The Effect of Chitin Size, Shape, Source and Purification Method on Immune Recognition
Abstract
:1. Introduction
2. Results and Discussion
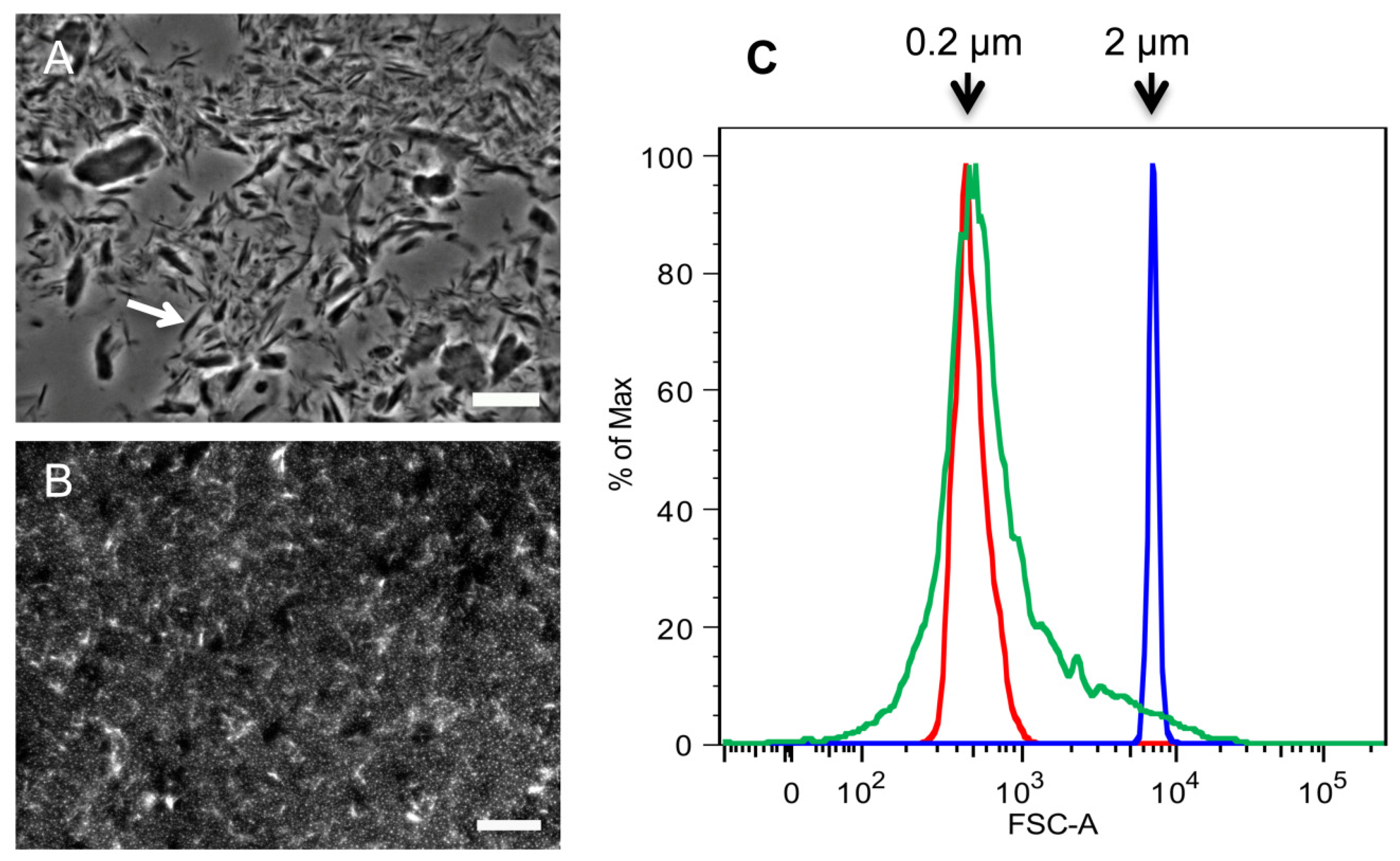
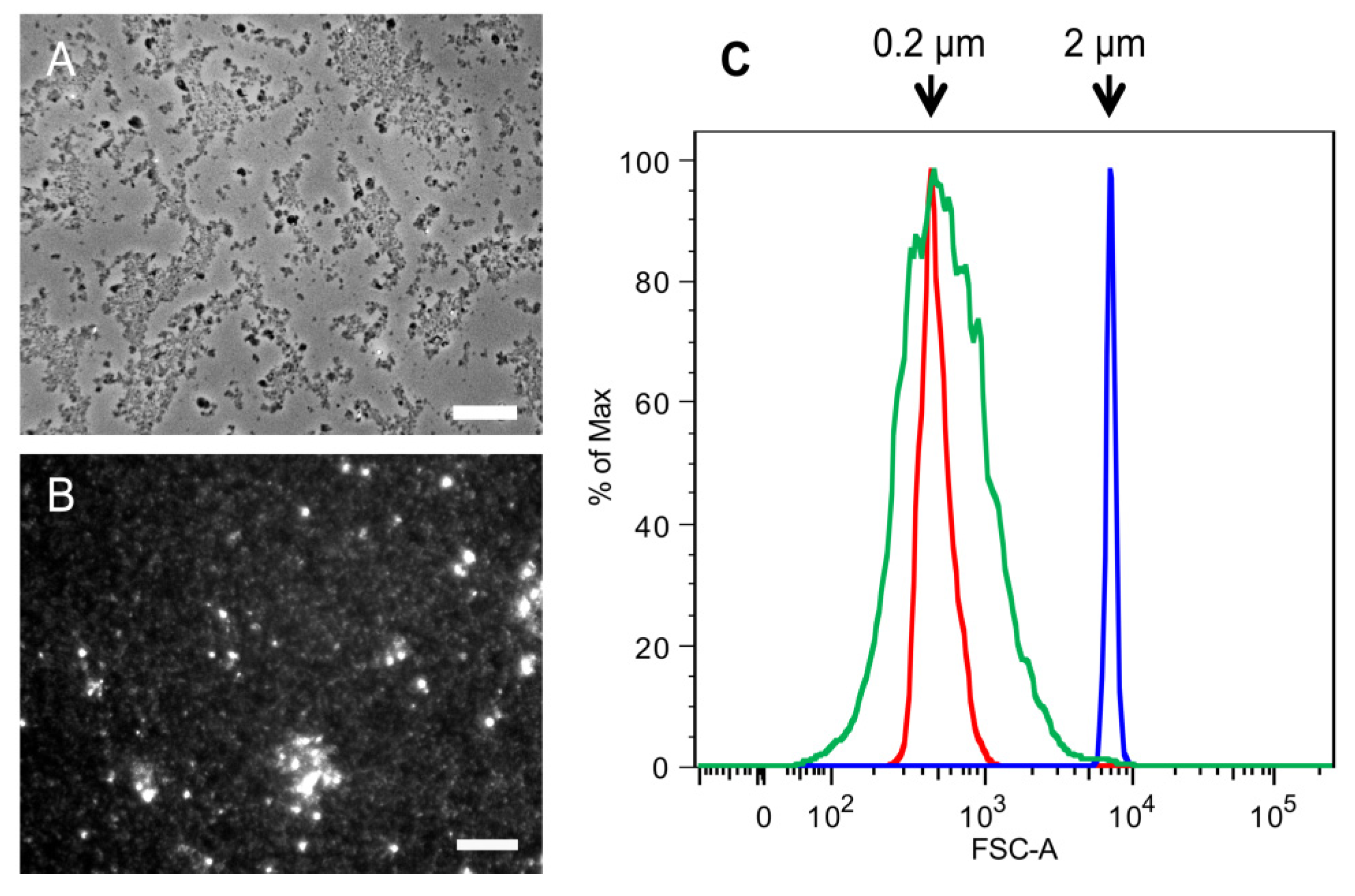
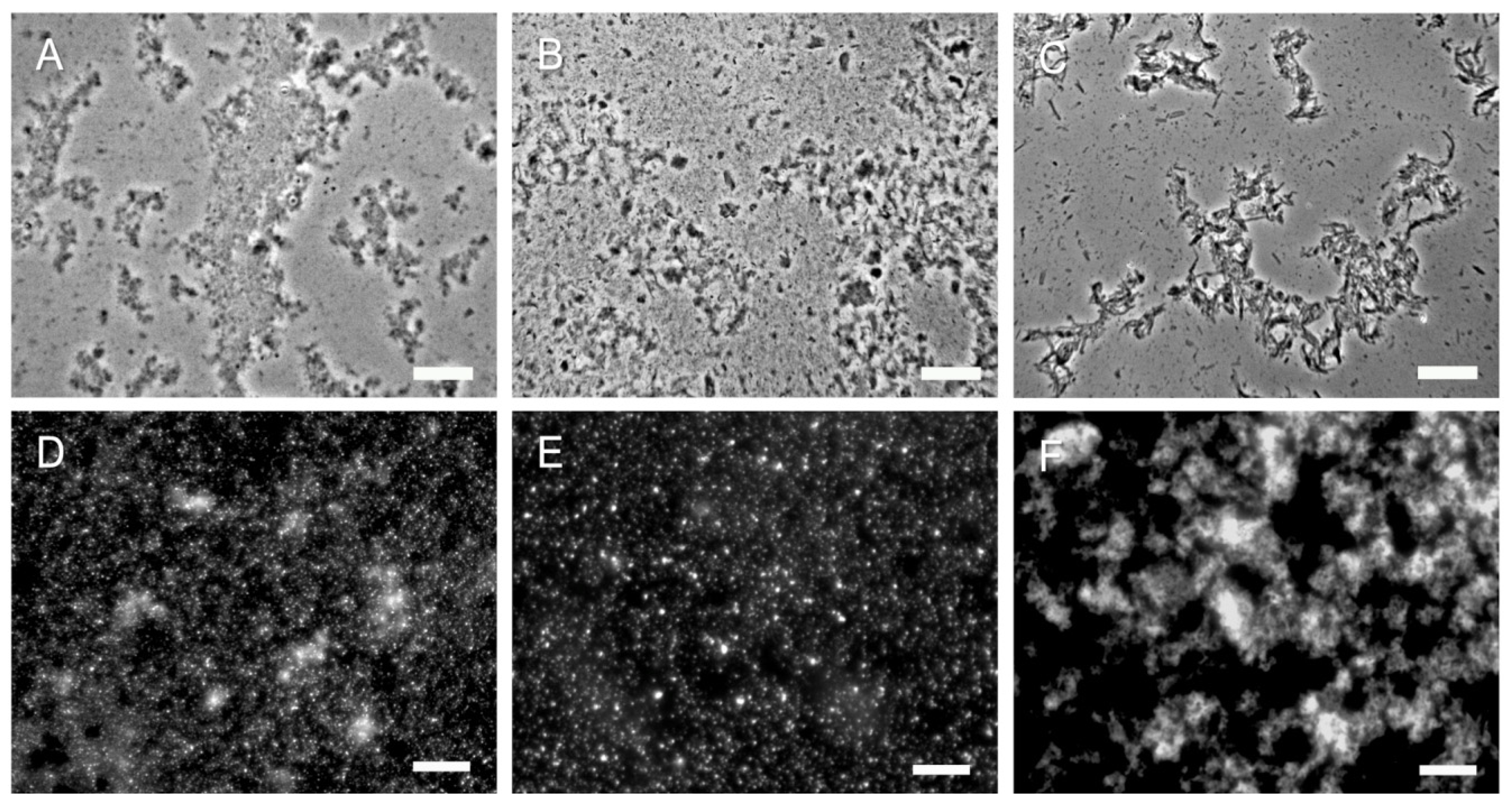
2.1. Purification of Commercial Chitin Results in Two Populations of Particles Very Different in Size and Homogeneity
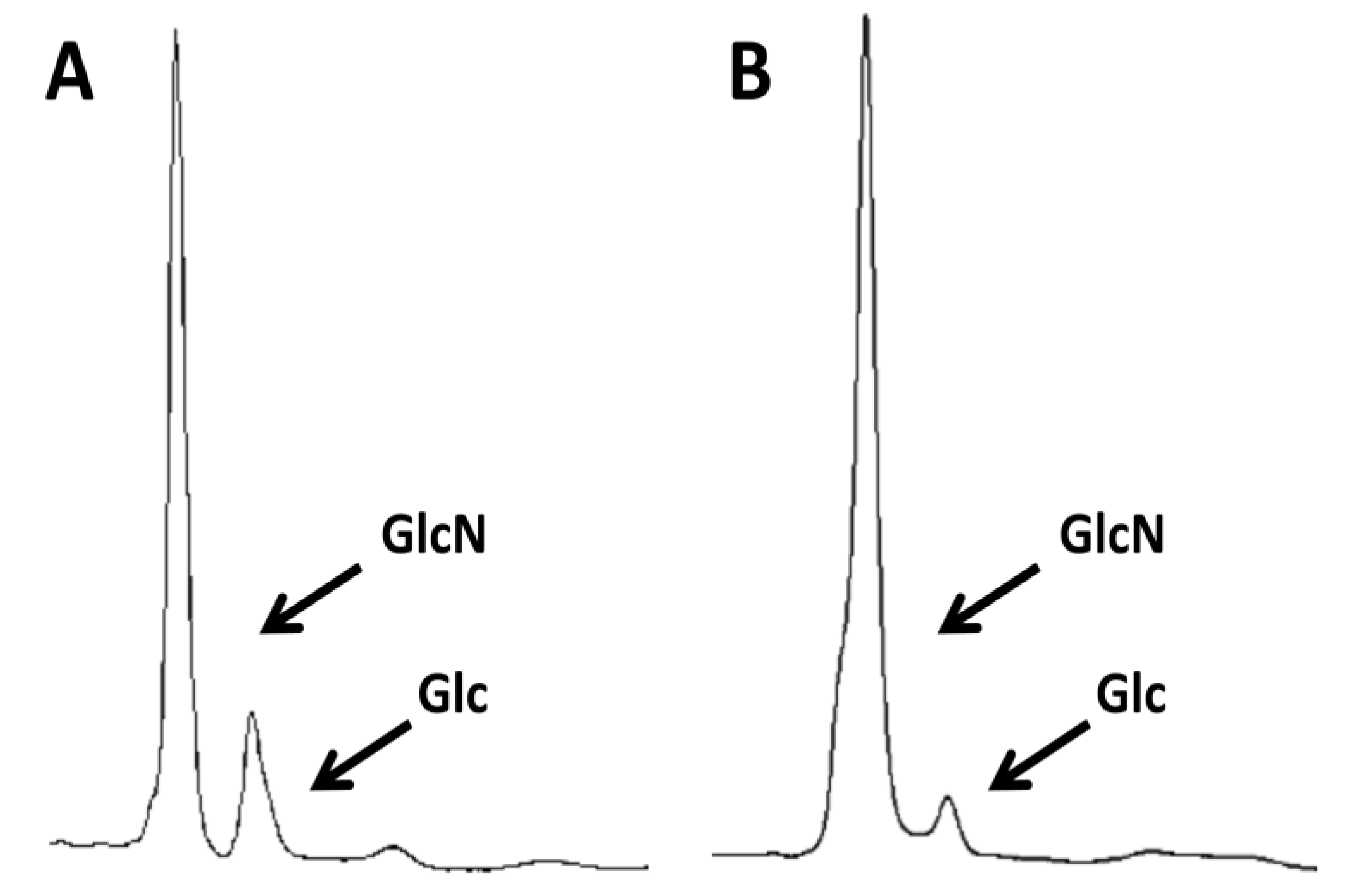
2.2. Differential Immune Response to 2 k Chitin and SSCs Purified from Commercial Crab Chitin
| Cytokine | SSCs (n = 20) | 2 k chitin (n = 9) | |
|---|---|---|---|
| IL-6 | control | 96 (80) | 61 (49) |
| chitin | 270 (540) | 2322 (1495) | |
| p | 0.173 | 0.0019 | |
| IL-1β | control | 29 (24) | 40 (0) |
| chitin | 28 (28) | 1543 (1200) | |
| p | 0.914 | 0.01 | |
| TNFα | control | 36 (7) | 15 (10) |
| chitin | 36 (14) | 78 (88) | |
| p | 0.972 | 0.02 | |
| IL-10 | control | 8 (1) | 56 (79) |
| chitin | 9 (5) | 629 (577) | |
| p | 0.798 | 0.06 | |
2.3. 2 k Chitin and SSCs from C. albicans Yeast Cells Show Significant Differences with Commercial Chitin
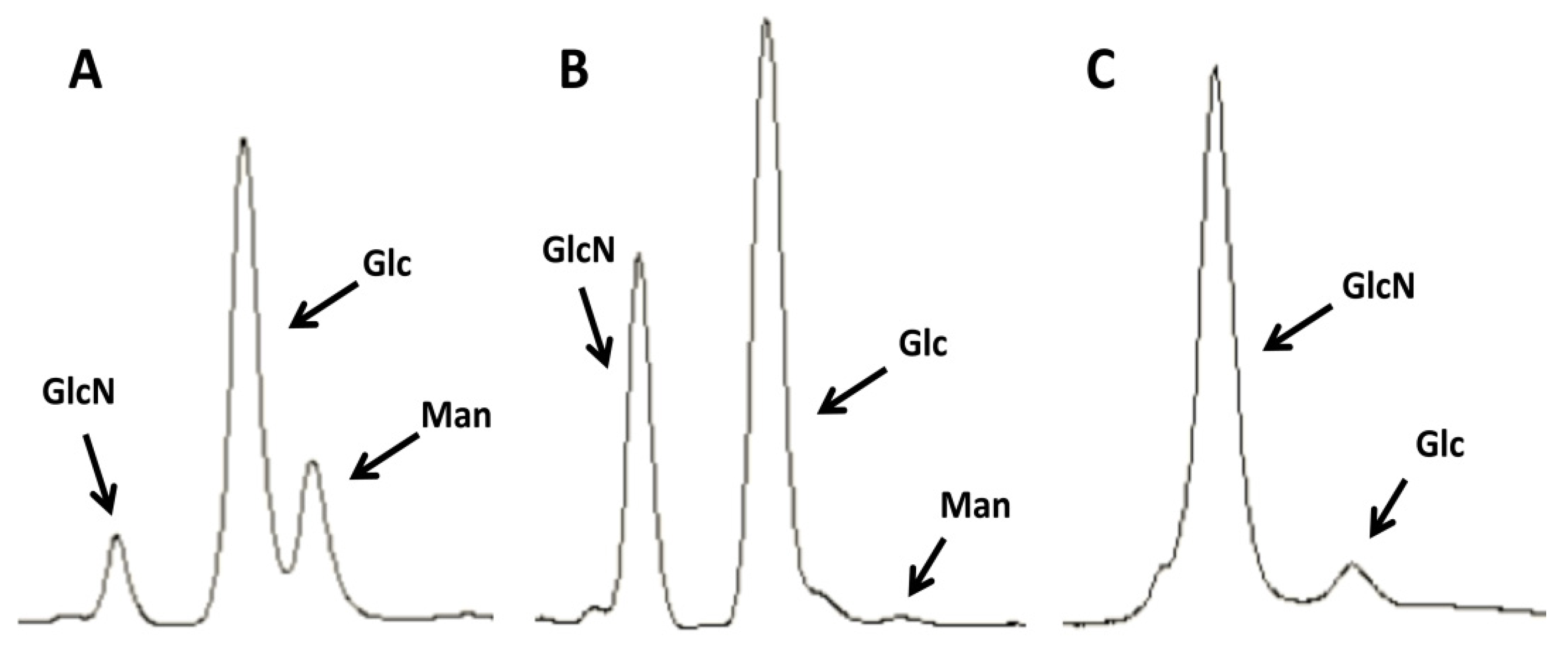
| Cytokine | C. albicans SSCs (n = 22–28) | C. albicans 2 k chitin (n = 18–36) | |
|---|---|---|---|
| IL-6 | control | 69 (57) | 164 (142) |
| chitin | 267 (530) | 379 (672) | |
| p | 0.060 | 0.073 | |
| IL-1β | control | 32 (21) | 42 (6) |
| chitin | 37 (27) | 187 (291) | |
| p | 0.576 | 0.008 | |
| TNFα | control | 34 (11) | 110 (59) |
| chitin | 25 (15) | 222 (246) | |
| p | 0.056 | 0.076 | |
| IL-10 | control | 19 (26) | 50 (76) |
| chitin | 21 (26) | 63 (89) | |
| p | 0.811 | 0.611 | |
2.4. Hyphal Chitin SSCs Behave Similar to C. albicans Yeast SSCs while the 2 k Fractions Show Differences Depending on Their Origin
| Cytokine | C. albicans (n = 18) | A. fumigatus (n = 26) | M. circinelloides (n = 9) | |
|---|---|---|---|---|
| IL-6 | control | 42 (32) | 97 (84) | 55 (75) |
| chitin | 56 (64) | 370 (708) | 46 (52) | |
