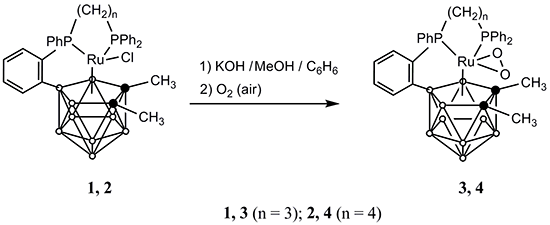
An Unusual Conversion of Paramagnetic [3-Cl-3,3,8-{Ph2P(CH2)nPPh-µ-(C6H4-ortho)}-1,2-(CH3)2-closo-3,1,2-RuIIIC2B9H8] (n = 3 and 4) to Form the First 18-Electron P-Phenylene ortho-Cycloboronated closo-Ruthenacarboranes with a Dioxygen Ligand
1. Introduction
2. Results and Discussion
 H8] (1,2-dimethyl-3-chloro-3,8-µ-{ortho-[phenyl-(3'-diphenylphosphinopropyl-κP(Ru))phosphino]phenyl-P(Ru),C1(B8)}-1,2-dicarba-3-ruthena-closo-dodecaborane (1) n = 3 [7] and 1,2-dimethyl-3-chloro-3,8-μ-{ortho-[phenyl-(4'-diphenylphosphinobutyl-κP(Ru))phosphino]phenyl-P(Ru),C1(B8)}-1,2-dicarba-3-ruthena-closo-dodecaborane (2); n = 4 [9]) with the hope of preparing either ortho-cycloboronated 11-vertex {RuC2B8} complexes or smaller clusters of these series. Accordingly, we examined the reaction of 1 and 2 with a fourfold molar excess of KOH at room temperature in a 1:1 benzene/methanol mixture in air.
H8] (1,2-dimethyl-3-chloro-3,8-µ-{ortho-[phenyl-(3'-diphenylphosphinopropyl-κP(Ru))phosphino]phenyl-P(Ru),C1(B8)}-1,2-dicarba-3-ruthena-closo-dodecaborane (1) n = 3 [7] and 1,2-dimethyl-3-chloro-3,8-μ-{ortho-[phenyl-(4'-diphenylphosphinobutyl-κP(Ru))phosphino]phenyl-P(Ru),C1(B8)}-1,2-dicarba-3-ruthena-closo-dodecaborane (2); n = 4 [9]) with the hope of preparing either ortho-cycloboronated 11-vertex {RuC2B8} complexes or smaller clusters of these series. Accordingly, we examined the reaction of 1 and 2 with a fourfold molar excess of KOH at room temperature in a 1:1 benzene/methanol mixture in air. H8] (3, n = 3; 4, n = 4), respectively (Scheme 1).
H8] (3, n = 3; 4, n = 4), respectively (Scheme 1).
| Compound | 3 | 4 | |
|---|---|---|---|
| A | B | ||
| Ru(3)-O(1) | 2.037(2) | 2.036(3) | 2.015(3) |
| Ru(3)-O(2) | 2.040(2) | 2.041(3) | 2.043(3) |
