RutheniumII(η6-arene) Complexes of Thiourea Derivatives: Synthesis, Characterization and Urease Inhibition
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization
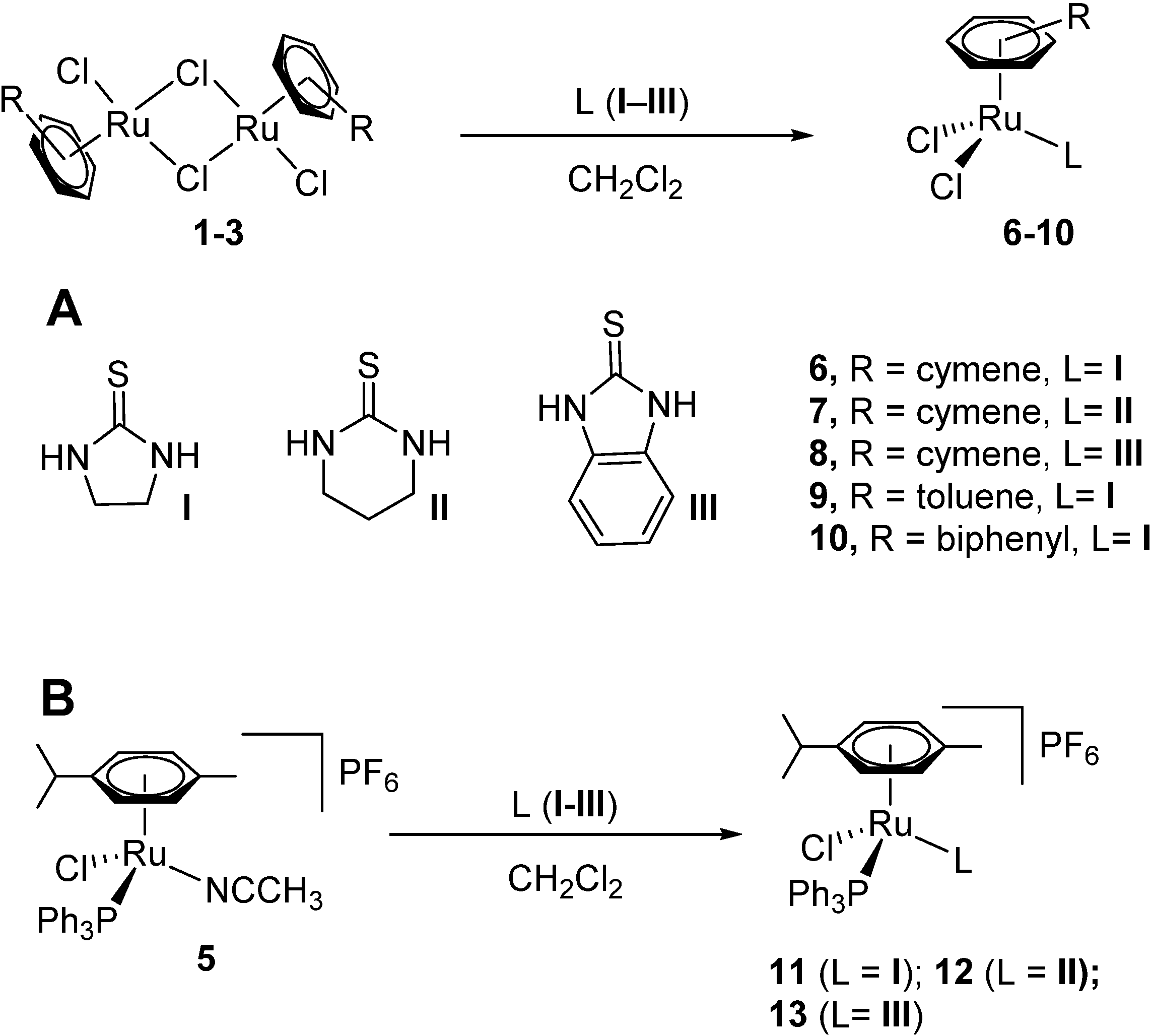
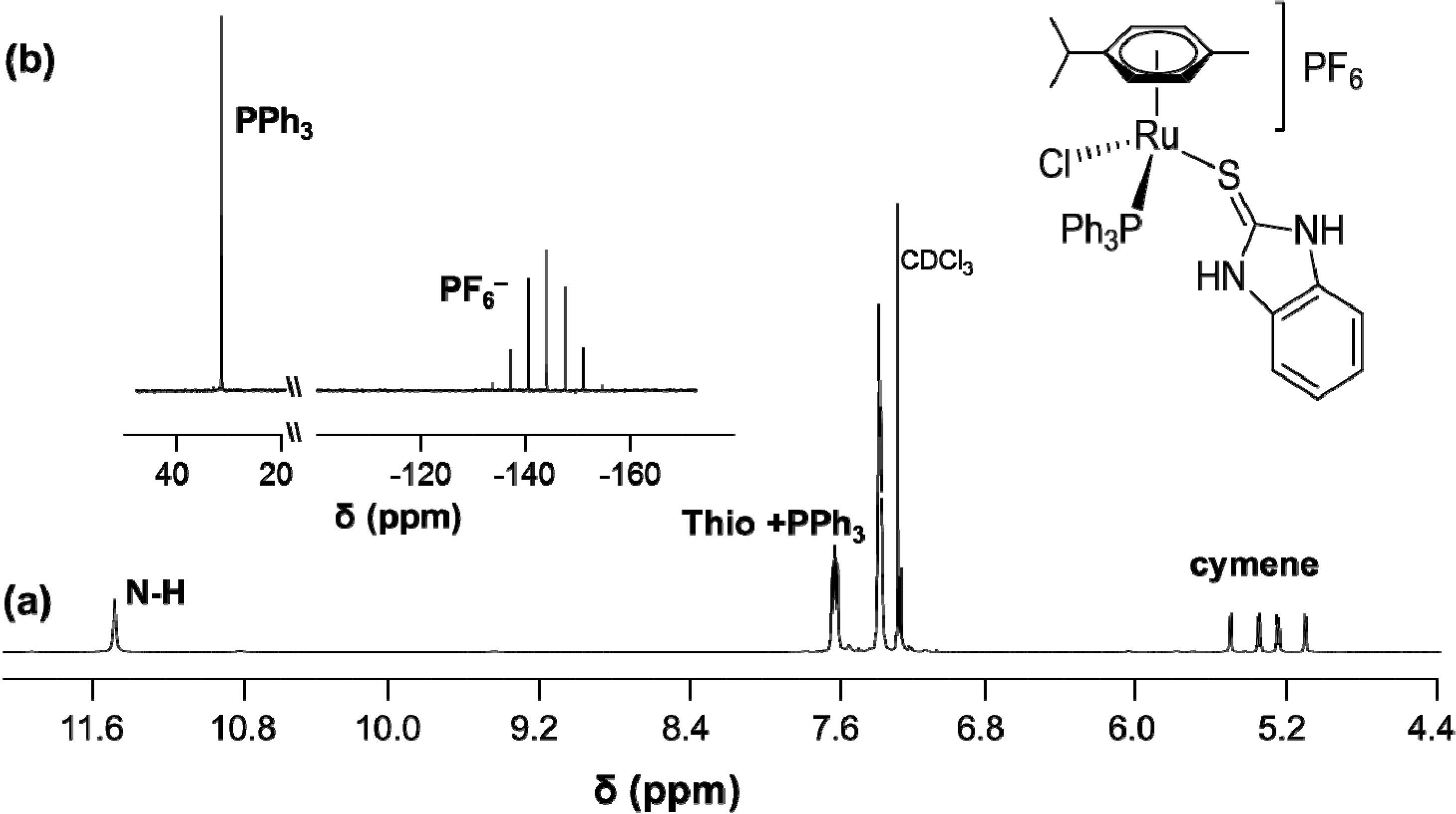
| Compound | 11 |
|---|---|
| CCDC N° | 1001627 |
| chemical formula | C31H35ClF6N2P2RuS·CH2Cl2 |
| M (g mol−1) | 865.06 |
| temperature (K) | 100(2) |
| crystal size (mm) | 0.23 × 0.20 × 0.02 |
| crystal color, habit | red, plate |
| crystal system | triclinic |
| space group | P-1 |
| a (Ǻ) | 9.7845(11) |
| b (Ǻ) | 9.9124(8) |
| c (Ǻ) | 18.1017(18) |
| V (Ǻ3) | 1729.5(3) |
| α (deg) | 81.773(5) |
| β (deg) | 86.024(6) |
| γ (deg) | 85.625(5) |
| Z | 2 |
| Dc (g cm−3) | 1.661 |
| μ (mm−1) | 0.898 |
| F(000) | 876.0 |
| Θ range (deg) | 2.23 to 25.34 |
| h range | −11/11 |
| k range | −11/10 |
| l range | −21/21 |
| No. unique refls. | 6034 |
| No. parameters | 425 |
| Rint | 0.1076 |
| R1 (obs.) | 0.0465 |
| wR2 (all data) | 0.1104 |
| S | 0.901 |

| Bond Lengths (Å) | |
|---|---|
| Ru–S | 2.399(13) |
| Ru–P | 2.369(12) |
| Ru–Cl | 2.415(11) |
| Ru–centroid | 1.739 |
| Bond Angles (°) | |
| S–Ru–P | 87.43(4) |
| S–Ru–Cl | 90.11(4) |
| P–Ru–Cl | 88.83(4) |
2.2. In Vitro Urease Inhibition Assay
| Compound | IC50 (μM) |
|---|---|
| I | 232 ± 27 |
| II | 118 ± 13 |
| III | ˃600 |
| 6 | 314 ± 62 |
| 7 | ˃600 |
| 8 | ˃600 |
| 9 | ˃600 |
| 10 | ˃600 |
| 11 | ˃600 |
| 12 | ˃600 |
| 13 | ˃600 |
| Thiourea | 22.1 ± 1.4 |
3. Experimental Section
3.1. Materials and Methods
3.2. Urease Inhibition Assay
General procedure for the synthesis of [RuII(η6-arene)(thiourea)Cl2] compounds 6–10
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Barry, N.P.E.; Sadler, P.J. Exploration of the medical periodic table: Towards new targets. Chem. Commun. 2013, 49, 5106–5131. [Google Scholar] [CrossRef]
