Phenoxyacetohydrazide Schiff Bases: β-Glucuronidase Inhibitors
Abstract
:1. Introduction
2. Results and Discussion
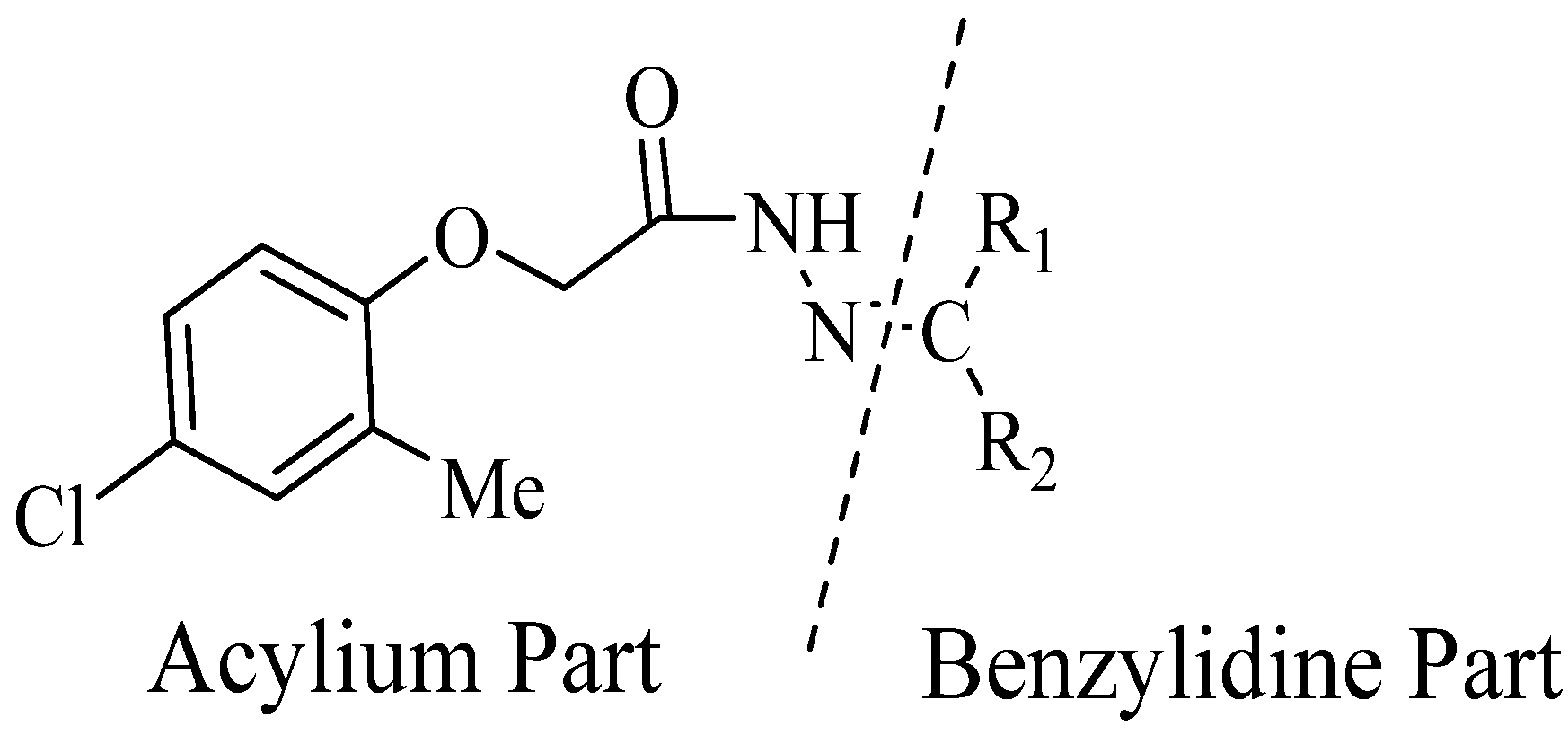
2.1. Chemistry
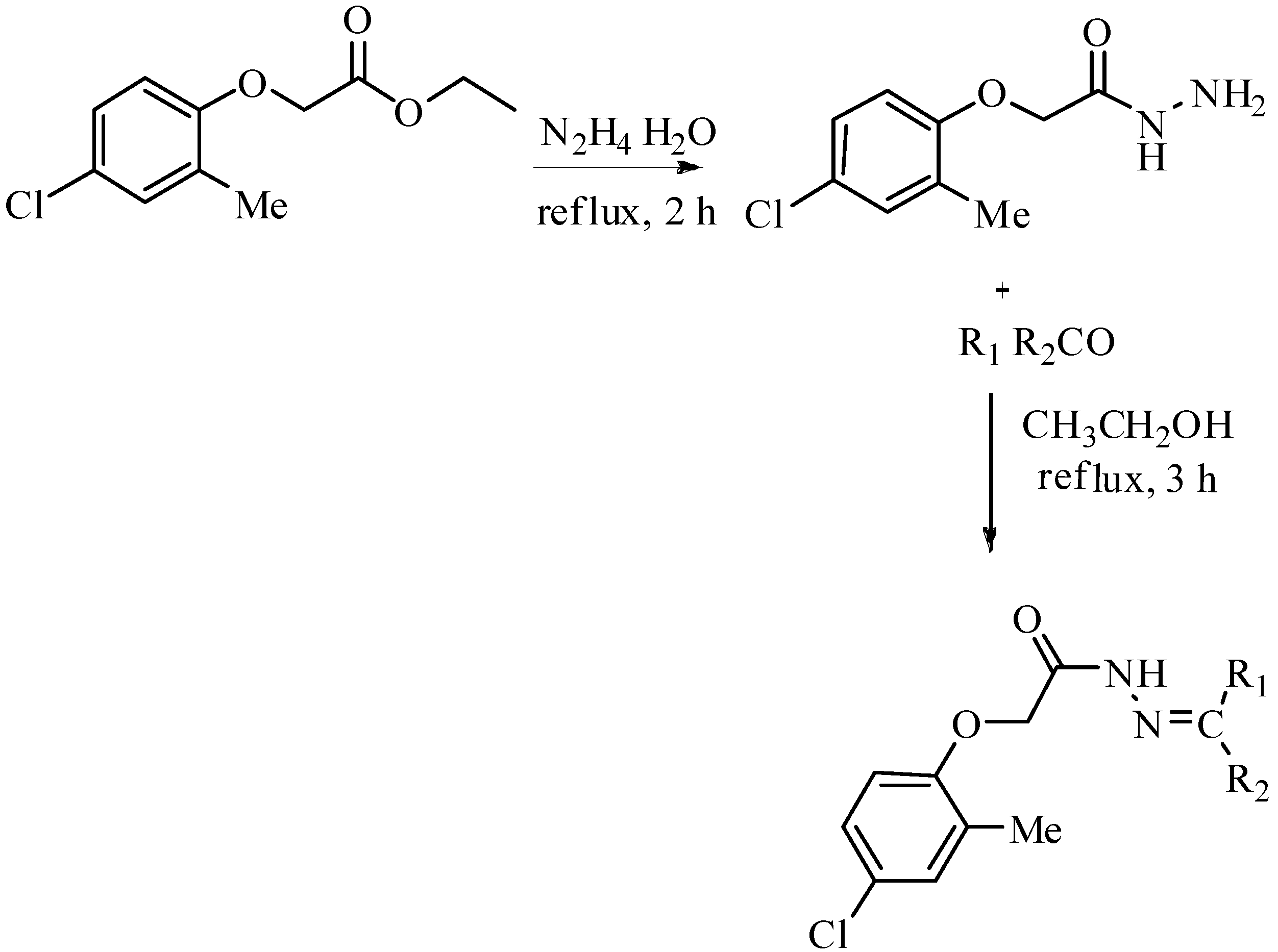
| Compound No. | R1 | R2 | Yield (%) | Compound No. | R1 | R2 | Yield (%) | ||
|---|---|---|---|---|---|---|---|---|---|
| 1 |  | H | 81 | 15 | 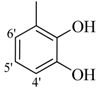 | H | 88 | ||
| 2 | 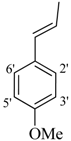 | H | 85 | 16 | 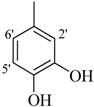 | H | 92 | ||
| 3 | 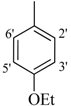 | H | 93 | 17 |  | CH3 | 94 | ||
| 4 | 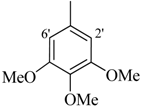 | H | 87 | 18 | 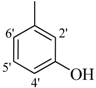 | H | 89 | ||
| 5 |  | H | 83 | 19 | 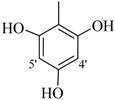 | CH3 | 87 | ||
| 6 |  | H | 86 | 20 |  | H | 84 | ||
| 7 |  | H | 89 | 21 |  | H | 94 | ||
| 8 | 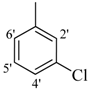 | H | 91 | 22 |  | H | 93 | ||
| 9 |  | H | 94 | 23 | 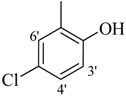 | H | 91 | ||
| 10 | 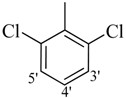 | H | 82 | 24 |  | CH3 | 95 | ||
| 11 | 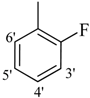 | H | 88 | 25 | 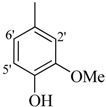 | H | 91 | ||
| 12 | 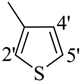 | H | 91 | 26 | 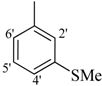 | H | 88 | ||
| 13 |  | H | 86 | 27 |  | H | 81 | ||
| 14 | 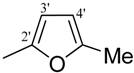 | H | 93 | 28 |  | H | 93 | ||
2.2. β-Glucuronidase
| Compounds | IC50 (μM ± SEM a) | Compounds | IC50 (μM ± SEM a) |
|---|---|---|---|
| 1 | 9.20 ± 0.32 | 15 | 12.0 ± 0.16 |
| 2 | NA b | 16 | NA b |
| 3 | NA b | 17 | NA b |
| 4 | NA b | 18 | NA b |
| 5 | 9.47 ± 0.16 | 19 | NA b |
| 6 | NA b | 20 | NA b |
| 7 | 14.7 ± 0.19 | 21 | 13.7 ± 0.40 |
| 8 | 15.4 ± 1.56 | 22 | 22.0 ± 0.14 |
| 9 | NA b | 23 | NA b |
| 10 | NA b | 24 | NA b |
| 11 | 19.6 ± 0.62 | 25 | NA b |
| 12 | 30.7 ± 1.49 | 26 | NA b |
| 13 | NA b | 27 | NA b |
| 14 | NA b | 28 | NA b |
