silva, R.S.; Bendhack, L.M. Vitamin C improves the effect of a new nitric oxide donor on the vascular smooth muscle from renal hypertensive rats. Nitric Oxide 2008, 18, 176–183. [Google Scholar] [CrossRef]
- Araujo, A.V.; Pereira, A.C.; Grando, M.D.; da Silva, R.S.; Bendhack, L.M. The new NO donor Terpy induces similar relaxation in mesenteric resistance arteries of renal hypertensive and normotensive rats. Nitric Oxide 2013, 35, 47–53. [Google Scholar] [CrossRef]
- Bonaventura, D.; Lunardi, C.N.; Rodrigues, G.J.; Neto, M.A.; Vercesi, J.A.; de Lima, R.G.; da Silva, R.S.; Bendhack, L.M. Endothelium negatively modulates the vascular relaxation induced by nitric oxide donor, due to uncoupling NO synthase. J. Inorg. Biochem. 2009, 103, 1366–1374. [Google Scholar] [CrossRef]
- Callera, G.E.; Bendhack, L.M. Contribution of sarcoplasmic reticulum uptake and L-type calcium channels to altered vascular responsiveness in the aorta of renal hypertensive rats. Gen. Pharmacol. 1999, 33, 457–466. [Google Scholar]
- Bonaventura, D.; de Lima, R.G.; Vercesi, J.A.; da Silva, R.S.; Bendhack, L.M. Comparison of the mechanisms underlying the relaxation induced by two nitric oxide donors: Sodium nitroprusside and a new ruthenium complex. Vasc. Pharmacol. 2007, 46, 215–222. [Google Scholar]
- Montezano, A.C.; Touyz, R.M. Reactive oxygen species, vascular Noxs, and hypertension: Focus on translational and clinical research. Antioxid. Redox Sign. 2014, 20, 164–182. [Google Scholar] [CrossRef]
- Da Rocha, Z.N.; Marcehsi, M.S.P.; Molin, J.C.; Lunardi, C.N.; Miranda, K.; Bendhack, L.M.; da Silva, R.S. The induced NO-vasodilatation by chemical reduction of coordinated nitric íon cis-[Ru(NO2)(L)(bpy)2]+ complex. J. Chem. Soc. 2008, 32, 4282–4287. [Google Scholar]
- Maranho, D.S.; de Lima, R.G.; Primo, F.L.; da Silva, R.S.; Tedesco, A.C. Photoinduced nitric oxide and singlet oxygen release from ZnPC liposome vehicle associated with the nitrosyl ruthenium complex: Synergistic effects in photodynamic therapy application. Photochem. Photobiol. 2009, 85, 705–713. [Google Scholar] [CrossRef]
- Allen, C.M.; Sharman, W.M.; van Lier, J.E. Current status of phthalocyanines in the photodynamic therapy of cancer. J. Porphyr. Phthalocyanines 2001, 5, 161–169. [Google Scholar] [CrossRef]
- Carneiro, Z.A.; de Moraes, J.C.; Rodrigues, F.P.; de Lima, R.G.; Curti, C.; da Rocha, Z.N.; Paulo, M.; Bendhack, L.M.; Tedesco, A.C.; Formiga, A.L.; et al. Photocytotoxic activity of a nitrosyl phthalocyanine ruthenium complex—A system capable of producing nitric oxide and singlet oxygen. J. Inorg. Biochem. 2011, 105, 1035–1043. [Google Scholar]
- Rodrigues, G.J.; Restini, C.B.; Lunardi, C.N.; Moreira, J.E.; Lima, R.G.; da Silva, R.S.; Bendhack, L.M. Caveolae dysfunction contributes to impaired relaxation induced by nitric oxide donor in aorta from renal hypertensive rats. J. Pharmacol. Exp. Ther. 2007, 323, 831–837. [Google Scholar] [CrossRef]
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Lima, R.G.; Silva, B.R.; Da Silva, R.S.; Bendhack, L.M. Ruthenium Complexes as NO Donors for Vascular Relaxation Induction. Molecules 2014, 19, 9628-9654. https://doi.org/10.3390/molecules19079628
De Lima RG, Silva BR, Da Silva RS, Bendhack LM. Ruthenium Complexes as NO Donors for Vascular Relaxation Induction. Molecules. 2014; 19(7):9628-9654. https://doi.org/10.3390/molecules19079628
Chicago/Turabian StyleDe Lima, Renata Galvão, Bruno Rodrigues Silva, Roberto Santana Da Silva, and Lusiane Maria Bendhack. 2014. "Ruthenium Complexes as NO Donors for Vascular Relaxation Induction" Molecules 19, no. 7: 9628-9654. https://doi.org/10.3390/molecules19079628