| Ru(3)-P(1) | 2.3412(7) | 2.3580(13) | 2.3693(13) |
| Ru(3)-P(2) | 2.2928(7) | 2.3201(13) | 2.3280(13) |
| Ru(3)-C(1) | 2.320(3) | 2.316(5) | 2.318(5) |
| Ru(3)-C(2) | 2.336(3) | 2.356(5) | 2.338(4) |
| Ru(3)-B(4) | 2.251(3) | 2.255(5) | 2.282(5) |
| Ru(3)-B(7) | 2.251(3) | 2.258(5) | 2.290(6) |
| Ru(3)-B(8) | 2.253(3) | 2.259(5) | 2.274(5) |
| O(1)-O(2) | 1.403(3) | 1.399(4) | 1.404(4) |
| C(1)-C(2) | 1.621(4) | 1.633(7) | 1.640(6) |
| O(1)-Ru(3)-O(2) | 40.26(8) | 40.14(12) | 40.49(12) |
| O(1)-Ru(3)-P(2) | 109.21(6) | 112.24(10) | 115.91(10) |
| O(2)-Ru(3)-P(2) | 79.80(6) | 81.07(10) | 83.70(9) |
| O(1)-Ru(3)-P(1) | 75.32(6) | 74.19(9) | 74.10(9) |
| O(2)-Ru(3)-P(1) | 100.35(6) | 98.09(10) | 95.59(10) |
| P(2)-Ru(3)-P(1) | 86.76(3) | 89.17(4) | 86.97(5) |
| O(2)-O(1)-Ru(3) | 69.97(11) | 69.76(18) | 68.68(18) |
| O(1)-O(2)-Ru(3) | 69.77(11) | 70.10(18) | 70.82(18) |
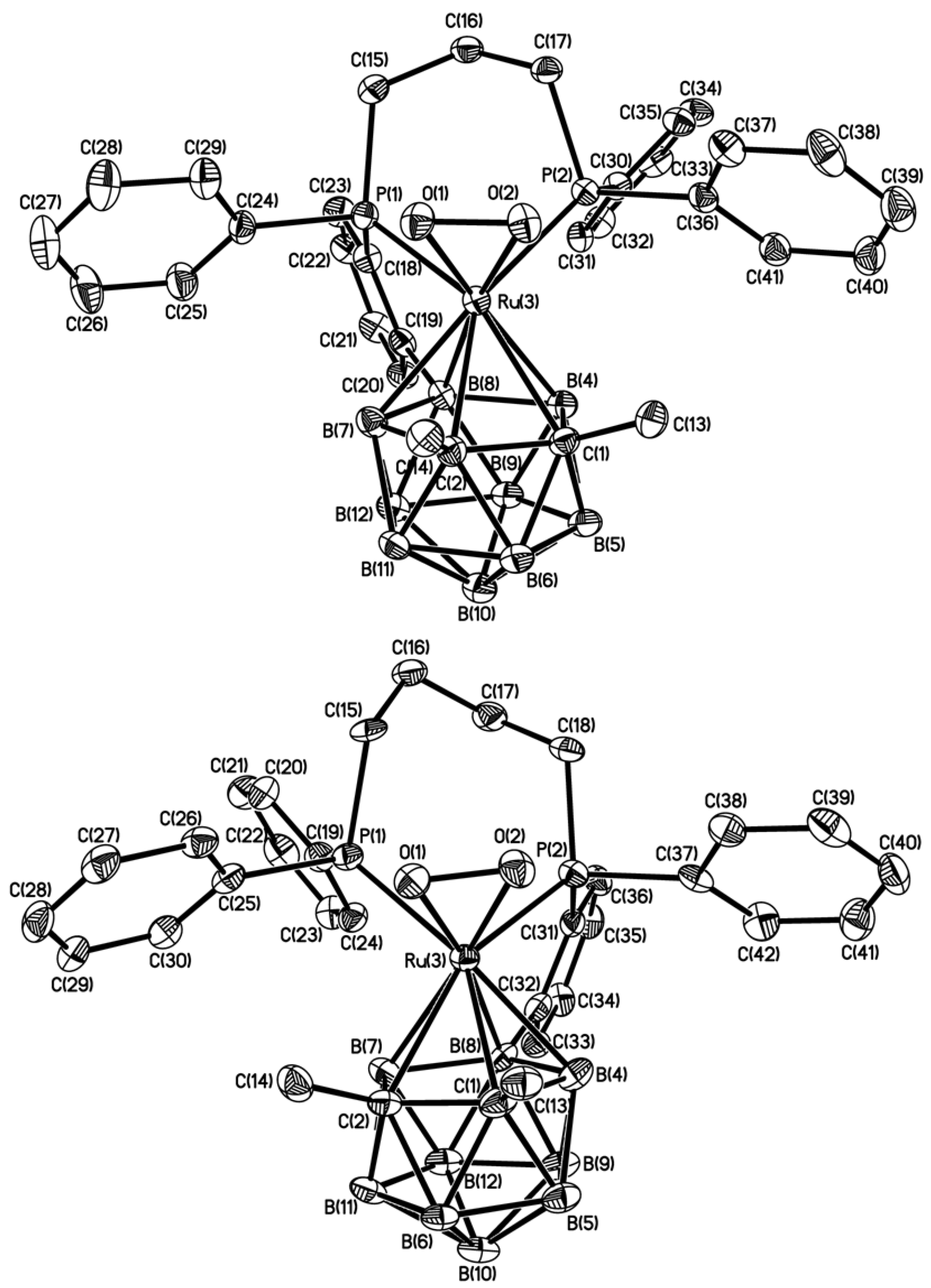
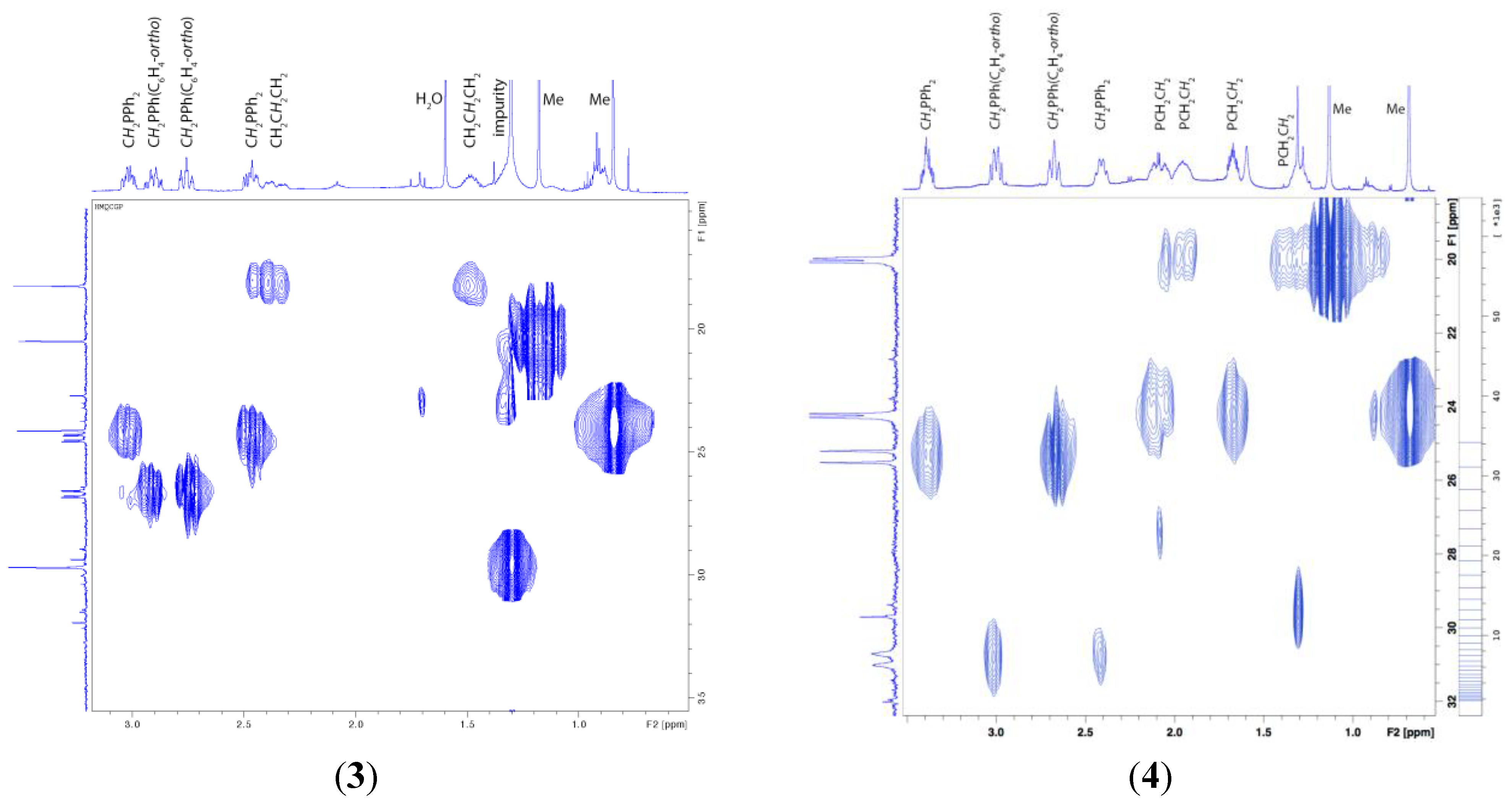
3. Experimental
3.1. General Information
3.2. Preparation of [3-(η2-O2)-3,3,8-[Ph2P(CH2)3PPh-µ-( ![Molecules 19 07094 i003]() H8] (3)
H8] (3)
3.3. Preparation of [3-(η2-O2)-3,3,8-[Ph2P(CH2)4PPh-µ-( ![Molecules 19 07094 i003]() H8] (4)
H8] (4)
3.4. X-ray Structure Determination for Complexes 3 and 4
| Compound | 3 | 4 |
|---|---|---|
| Molecular formula | C31H39B9O2P2Ru•2(CH2Cl2) | C32H41B9O2P2Ru•0.25(CH2Cl2) |
| Formula weight | 873.77 | 739.18 |
| Dimension, mm3 | 0.20 × 0.15 × 0.10 | 0.28 × 0.14 × 0.07 |
| Crystal system | triclinic | triclinic |
| Temperature, K | 120 | 100 |
| Space group | P?1 | P?1 |
| a, Å | 9.791(2) | 10.361(2) |
| b, Å | 10.063(4) | 16.496(4) |
| c, Å | 20.831(1) | 21.721(5) |
| α, deg. | 82.764(1) | 76.958(4) |
| β, deg. | 84.678(1) | 81.790(4) |
| γ, deg. | 62.696(1) | 79.194(4) |
| V, Å3 | 1894.6(1) | 3533(1) |
| Z | 2 | 4 |