| D-saccharic acid-1,4-lactone c | 48.4 ± 1.25 | - | - |
3. Experimental
3.1. General Information
3.2. Biological Assays
3.3. Assay for β-D-Glucuronidase
3.4. Typical Method for the Synthesis of Compounds 1–28
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Küçükgüzel, S.G.; Mazi, A.; Sahin, F.; Öztürk, S.; Stables, J. Synthesis and biological activities of diflunisal hydrazide hydrazones. Eur. J. Med. Chem. 2003, 38, 1005–1013. [Google Scholar] [CrossRef]
- Melnyk, P.; Leroux, V.; Sergheraert, C.; Grellier, P. Design, synthesis and in vitro antimalarial activity of an acylhydrazone library. Bioorg. Med. Chem. Lett. 2006, 16, 31–35. [Google Scholar] [CrossRef]
- Lima, P.C.; Lima, L.M.; da Silva, K.C.M.; Léda, P.H.O.; Miranda, A.L.P.; Fraga, C.A.M.; Barreiro, E.J. Synthesis and analgesic activity of novel N-acyl aryl hydrazones and isosters, derived from natural safrole. Eur. J. Med. Chem. 2000, 35, 187–203. [Google Scholar]
- Cunha, A.C.; Figueiredo, J.M.; Tributino, J.L.M.; Miranda, A.L.P.; Castro, H.C.; Zingali, R.B.; Fraga, C.A.M.; de Souza, M.C.B.V.; Ferreira, V.F.; Barreiro, E. Antiplatelet properties of novel N-Substituted-phenyl-1,2,3-triazole-4-acylhydrazone Derivatives. J. Bioorg. Med. Chem. 2003, 11, 2051–2059. [Google Scholar]
- Bedia, K.K.; Elçin, O.; Seda, U.; Fatma, K.; Nathaly, S.; Sevim, R.; Dimoglo, A. Synthesis and characterization of novel hydrazide-hydrazones and the study of their structure antituberculosis activity. Eur. J. Med. Chem. 2006, 41, 1253–1261. [Google Scholar]
- Terzioglu, N.; Gürsoy, A. Synthesis and anticancer evaluation of some new hydrazone derivatives of 2,6-dimethylimidazo[2,1-b][1,3,4]thiadiazole-5-carbohydrazide. Eur. J. Med. Chem. 2003, 38, 781–786. [Google Scholar] [CrossRef]
- Beraldo, H.; Gambino, D. The wide pharmacological versatility of semicarbazones, thiosemicarbazones and their metal complexes. Mini-Rev. Med. Chem. 2004, 4, 31–39. [Google Scholar]