- Gasser, G.; Metzler-Nolte, N. Metal compounds as enzyme inhibitors. In Bioinorganic Medicinal Chemistry; Alessio, E., Ed.; Wiley-VCH: Weinheim, Germany, 2011; pp. 351–382. [Google Scholar]
- Meggers, E. Targeting proteins with metal complexes. Chem. Commun. 2009, 1001–1010. [Google Scholar] [CrossRef]
- Kilpin, K.J.; Dyson, P.J. Enzyme inhibition by metal complexes: Concepts, strategies and applications. Chem. Sci. 2013, 4, 1410–1419. [Google Scholar] [CrossRef]
- Vock, C.A.; Ang, W.H.; Scolaro, C.; Phillips, A.D.; Lagopoulos, L.; Juillerat-Jeanneret, L.; Sava, G.; Scopelliti, R.; Dyson, P.J. Development of ruthenium antitumor drugs that overcome multidrug resistance mechanisms. J. Med. Chem. 2007, 50, 2166–2175. [Google Scholar] [CrossRef]
- Salmon, A.J.; Williams, M.L.; Hofmann, A.; Poulsen, S.A. Protein crystal structures with ferrocene and ruthenocene-based enzyme inhibitors. Chem. Commun. 2012, 48, 2328–2330. [Google Scholar]
- Giannini, F.; Furrer, J.; Ibao, A.F.; Suss-Fink, G.; Therrien, B.; Zava, O.; Baquie, M.; Dyson, P.J.; Stepnicka, P. Highly cytotoxic trithiophenolatodiruthenium complexes of the type [(η6-p-MeC6H4Pri)2Ru2(SC6H4-p-X)3]+: Synthesis, molecular structure, electrochemistry, cytotoxicity, and glutathione oxidation potential. J. Biol. Inorg. Chem. 2012, 17, 951–960. [Google Scholar] [CrossRef]
- Gras, M.; Therrien, B.; Suss-Fink, G.; Zava, O.; Dyson, P.J. Thiophenolato-bridged dinuclear arene ruthenium complexes: A new family of highly cytotoxic anticancer agents. Dalton Trans. 2010, 39, 10305–10313. [Google Scholar]