| ρcalc, g·cm−3 | 1.532 | 1.390 |
| 2 θmax, deg. | 60 | 54 |
| Linear absorption ( µ), cm−1 | 8.13 | 7.95 |
| No. unique refl. ( Rint) | 10975 (0.0306) | 15322 (0.1018) |
| No. observed refl. ( I > 2σ(I)) | 8254 | 8960 |
| No. parameters | 470 | 877 |
| R1 (on F for observed refl.) a | 0.0456 | 0.0534 |
| wR2 (on F2 for all refl.) b | 0.1017 | 0.1197 |
| GOOF | 0.980 | 0.907 |
4. Conclusions
 H8] (3, n = 3) and (4, n = 4). They were prepared by a facile and potentially useful method starting from paramagnetic species [3-Cl-3,3,8-{Ph2P(CH2)nPPh-µ-(
H8] (3, n = 3) and (4, n = 4). They were prepared by a facile and potentially useful method starting from paramagnetic species [3-Cl-3,3,8-{Ph2P(CH2)nPPh-µ-(  H8] (n = 3 and 4), which formally undergo a mild base-mediated chlorine-oxygen displacement reaction. Based on these and the literature data, it is envisaged that suitably constituted paramagnetic ruthenacarborane complexes as the potential precursors might open up extensive applications in cluster ruthenacarborane chemistry as well as in the homogeneous metallacarborane catalysis.
H8] (n = 3 and 4), which formally undergo a mild base-mediated chlorine-oxygen displacement reaction. Based on these and the literature data, it is envisaged that suitably constituted paramagnetic ruthenacarborane complexes as the potential precursors might open up extensive applications in cluster ruthenacarborane chemistry as well as in the homogeneous metallacarborane catalysis.Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Crook, J.E.; Greenwood, N.N.; Kennedy, J.D.; McDonald, W.S. A novel exo-bicyclic closo-six-vertex dimetalla-hexaborane: [1,1,2-(CO)3–1-(PPh3)-2,2-(Ph2P
H2–3,5-)]•CH2Cl2. J. Chem. Soc. Chem. Commun. 1982. [Google Scholar] [CrossRef]