| p | 0.534 | 0.064 | 0.766 | |
| IL-1β | control | 20 (0) | 28 (25) | 35 (25) |
| chitin | 24 (18) | 35 (42) | 27 (10) | |
| p | 0.475 | 0.624 | 0.363 | |
| TNFα | control | 36 (8) | 37 (7) | 41 (3) |
| chitin | 38 (11) | 49 (36) | 40 (0) | |
| p | 0.583 | 0.116 | 0.332 | |
| IL-10 | control | 9 (2) | 9 (1) | 10 (3) |
| chitin | 8 (3) | 10 (6) | 11 (7) | |
| p | 0.953 | 0.180 | 0.673 | |
| Cytokine | C. albicans (n = 7) | A. fumigatus (n = 16) | M. circinelloides (n = 16) | |
|---|---|---|---|---|
| IL-6 | control | 65 (55) | 61 (49) | 61 (49) |
| chitin | 394 (922) | 105 (209) | 1681 (1419) | |
| p | 0.383 | 0.428 | 0.0004 | |
| IL-1β | control | 40 (0) | 40 (0) | 40 (0) |
| chitin | 195 (410) | 61 (58) | 898 (1081) | |
| p | 0.456 | 0.158 | 0.006 | |
| TNFα | control | 41 (4) | 50 (17) | 50 (17) |
| chitin | 137 (254) | 47 (14) | 545 (1063) | |
| p | 0.361 | 0.6 | 0.082 | |
| IL-10 | control | 12 (5) | 16 (10) | 16 (10) |
| chitin | 16 (14) | 12 (8) | 66 (120) | |
| p | 0.475 | 0.469 | 0.119 | |
3. Experimental
3.1. Fungal Strains, Growth Conditions and Source of Commercial Crab Chitin
3.2. Determination of SSC Size by Flow Cytometry
3.3. Purification of Chitin from Fungal Cells
3.4. HPLC Determination of Chitin Purity and Glucosamine Content
3.5. Cytokine Release by hPBMCs
3.6. Statistical Analysis
4. Conclusions
Abbreviations
| SSCs | super small chitin particles |
| hPBMCs | human peripheral blood mononuclear cells |
| GlcNAc | N-acetylglucosamine |
| GlcN | glucosamine |
| CFW | calcofluor white |
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Blumenthal, H.J.; Roseman, S. Quantitative estimation of chitin in fungi. J. Bacteriol. 1957, 74, 222–224. [Google Scholar]
- Wagner, G.P.; Lo, J.; Laine, R.; Almeder, M. Chitin in the epidermal cuticle of a vertebrate (paralipophrys trigloides, blenniidae, teleostei). Experientia 1993, 49, 317–319. [Google Scholar] [CrossRef]