- Gupta, G.; Garci, A.; Murray, B.S.; Dyson, P.J.; Fabre, G.; Trouillas, P.; Giannini, F.; Furrer, J.; Suss-Fink, G.; Therrien, B. Synthesis, molecular structure, computational study and in vitro anticancer activity of dinuclear thiolato-bridged pentamethylcyclopentadienyl Rh(III) and Ir(III) complexes. Dalton Trans. 2013, 42, 15457–15463. [Google Scholar] [CrossRef]
- Hausinger, R.P. Biochemistry of Nickel, 1st ed.; Plenum Press: New York, NY, USA, 1993; Volume 12, p. 280. [Google Scholar]
- You, Z.L.; Ni, L.L.; Shi, D.H.; Bai, S. Synthesis, structures, and urease inhibitory activities of three copper(II) and zinc(II) complexes with 2-{[2-(2-hydroxyethylamino)ethylimino]methyl}-4-nitrophenol. Eur. J. Med. Chem. 2010, 45, 3196–3199. [Google Scholar] [CrossRef]
- Zhang, L.; Mulrooney, S.B.; Leung, A.F.K.; Zeng, Y.; Ko, B.B.C.; Hausinger, R.P.; Sun, H. Inhibition of urease by bismuth(III): Implications for the mechanism of action of bismuth drugs. BioMetals 2006, 19, 503–511. [Google Scholar] [CrossRef]
- Bacelar, A.H.; Carvalho, M.A.; Proenca, M.F. Synthesis and in vitro evaluation of substituted pyrimido[5,4-d]pyrimidines as a novel class of antimycobacterium tuberculosis agents. Eur. J. Med. Chem. 2010, 45, 3234–3239. [Google Scholar] [CrossRef]
- Chaplin, A.B.; Fellay, C.; Laurenczy, G.; Dyson, P.J. Mechanistic studies on the formation of η2-diphosphine (η6-p-cymene)ruthenium(II) compounds. Organometallics 2007, 26, 586–593. [Google Scholar] [CrossRef]
- Khan, K.M.; Naz, F.; Taha, M.; Khan, A.; Perveen, S.; Choudhary, M.I.; Voelter, W. Synthesis and in vitro urease inhibitory activity of N,N′-disubstituted thioureas. Eur. J. Med. Chem. 2014, 74, 314–323. [Google Scholar] [CrossRef]
- Hanif, M.; Nazarov, A.A.; Hartinger, C.G. Synthesis of [RuII(η6-p-cymene)(PPh3)(L)Cl]PF6 complexes with carbohydrate-derived phosphites, imidazole or indazole co-ligands. Inorg. Chim. Acta 2012, 380, 211–215. [Google Scholar] [CrossRef]