- Elrington, M.; Greenwood, N.N.; Kennedy, J.D.; Thornton-Pett, M. Preparation and nuclear magnetic resonance properties of eleven-vertex closo-type osmaundecaboranes and the X-ray crystal structure of the ortho-cycloboronated compound [2,5-(OEt)2-1-(PPh3)-1-(o-Ph2P
]. J. Chem. Soc. Dalton Trans. 1986. [Google Scholar] [CrossRef]
- Bould, J.; Brint, P.; Kennedy, J.D.; Thornton-Pett, M. Metallaborane reaction chemistry. Part 1. Two interesting closed cluster compounds from the reaction of acetylene with an open nido-6-iridadecaborane. J. Chem. Soc. Dalton Trans. 1993. [Google Scholar] [CrossRef]
- Crook, J.E.; Greenwood, N.N.; Kennedy, J.D.; McDonald, W.S. A novel oxidative insertion of a metal centre into a degraded closo-borane cluster; crystal and molecular structure of the ten-vertex iso-nido cluster [{IrC(OH)
H4PPh2)(PPh3)]. J. Chem. Soc. Chem. Commun. 1981. [Google Scholar] [CrossRef]
- Pisareva, I.V.; Konoplev, V.E.; Balagurova, E.V.; Yanovsky, A.I.; Dolgushin, F.M.; Cheredilin, D.N.; Chizhevsky, I.T.; Franken, A.; Carr, M.J.; Thornton-Pett, M.; et al. Metal-assisted polyhedral contraction reactions of monocarbon and dicarbon carborane species involving nido-{CB10}, nido-{CB9}, nido-C2B8} and exo-nido-C2B9Os} systems. In Boron Chemistry at the Beginning of the 21st Century; Bubnov, Y., Ed.; Editorial URSS: Moscow, Russian, 2003; pp. 271–278. [Google Scholar]
- Cheredilin, D.N.; Kadyrov, R.; Dolgushin, F.M.; Balagurova, E.V.; Godovikov, I.A.; Solodovnikov, S.P.; Chizhevsky, I.T. Chiral paramagnetic closo-ruthenacarboranes via phosphine-diphosphine displacement reaction of “three-bridge” exo-nido-ruthenacarboranes: Molecular Structure of (−)-[closo-3-Cl-3,3-{(Ph2PCHCH3)2CH2}-3,1,2-RuC2B9H11] and its ortho-cycloboronated derivative. Inorg. Chem. Commun. 2005, 8, 614–618. [Google Scholar]