- Costa, R.F.F.; Rebolledo, A.P.; Matencio, T.; Calado, H.D.R.; Ardisson, J.D.; Cortes, M.E.; Rodrigues, B.L.; Beraldo, H. Metal complexes of 2-benzoylpyridine-derived thiosemicarbazones: Structural, electrochemical and biological studies. J. Coord. Chem. 2005, 58, 1307–1319. [Google Scholar] [CrossRef]
- Hussain, Z.; Khan, K.M.; Perveen, S.; Nawaz, Y.; Bukhari, I.H. Antifungal activity of the pyrolyzate of glucose, sucrose and starch in comparison to paper pyrolyzate. J. Chem. Soc. Pak. 2011, 33, 694–697. [Google Scholar]
- Bondock, S.; Khalifa, W.; Fadda, A.A. Synthesis and antimicrobial evaluation of some new thiazole, thiazolidinone and thiazoline derivatives starting from 1-chloro-3,4-dihydronaphthalene-2-carboxaldehyde. Eur. J. Med. Chem. 2007, 42, 948–954. [Google Scholar]
- Bernardino, A.M.R.; Gomes, A.O.; Charret, K.S.; Freitas, A.C.C.; Machado, G.M.C.; Canto-Cavalheiro, M.M.; Leon, L.L.; Amaral, V.F. Synthesis and leishmanicidal activities of 1-(4-X-phenyl)-N′-[(4-Y-phenyl)methylene]-1H-pyrazole-4-carbohydrazides. Eur. J. Med. Chem. 2006, 41, 80–87. [Google Scholar] [CrossRef]
- Xia, Y.; Fan, C.-D.; Zhao, B.-X.; Zhao, J.; Shin, D.-S.; Miao, J.-Y. Synthesis and structure activity relationships of novel 1-arylmethyl-3-aryl-1H-pyrazole-5-carbohydrazide hydrazone derivatives as potential agents against A549 lung cancer cells. Eur. J. Med. Chem. 2008, 43, 2347–2353. [Google Scholar] [CrossRef]
- Zheng, L.W.; Wu, L.L.; Zhao, B.-X.; Dong, W.L.; Miao, J.Y. Synthesis of novel substituted pyrazole-5-carbohydrazide hydrazone derivatives and discovery of a potent apoptosis inducer in A549 lung cancer cells. Bioorg. Med. Chem. 2009, 17, 1957–1962. [Google Scholar] [CrossRef]
- Todeschini, A.R.; Miranda, A.L.; Silva, C.M.; Parrini, S.C.; Barreiro, E.J. Synthesis and evaluation of analgesic, antiinflammatory and antiplatelet properties of new 2-pyridylarylhydrazone derivatives. Eur. J. Med. Chem. 1998, 33, 189–199. [Google Scholar] [CrossRef]