- Kurita, K. Chitin and chitosan: Functional biopolymers from marine crustaceans. Mar. Biotechnol. 2006, 8, 203–226. [Google Scholar] [CrossRef]
- Jang, M.-K.; Kong, B.-G.; Jeong, Y.-I.; Lee, C.H.; Nah, J.-W. Physicochemical characterization of alpha-chitin, beta-chitin, and gamma-chitin separated from natural resources. J. Polym. Sci. 2004, 42, 3423–3432. [Google Scholar] [CrossRef]
- Khoushab, F.; Yamabhai, M. Chitin research revisited. Mar. Drugs 2010, 8, 1988–2012. [Google Scholar] [CrossRef]
- Nishiyama, A.; Tsuji, S.; Yamashita, M.; Henriksen, R.A.; Myrvik, Q.N.; Shibata, Y. Phagocytosis of n-acetyl-d-glucosamine particles, a th1 adjuvant, by raw 264.7 cells results in mapk activation and tnf-alpha, but not il-10, production. Cell. Immunol. 2006, 239, 103–112. [Google Scholar] [CrossRef]
- Nishiyama, A.; Shinohara, T.; Pantuso, T.; Tsuji, S.; Yamashita, M.; Shinohara, S.; Myrvik, Q.N.; Henriksen, R.A.; Shibata, Y. Depletion of cellular cholesterol enhances macrophage mapk activation by chitin microparticles but not by heat-killed mycobacterium bovis bcg. Am. J. Physiol. Cell Physiol. 2008, 295, C341–C349. [Google Scholar] [CrossRef]
- Kogiso, M.; Nishiyama, A.; Shinohara, T.; Nakamura, M.; Mizoguchi, E.; Misawa, Y.; Guinet, E.; Nouri-Shirazi, M.; Dorey, C.K.; Henriksen, R.A.; et al. Chitin particles induce size-dependent but carbohydrate-independent innate eosinophilia. J. Leukoc. Biol. 2011, 90, 167–176. [Google Scholar] [CrossRef]
- Reese, T.A.; Liang, H.E.; Tager, A.M.; Luster, A.D.; van Rooijen, N.; Voehringer, D.; Locksley, R.M. Chitin induces accumulation in tissue of innate immune cells associated with allergy. Nature 2007, 447, 92–96. [Google Scholar] [CrossRef]
- Shibata, Y.; Honda, I.; Justice, J.P.; van Scott, M.R.; Nakamura, R.M.; Myrvik, Q.N. Th1 adjuvant n-acetyl-d-glucosamine polymer up-regulates th1 immunity but down-regulates th2 immunity against a mycobacterial protein (mpb-59) in interleukin-10-knockout and wild-type mice. Infect. Immun. 2001, 69, 6123–6130. [Google Scholar] [CrossRef]