- Kolyakina, E.V.; Grishin, I.D.; Cheredilin, D.N.; Dolgushin, F.M.; Chizhevsky, I.T.; Grishin, D.F. Ruthenium carborane complexes in the controlled radical polymerization of methyl methacrylate. Russ. Chem. Bull. 2006, 55, 89–93. [Google Scholar] [CrossRef]
- Grishin, I.D.; D’yachihin, D.I.; Piskunov, A.V.; Dolgushin, F.M.; Smol’yakov, A.F.; Il’in, M.M.; Davankov, V.A.; Chizhevsky, I.T.; Grishin, D.F. Carborane complexes of ruthenium(III): Studies on thermal reaction chemistry and the catalyst design for atom transfer radical polymerization of methyl methacrylate. Inorg. Chem. 2011, 50, 7574–7585. [Google Scholar] [CrossRef]
- Grishin, I.D.; Turmina, E.S.; D’yachihin, D.I.; Vinogradov, D.S.; Piskunov, A.V.; Smol’yakov, A.F.; Dolgushin, F.M.; Chizhevsky, I.T.; Grishin, D.F. Efficient catalytic systems based on paramagnetic closo-ruthenacarboranes for the controlled synthesis of polymers. Russ. Chem. Bull. 2011, 60, 2375–2383. [Google Scholar] [CrossRef]
- Grishin, I.D.; Turmina, E.S.; D’yachihin, D.I.; Chizhevsky, I.T.; Grishin, D.F. Carborane complexes of ruthenium with long-chain diphosphine ligands as effective catalysts of controlled radical polymerization. Polym. Sci. B 2014, 56, 1–10. [Google Scholar]
- Grishin, I.D.; D’yachihin, D.I.; Turmina, E.S.; Dolgushin, F.M.; Smol’yakov, A.F.; Piskunov, A.V.; Chizhevsky, I.T.; Grishin, D.F. Mononuclear closo-ruthenacarborane complexes containing a rare eight-membered metal-diphosphine ring. J. Organomet. Chem. 2012, 721–722, 113–118. [Google Scholar] [CrossRef]
- We note that a series of some other closo-bis(phosphine)hydridoruthenacarboranes with monoanionic charge-compensated cage ligands [13] were also found to display very high activity as Kharasch catalysts [14], where the importance of Ru (II) → Ru (III) transformation in this catalytic process is stressed.