- Anouar, E.H.; Raweh, S.; Bayach, I.; Taha, M.; Baharudin, M.S.; Meo, F.D.; Hasan, M.H.; Adam, A.; Ismail, N.H.; Weber, J.F.; et al. Antioxidant properties of phenolic Schiff bases: Structure-activity relationship and mechanism of action. J. Comput. Aided. Mol. Des. 2013, 27, 951–964. [Google Scholar]
- Taha, M.; Ismail, N.H.; Jamil, W.; Yousuf, S.; Jaafar, F.M.; Ali, M.I.; Kashif, S.M.; Hussain, E. Synthesis, evaluation of antioxidant activity and crystal structure of 2,4-dimethylbenzoylhydrazones. Molecules 2013, 18, 10912–10929. [Google Scholar]
- Khan, K.M.; Shah, Z.; Ahmad, V.U.; Khan, M.; Taha, M.; Ali, S.; Perveen, S.; Choudhary, M.I.; Voelter, W. 2,4,6-Trichlorophenylhydrazine Schiff bases as DPPH Radical and super oxide anion scavengers. Med. Chem. 2012, 8, 452–461. [Google Scholar] [CrossRef]
- Khan, K.M.; Taha, M.; Naz, F.; Ali, S.; Perveen, S.; Choudhary, M.I. Synthesis of acylhydrazide Schiff bases and their anti-oxidant activity. Med. Chem. 2012, 8, 705–710. [Google Scholar] [CrossRef]
- Khan, K.M.; Shah, Z.; Ahmad, V.U.; Khan, M.; Taha, M.; Rahim, F.; Jahan, H.; Perveen, H.; Choudhary, M.I. Synthesis of 2,4,6-trichlorophenyl hydrazones and their inhibitory potential against glycation of protein. Med. Chem. 2011, 7, 572–580. [Google Scholar] [CrossRef]
- Khan, K.M.; Rahim, F.; Ambreen, N.; Taha, M.; Khan, M.; Jahan, H.; Najeebullah, U.; Shaikh, A.; Iqbal, S.; Perveen, S.; et al. Synthesis of benzophenonehydrazone Schiff bases and their in vitro antiglycating activities. Med. Chem. 2013, 9, 588–595. [Google Scholar] [CrossRef]
- Taha, M.; Naz, H.; Rasheed, S.; Ismail, N.H.; Rahman, A.A.; Yousuf, S.; Choudhary, M.I. Synthesis of 4-Methoxybenzoylhydrazones and Evaluation of Their Antiglycation Activity. Molecules 2014, 19, 1286–1301. [Google Scholar] [CrossRef]
- Khan, K.M.; Taha, M.; Rahim, F.; Fakhri, M.I.; Jamil, W.; Khan, M.; Rasheed, S.; Karim, A.; Perveen, S.; Choudhary, M.I. Acylhydrazide Schiff bases: Synthesis and antiglycation activity. J. Pak. Chem Soc. 2013, 35, 929–937. [Google Scholar]