- Shibata, Y.; Foster, L.A.; Metzger, W.J.; Myrvik, Q.N. Alveolar macrophage priming by intravenous administration of chitin particles, polymers of n-acetyl-d-glucosamine, in mice. Infect. Immun. 1997, 65, 1734–1741. [Google Scholar]
- Shibata, Y.; Metzger, W.J.; Myrvik, Q.N. Chitin particle-induced cell-mediated immunity is inhibited by soluble mannan: Mannose receptor-mediated phagocytosis initiates il-12 production. J. Immunol. 1997, 159, 2462–2467. [Google Scholar]
- Da Silva, C.A.; Hartl, D.; Liu, W.; Lee, C.G.; Elias, J.A. Tlr-2 and il-17a in chitin-induced macrophage activation and acute inflammation. J. Immunol. 2008, 181, 4279–4286. [Google Scholar]
- Da Silva, C.A.; Pochard, P.; Lee, C.G.; Elias, J.A. Chitin particles are multifaceted immune adjuvants. Am. J. Respir. Crit. Care Med. 2010, 182, 1482–1491. [Google Scholar] [CrossRef]
- Koller, B.; Muller-Wiefel, A.S.; Rupec, R.; Korting, H.C.; Ruzicka, T. Chitin modulates innate immune responses of keratinocytes. PLoS One 2011, 6, e16594. [Google Scholar]
- Roy, R.M.; Paes, H.C.; Nanjappa, S.G.; Sorkness, R.; Gasper, D.; Sterkel, A.; Wuthrich, M.; Klein, B.S. Complement component 3c3 and c3a receptor are required in chitin-dependent allergic sensitization to aspergillus fumigatus but dispensable in chitin-induced innate allergic inflammation. MBio 2013, 4. [Google Scholar] [CrossRef]
- Suzuki, K.; Okawa, Y.; Suzuki, S.; Suzuki, M. Candidacidal effect of peritoneal exudate cells in mice administered with chitin or chitosan: The role of serine protease on the mechanism of oxygen-independent candidacidal effect. Microbiol. Immunol. 1987, 31, 375–379. [Google Scholar] [CrossRef]
- Suzuki, K.; Okawa, Y.; Hashimoto, K.; Suzuki, S.; Suzuki, M. Protecting effect of chitin and chitosan on experimentally induced murine candidiasis. Microbiol. Immunol. 1984, 28, 903–912. [Google Scholar] [CrossRef]