- Peacock, A.F.A.; Melchart, M.; Deeth, R.J.; Habtemariam, A.; Parsons, S.; Sadler, P.J. Osmium(II) and ruthenium(II) arene maltolato complexes: Rapid hydrolysis and nucleobase binding. Chem. Eur. J. 2007, 13, 2601–2613. [Google Scholar] [CrossRef]
- Scolaro, C.; Chaplin, A.B.; Hartinger, C.G.; Bergamo, A.; Cocchietto, M.; Keppler, B.K.; Sava, G.; Dyson, P.J. Tuning the hydrophobicity of ruthenium(II)-arene (RAPTA) drugs to modify uptake, biomolecular interactions and efficacy. Dalton Trans. 2007, 5065–5072. [Google Scholar]
- Bennett, M.A.; Smith, A.K. Arene ruthenium(II) complexes formed by dehydrogenation by cyclohexadienes with ruthenium(III) trichloride. J. Chem. Soc. Dalton Trans. 1974, 233–241. [Google Scholar] [CrossRef]
- Scolaro, C.; Bergamo, A.; Brescacin, L.; Delfino, R.; Cocchietto, M.; Laurenczy, G.; Geldbach, T.J.; Sava, G.; Dyson, P.J. In vitro and in vivo evaluation of ruthenium(II)-arene pta complexes. J. Med. Chem. 2005, 48, 4161–4171. [Google Scholar] [CrossRef]
- Ashraf, W.; Ahmad, S.; Isab, A.A. Silver cyanide complexes of heterocyclic thiones. Transition Met. Chem. 2004, 29, 400–404. [Google Scholar] [CrossRef]
- Chaplin, A.B. Catalytic activity of bis-phosphine ruthenium(II)-arene compounds. Chimia 2008, 62, 217–220. [Google Scholar] [CrossRef]
- Pressprich, M.R.; Chambers, J. Saint + Integration Engine, Program for Crystal Structure Integration; Bruker Analytical X-ray systems: Madison, WI, USA, 2004. [Google Scholar]
- Sheldrick, G.M. SHELXL-97, Program for Crystal Structure Refinement; University Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A: Found. Crystallogr. 2008, A64, 112–122. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. Olex2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0—new features for the visualization and investigation of crystal structures. J. Appl. Crystallogr. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Weatherburn, M.W. Phenol-hypochlorite reaction for determination of ammonia. Anal. Chem. 1967, 39, 971–974. [Google Scholar] [CrossRef]