- Taha, M.; Baharudin, M.S.; Ismail, N.H.; Khan, K.M.; Jaafar, F.M.; Samreen; Siddiqui, S.; Choudhary, M.I. Synthesis of 2-methoxybenzoylhydrazone and evaluation of their antileishmanial activity. Bioorg. Med. Chem. Lett. 2013, 23, 3463–3466. [Google Scholar] [CrossRef]
- Musharraf, S.G.; Bibi, A.; Shahid, N.; Najam-ul-Haq, M.; Khan, M.; Taha, M.; Mughal, U.R.; Khan, K.M. Acylhydrazide and isatin Schiff bases as alternate UV-laser desorption ionization (LDI) matrices for low molecular weight (LMW) peptides analysis. Am. J. Anal. Chem. 2012, 3, 779–789. [Google Scholar] [CrossRef]
- De Graaf, M.; Boven, E.; Scheeren, H.W.; Haisma, H.J.; Pinedo, H.M. β-Glucuronidase-mediated drug release. Curr. Pharm. Design. 2002, 8, 1391–1403. [Google Scholar]
- Sperker, B.; Backman, J.T.; Kromer, K. The role of β-glucuronidase in drug disposition and drug targeting in humans. Clin. Pharmacokinet. 1997, 33, 18–31. [Google Scholar]
- Wakabayyashi, M. Metabolic Conjugation and Metabolic Hydrolysis; Fishman, W.H., Ed.; Academic Press: New York, NY, USA, 1970; p. 519. [Google Scholar]
- Paigen, K. Mammalian β-Glucuronidase: Genetics, molecular biology, and cell biology. Prog. Nucleic Acid Res. Mol. Biol. 1989, 37, 155–205. [Google Scholar] [CrossRef]
- Murdter, T.E.; Sperker, B.; Kivisto, K.T.; McClellan, M.; Fritz, P.; Friedel, G.; Linder, A.; Bosslet, K.; Toomes, H.; Dierkesmann, R.; et al. Enhanced uptake of doxorubicin into bronchial carcinoma: α-Glucuronidase mediates release of doxorubicin from a glucuromde prodrug (hmr 1826) at the tumor site. Cancer Res. 1997, 57, 2440. [Google Scholar]
- Sly, W.S.; Quinton, B.A.; McAlister, W.H.; Rimoin, D.L. β-Glucuronidase deficiency: Report of clinical, radiologic, and biochemical features of a new mucopolysaccharidosis. J. Pediatr. 1973, 82, 249–257. [Google Scholar] [CrossRef]
- Hall, C.W.; Cantz, M.; Neufeld, E.F. A β-Glucuronidase deficiency mucopolysaccharidosis: Studies in cultured fibroblasts. Arch. Biochem. Biophys. 1973, 155, 32–38. [Google Scholar]