- Rementeria, A.; Abaitua, F.; Garcia-Tobalina, R.; Hernando, F.; Ponton, J.; Sevilla, M.J. Resistance to candidiasis and macrophage activity in chitin-treated mice. FEMS Immunol. Med. Microbiol. 1997, 19, 223–230. [Google Scholar] [CrossRef]
- Van Dyken, S.J.; Garcia, D.; Porter, P.; Huang, X.; Quinlan, P.J.; Blanc, P.D.; Corry, D.B.; Locksley, R.M. Fungal chitin from asthma-associated home environments induces eosinophilic lung infiltration. J. Immunol. 2011, 187, 2261–2267. [Google Scholar] [CrossRef]
- Cartier, A.; Lehrer, S.B.; Horth-Susin, L.; Swanson, M.; Neis, B.; Howse, D.; Jong, M. Prevalence of crab asthma in crab plant workers in newfoundland and labrador. Int. J. Circumpolar Health 2004, 63, 333–336. [Google Scholar]
- Vo, T.S.; Ngo, D.H.; Ta, Q.V.; Wijesekara, I.; Kong, C.S.; Kim, S.K. Protective effect of chitin oligosaccharides against lipopolysaccharide-induced inflammatory response in bv-2 microglia. Cell. Immunol. 2012, 277, 14–21. [Google Scholar] [CrossRef]
- Nagatani, K.; Wang, S.; Llado, V.; Lau, C.W.; Li, Z.; Mizoguchi, A.; Nagler, C.R.; Shibata, Y.; Reinecker, H.C.; Mora, J.R.; et al. Chitin microparticles for the control of intestinal inflammation. Inflamm. Bowel Dis. 2012, 18, 1698–1710. [Google Scholar] [CrossRef]
- Da Silva, C.A.; Chalouni, C.; Williams, A.; Hartl, D.; Lee, C.G.; Elias, J.A. Chitin is a size-dependent regulator of macrophage tnf and il-10 production. J. Immunol. 2009, 182, 3573–3582. [Google Scholar] [CrossRef]
- Romani, L. Immunity to fungal infections. Nat. Rev. Immunol. 2011, 11, 275–288. [Google Scholar] [CrossRef]
- Netea, M.G.; Brown, G.D.; Kullberg, B.J.; Gow, N.A. An integrated model of the recognition of candida albicans by the innate immune system. Nat. Rev. Microbiol. 2008, 6, 67–78. [Google Scholar] [CrossRef]
- No, H.K.; Meyers, S.P. Preparation and production of chitin and chitosan. In Chitin Handbook; Muzzarelli, R.A.A., Peter, M.G., Eds.; Atec: Grottammare, Italy, 1997. [Google Scholar]