- Aslam, M.A.S.; Mahmood, S.; Shahid, M.; Saeed, A.; Iqbal, J. Synthesis, biological assay in vitro and molecular docking studies of new schiff base derivatives as potential urease inhibitors. Eur. J. Med. Chem. 2011, 46, 5473–5479. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.
© 2014 by the authors. licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hanif, M.; Nawaz, M.A.H.; Babak, M.V.; Iqbal, J.; Roller, A.; Keppler, B.K.; Hartinger, C.G. RutheniumII(η6-arene) Complexes of Thiourea Derivatives: Synthesis, Characterization and Urease Inhibition. Molecules 2014, 19, 8080-8092. https://doi.org/10.3390/molecules19068080
Hanif M, Nawaz MAH, Babak MV, Iqbal J, Roller A, Keppler BK, Hartinger CG. RutheniumII(η6-arene) Complexes of Thiourea Derivatives: Synthesis, Characterization and Urease Inhibition. Molecules. 2014; 19(6):8080-8092. https://doi.org/10.3390/molecules19068080
Chicago/Turabian StyleHanif, Muhammad, Muhammad Azhar Hayat Nawaz, Maria V. Babak, Jamshed Iqbal, Alexander Roller, Bernhard K. Keppler, and Christian G. Hartinger. 2014. "RutheniumII(η6-arene) Complexes of Thiourea Derivatives: Synthesis, Characterization and Urease Inhibition" Molecules 19, no. 6: 8080-8092. https://doi.org/10.3390/molecules19068080
APA StyleHanif, M., Nawaz, M. A. H., Babak, M. V., Iqbal, J., Roller, A., Keppler, B. K., & Hartinger, C. G. (2014). RutheniumII(η6-arene) Complexes of Thiourea Derivatives: Synthesis, Characterization and Urease Inhibition. Molecules, 19(6), 8080-8092. https://doi.org/10.3390/molecules19068080