- Khan, K.M.; Taha, M.; Ali, M.; Perveen, S. A mild and alternative approach towards symmetrical disulfides using H3IO5/NaHSO3 combination. Lett. Org. Chem. 2009, 6, 319–320. [Google Scholar]
- Khan, K.M.; Khan, M.Z.; Taha, M.; Maharvi, G.M.; Saify, Z.S.; Parveen, S.; Choudhary, M.I. Leishmanicidal potential of N-substituted morpholine derivatives: Synthesis and structure-activity relationships. Nat. Prod. Res. 2009, 23, 479–484. [Google Scholar] [CrossRef]
- Taha, M.; Ismail, N.H.; Imran, S.; Khan, K.M. 4-[5-(2-Methoxyphenyl)-1,3,4-oxadiazol-2-yl] benzohydrazide. Molbank 2014, 2014, M826. [Google Scholar] [CrossRef]
- Khan, K.M.; Shujaat, S.; Rahat, S.; Hayat, S.; Atta-ur-Rahman; Choudhary, M.I. β-N-Cyanoethyl acyl hydrazide derivatives: A new class of β-glucuronidase inhibitors. Chem. Pharm. Bull. 2002, 50, 1443–1446. [Google Scholar] [CrossRef]
- Ahmed, W.; Rani, M.; Khan, I.A.; Iqbal, A.; Khan, K.M.; Haleem, M.A.; Azim, M.K. Characterization of hydrazides and hydrazine derivatives as novel aspartic protease inhibitors. J. Enz. Inhib. Med. Chem. 2010, 25, 673–678. [Google Scholar] [CrossRef]
- Khan, K.M.; Taha, M.; Naz, F.; Khan, M.; Rahim, F.; Samreen ; Perveen, S.; Choudhary, M.I. Synthesis and in vitro leishmanicidal activity of disulfide derivatives. Med. Chem. 2011, 7, 704–710. [Google Scholar] [CrossRef]
- Khan, K.M.; Rasheed, M.; Zia-Ullah; Hayat, S.; Kaukab, F.; Choudhary, M.I.; Atta-ur-Rahman. Synthesis and in vitro leishmanicidal activity of some hydrazides and their analogs. Bioorg. Med. Chem. 2003, 11, 1381–1387. [Google Scholar] [CrossRef]
- Khan, K.M.; Naz, F.; Taha, M.; Khan, A.; Perveen, S.; Choudhary, M.I.; Voelter, W. Synthesis and in vitro urease inhibitory activity of N,N'-disubsituted thioureas. Eur. J. Med. Chem. 2014, 74, 314–323. [Google Scholar]
- Khan, K.M.; Rahim, F.; Wadood, A.; Taha, M.; Khan, M.; Naureen, S.; Ambreen, N.; Hussain, S.; Perveen, S.; Iqbal, M.C. Evaluation of bisindole as potent β-glucuronidase inhibitors: Synthesis and in silico based studies. Bioorg. Med. Chem. Lett. 2014, 24, 1825–1829. [Google Scholar]