- Arbia, W.; Arbia, L.; Adour, L.; Amrane, A. Chitin extraction from crustacean shells using biological methods—A review. Food Technol. Biotechnol. 2013, 51, 12–25. [Google Scholar]
- Mora-Montes, H.M.; Netea, M.G.; Ferwerda, G.; Lenardon, M.D.; Brown, G.D.; Mistry, A.R.; Kullberg, B.J.; O’Callaghan, C.A.; Sheth, C.C.; Odds, F.C.; et al. Recognition and blocking of innate immunity cells by candida albicans chitin. Infect. Immun. 2011, 79, 1961–1970. [Google Scholar] [CrossRef]
- Gow, N.A.R.; Gooday, G.W.; Newsam, R.J.; Gull, K. Ultraestructure of the septum in candida albicans. Curr. Microbiol. 1980, 4, 357–359. [Google Scholar] [CrossRef]
- Wang, H.X.; Douglas, L.M.; Aimanianda, V.; Latge, J.P.; Konopka, J.B. The candida albicans sur7 protein is needed for proper synthesis of the fibrillar component of the cell wall that confers strength. Eukaryot. Cell 2011, 10, 72–80. [Google Scholar] [CrossRef]
- Walker, L.A.; Munro, C.A.; de Bruijn, I.; Lenardon, M.D.; McKinnon, A.; Gow, N.A. Stimulation of chitin synthesis rescues candida albicans from echinocandins. PLoS Pathog. 2008, 4, e1000040. [Google Scholar] [CrossRef]
- Smeekens, S.P.; van de Veerdonk, F.L.; van der Meer, J.W.; Kullberg, B.J.; Joosten, L.A.; Netea, M.G. The candida th17 response is dependent on mannan- and beta-glucan-induced prostaglandin e2. Int. Immunol. 2010, 22, 889–895. [Google Scholar] [CrossRef]
- Acosta-Rodriguez, E.V.; Napolitani, G.; Lanzavecchia, A.; Sallusto, F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human t helper cells. Nat. Immunol. 2007, 8, 942–949. [Google Scholar]
- McGreal, E.P.; Miller, J.L.; Gordon, S. Ligand recognition by antigen-presenting cell c-type lectin receptors. Curr. Opin. Immunol. 2005, 17, 18–24. [Google Scholar] [CrossRef]