- Tatusaus, O.; Núñez, R.; Viñas, C.; Teixidor, F.; Mata, I.; Molins, E. Synthesis, characterization, and dinamic studies of 12-vertex η5-ruthenium(II) closo-Phosphine complexes with monoanionic [10-L-nido-7-R-7,8-C2B9H9]-ligands. Inorg. Chem. 2004, 43, 6067–6074. [Google Scholar] [CrossRef]
- Tatusaus, O.; Viñas, C.; Núñez, R.; Teixidor, F.; Demonceau, A.; Delfosse, S.; Noels, A.F.; Mata, I.; Molins, E. The modulation possibilities of dicarbollide clusters: Optimizing the Kharasch catalysts. J. Am. Chem. Soc. 2003, 125, 11830–11831. [Google Scholar] [CrossRef]
- Jones, C.J.; Francis, J.N.; Hawthorne, M.F. New 10- and 11-atom polyhedral metallocarboranes prepared by polyhedral contraction. J. Am. Chem. Soc. 1972, 94, 8391–8399. [Google Scholar] [CrossRef]
- Jones, C.J.; Francis, J.N.; Hawthorne, M.F. The chemistry of an 11-atom polyhedral metallocarborane prepared by polyhedral contraction. J. Chem. Soc. Chem. Commun. 1972. [Google Scholar] [CrossRef]
- Dustin, D.F.; Hawthorne, M.F. Synthesis of nine-vertex, monocarbon metallocarboranes by polyhedral contraction. Inorg. Chem. 1973, 12, 1380–1383. [Google Scholar] [CrossRef]
- Hanusa, T.P.; Huffman, J.C.; Curtis, T.L.; Todd, L.J. Synthesis of (π-arene)metallacarbaboranes containing ruthenium or osmium and a (π-cyclohexadienyl)cobaltacarbaborane. Crystal structure of 2,5,6-(η-C6H6)RuC2B7H11. Inorg. Chem. 1985, 24, 787–792. [Google Scholar] [CrossRef]
- Kirchner, K.; Mauthner, K.; Mereiter, K.; Schmid, R. Irreversible binding of dioxygen and other gases to a half-sandwich ruthenium(II) complex: X-ray structure of [Ru(η2-O2)(η5-C5Me5)(Ph2PCH2CH2PPh2)]PF6. J. Chem. Soc. Chem. Commun. 1993. [Google Scholar] [CrossRef]
- Jia, G.; Ng, W.S.; Chu, H.S.; Wong, W.-T.; Yu, N.-T.; Williams, I.D. Dioxygen Complexes from the Reactions of [Cp*RuH2(PP)]+ (PP = dppm, dppe) with Air. Organometallics 1999, 18, 3597–3602. [Google Scholar] [CrossRef]
- Teixidor, F.; Ayllón, J.A.; Viñas, C.; Sillanpää, R.; Kivekäs, R.; Casabó, J. Iridium coordination to exo-dithio-7,8-dicarba-nido-undecaborate derivatives. Inorg. Chem. 1994, 33, 4815–4818. [Google Scholar] [CrossRef]
- Yanovsky, A.I. Metallocarbaboranes. Structural studies in recent years. Russ. Chem. Rev. 1985, 54, 515–531. [Google Scholar] [CrossRef]
- SMART v5.051 and SAINT v 5.00, Area Detector Control and Integration Software, Bruker AXS Inc.: Madison, WI, USA, 1998.
- APEX II Software Package, Bruker AXS Inc.: Madison, WI, USA, 2005.
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 1–4 are available from the authors.
© 2014 by the authors. licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kostukovich, A.Y.; D'yachihin, D.I.; Dolgushin, F.M.; Smol'yakov, A.F.; Godovikov, I.A.; Chizhevsky, I.T. An Unusual Conversion of Paramagnetic [3-Cl-3,3,8-{Ph2P(CH2)nPPh-µ-(C6H4-ortho)}-1,2-(CH3)2-closo-3,1,2-RuIIIC2B9H8] (n = 3 and 4) to Form the First 18-Electron P-Phenylene ortho-Cycloboronated closo-Ruthenacarboranes with a Dioxygen Ligand. Molecules 2014, 19, 7094-7103. https://doi.org/10.3390/molecules19067094
Kostukovich AY, D'yachihin DI, Dolgushin FM, Smol'yakov AF, Godovikov IA, Chizhevsky IT. An Unusual Conversion of Paramagnetic [3-Cl-3,3,8-{Ph2P(CH2)nPPh-µ-(C6H4-ortho)}-1,2-(CH3)2-closo-3,1,2-RuIIIC2B9H8] (n = 3 and 4) to Form the First 18-Electron P-Phenylene ortho-Cycloboronated closo-Ruthenacarboranes with a Dioxygen Ligand. Molecules. 2014; 19(6):7094-7103. https://doi.org/10.3390/molecules19067094
Chicago/Turabian StyleKostukovich, Alexander Y., Dmitrii I. D'yachihin, Fedor M. Dolgushin, Alexander F. Smol'yakov, Ivan A. Godovikov, and Igor T. Chizhevsky. 2014. "An Unusual Conversion of Paramagnetic [3-Cl-3,3,8-{Ph2P(CH2)nPPh-µ-(C6H4-ortho)}-1,2-(CH3)2-closo-3,1,2-RuIIIC2B9H8] (n = 3 and 4) to Form the First 18-Electron P-Phenylene ortho-Cycloboronated closo-Ruthenacarboranes with a Dioxygen Ligand" Molecules 19, no. 6: 7094-7103. https://doi.org/10.3390/molecules19067094