- Khan, K.M.; Ambreen, N.; Taha, M.; Halim, S.A.; Zaheer-ul-Haq; Naureen, S.; Rasheed, S.; Perveen, S.; Ali, S.; Choudhary, M.I. Structure-based design, synthesis and biological evaluation of β-glucuronidase Inhibitors. J. Comput. Aided Mol. Des. 2014, 28, 577–585. [Google Scholar]
- Khan, K.M.; Rahim, F.; Wadood, A.; Kosar, N.; Taha, M.; Khan, A.; Fakhri, M.I.; Junaid, M.; Rehman, W.; Khan, M.; et al. Synthesis and molecular docking studies of potentn α-glucosidase inhibitors based on biscoumarin skeleton. Eur. J. Med. Chem. 2014, 81, 245–252. [Google Scholar]
- Collins, R.A.; Ng, T.B.; Fong, W.P.; Wan, C.C.; Yeung, H.W. Inhibition of glycohydrolase enzymes by aqueous extracts of chinese medicinal herbs in a microplate format. IUBMB Life 1997, 42, 1163–1169. [Google Scholar]
- Sample Availability: Not available
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamil, W.; Perveen, S.; Shah, S.A.A.; Taha, M.; Ismail, N.H.; Perveen, S.; Ambreen, N.; Khan, K.M.; Choudhary, M.I. Phenoxyacetohydrazide Schiff Bases: β-Glucuronidase Inhibitors. Molecules 2014, 19, 8788-8802. https://doi.org/10.3390/molecules19078788
Jamil W, Perveen S, Shah SAA, Taha M, Ismail NH, Perveen S, Ambreen N, Khan KM, Choudhary MI. Phenoxyacetohydrazide Schiff Bases: β-Glucuronidase Inhibitors. Molecules. 2014; 19(7):8788-8802. https://doi.org/10.3390/molecules19078788
Chicago/Turabian StyleJamil, Waqas, Shagufta Perveen, Syed Adnan Ali Shah, Muhammad Taha, Nor Hadiani Ismail, Shahnaz Perveen, Nida Ambreen, Khalid M. Khan, and Muhammad I. Choudhary. 2014. "Phenoxyacetohydrazide Schiff Bases: β-Glucuronidase Inhibitors" Molecules 19, no. 7: 8788-8802. https://doi.org/10.3390/molecules19078788
APA StyleJamil, W., Perveen, S., Shah, S. A. A., Taha, M., Ismail, N. H., Perveen, S., Ambreen, N., Khan, K. M., & Choudhary, M. I. (2014). Phenoxyacetohydrazide Schiff Bases: β-Glucuronidase Inhibitors. Molecules, 19(7), 8788-8802. https://doi.org/10.3390/molecules19078788