- Cheng, S.C.; van de Veerdonk, F.L.; Lenardon, M.; Stoffels, M.; Plantinga, T.; Smeekens, S.; Rizzetto, L.; Mukaremera, L.; Preechasuth, K.; Cavalieri, D.; et al. The dectin-1/inflammasome pathway is responsible for the induction of protective t-helper 17 responses that discriminate between yeasts and hyphae of candida albicans. J. Leukoc. Biol. 2011, 90, 357–366. [Google Scholar] [CrossRef]
- Wessels, J.G.H.; Sietsma, J.H.; Sonnenberg, A.S.M. Wall synthesis and assembly during hyphal morphogenesis in schizophyllum commune. J. Gen. Microbiol. 1983, 129, 1607–1616. [Google Scholar]
- Dubey, L.K.; Moeller, J.B.; Schlosser, A.; Sorensen, G.L.; Holmskov, U. Induction of innate immunity by aspergillus fumigatus cell wall polysaccharides is enhanced by the composite presentation of chitin and beta-glucan. Immunobiology 2013, 219, 179–188. [Google Scholar]
- Granja, L.F.; Pinto, L.; Almeida, C.A.; Alviano, D.S.; da Silva, M.H.; Ejzemberg, R.; Alviano, C.S. Spores of mucor ramosissimus, mucor plumbeus and mucor circinelloides and their ability to activate human complement system in vitro. Med. Mycol. 2010, 48, 278–284. [Google Scholar] [CrossRef]
- Brand, A.; MacCallum, D.M.; Brown, A.J.; Gow, N.A.; Odds, F.C. Ectopic expression of ura3 can influence the virulence phenotypes and proteome of candida albicans but can be overcome by targeted reintegration of ura3 at the rps10 locus. Eukaryot. Cell 2004, 3, 900–909. [Google Scholar] [CrossRef]
- Mellado, E.; Dubreucq, G.; Mol, P.; Sarfati, J.; Paris, S.; Diaquin, M.; Holden, D.W.; Rodriguez-Tudela, J.L.; Latge, J.P. Cell wall biogenesis in a double chitin synthase mutant (chsg-/chse-) of aspergillus fumigatus. Fungal Genet. Biol. 2003, 38, 98–109. [Google Scholar] [CrossRef]
- Li, C.H.; Cervantes, M.; Springer, D.J.; Boekhout, T.; Ruiz-Vazquez, R.M.; Torres-Martinez, S.R.; Heitman, J.; Lee, S.C. Sporangiospore size dimorphism is linked to virulence of mucor circinelloides. PLoS Pathog. 2011, 7, e1002086. [Google Scholar] [CrossRef]
- Araujo, R.; Rodrigues, A.G. Variability of germinative potential among pathogenic species of aspergillus. J. Clin. Microbiol. 2004, 42, 4335–4337. [Google Scholar] [CrossRef]
- Muzzarelli, R.A. Colorimetric determination of chitosan. Anal. Biochem. 1998, 260, 255–257. [Google Scholar] [CrossRef]
- Plaine, A.; Walker, L.; da Costa, G.; Mora-Montes, H.M.; McKinnon, A.; Gow, N.A.; Gaillardin, C.; Munro, C.A.; Richard, M.L. Functional analysis of candida albicans gpi-anchored proteins: Roles in cell wall integrity and caspofungin sensitivity. Fungal Genet. Biol. 2008, 45, 1404–1414. [Google Scholar] [CrossRef]
- Endres, S.; Ghorbani, R.; Lonnemann, G.; van der Meer, J.W.; Dinarello, C.A. Measurement of immunoreactive interleukin-1 beta from human mononuclear cells: Optimization of recovery, intrasubject consistency, and comparison with interleukin-1 alpha and tumor necrosis factor. Clin. Immunol. Immunopathol. 1988, 49, 424–438. [Google Scholar] [CrossRef]
- Roy, R.M.; Wuthrich, M.; Klein, B.S. Chitin elicits ccl2 from airway epithelial cells and induces ccr2-dependent innate allergic inflammation in the lung. J. Immunol. 2012, 189, 2545–2552. [Google Scholar] [CrossRef]
- Gow, N.A.R.; Gooday, G.W.; Russell, J.D.; Wilson, M.J. Infrared and x-ray diffraction data on chitins of variable structure. Carbohydr. Res. 1987, 165, 105–110. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Alvarez, F.J. The Effect of Chitin Size, Shape, Source and Purification Method on Immune Recognition. Molecules 2014, 19, 4433-4451. https://doi.org/10.3390/molecules19044433
Alvarez FJ. The Effect of Chitin Size, Shape, Source and Purification Method on Immune Recognition. Molecules. 2014; 19(4):4433-4451. https://doi.org/10.3390/molecules19044433
Chicago/Turabian StyleAlvarez, Francisco J. 2014. "The Effect of Chitin Size, Shape, Source and Purification Method on Immune Recognition" Molecules 19, no. 4: 4433-4451. https://doi.org/10.3390/molecules19044433




