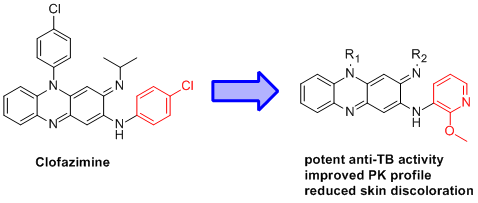
Synthesis and Biological Evaluation of Novel 2-Methoxypyridylamino-Substituted Riminophenazine Derivatives as Antituberculosis Agents
Abstract
:1. Introduction
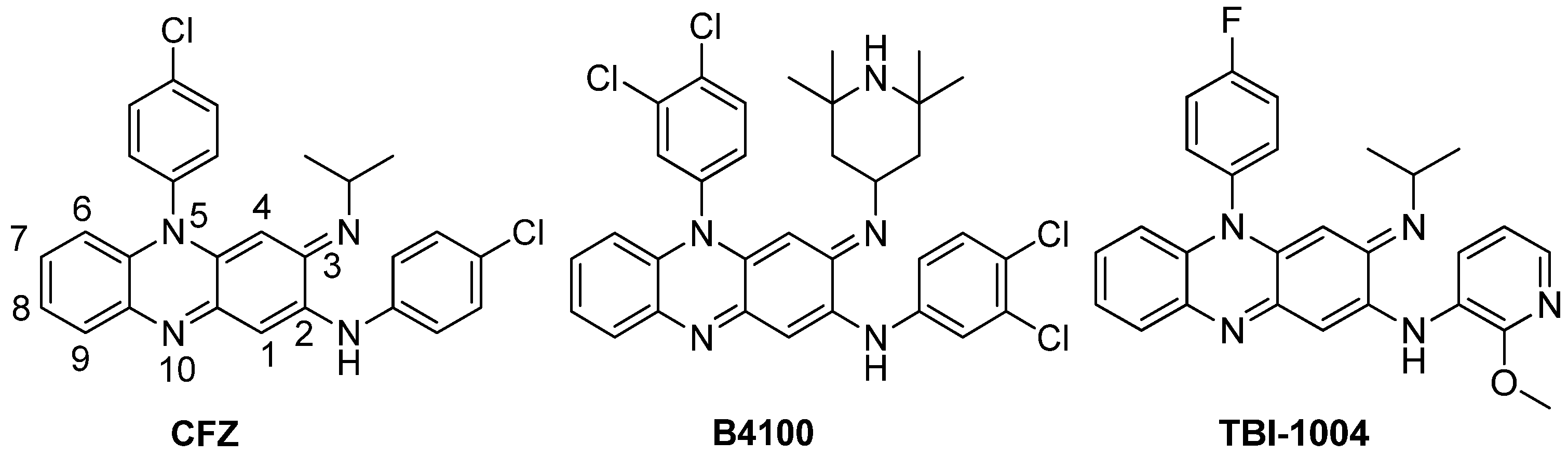
2. Results and Discussion
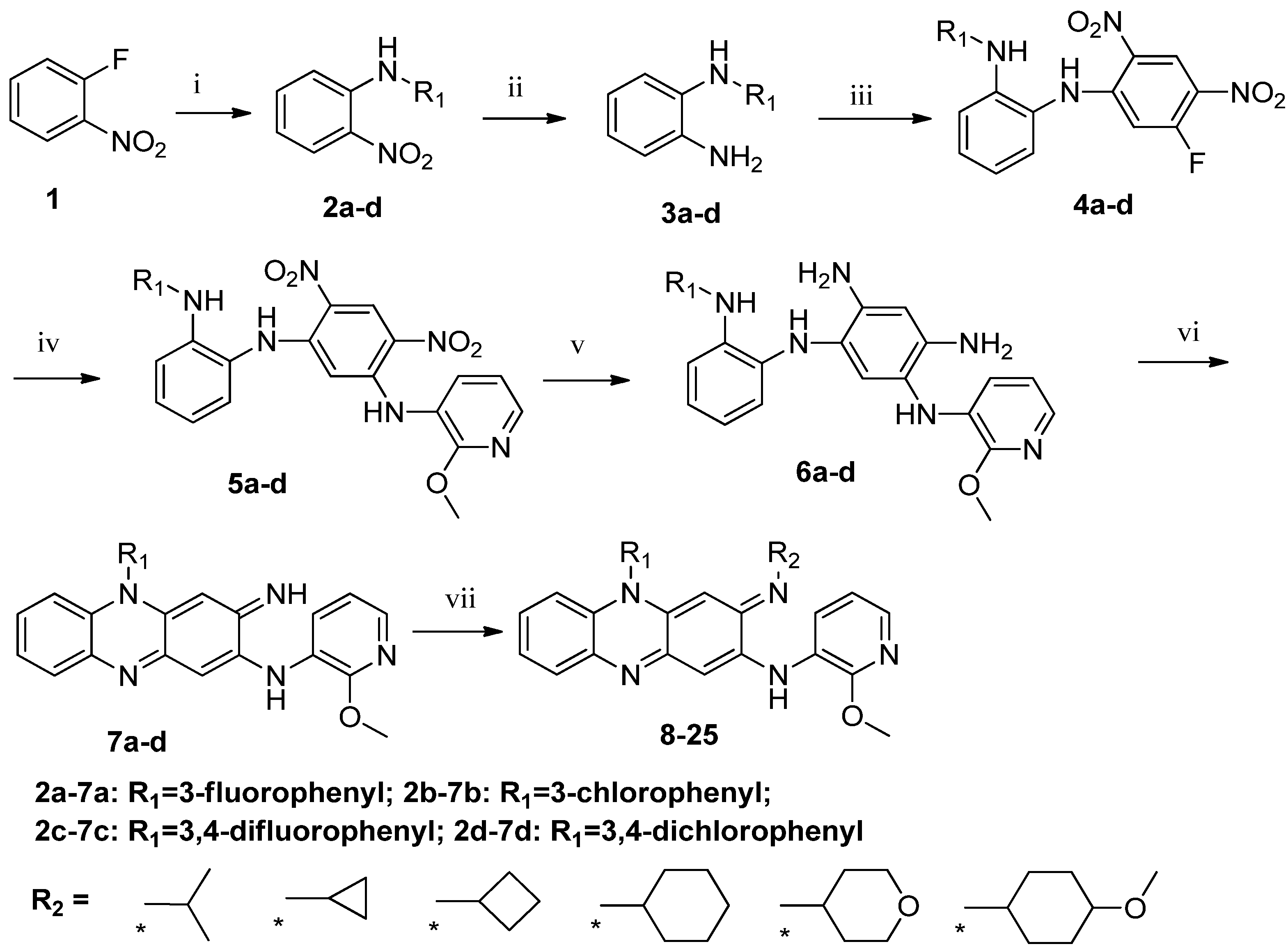
2.1. Chemistry
2.2. Biological Results and Discussion
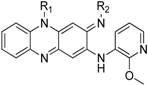
| Compound | R1 | R2 | ClogP a | MIC(μg/mL) | IC50(μg/mL) | SI b |
|---|---|---|---|---|---|---|
| CFZ | 7.50 | 0.12 | 68.6 | 572 | ||
| TBI-1004 | 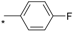 | 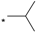 | 5.90 | 0.038 | >64 | 1684 |
| 8 | 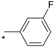 | 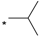 | 5.90 | 0.06 | 2.65 | 44 |
| 9 | 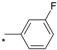 | 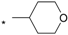 | 4.70 | 0.061 | 9.21 | 151 |
| 10 | 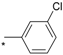 | 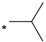 | 6.47 | 0.250 | 5.10 | 20 |
| 11 | 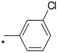 | 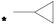 | 5.99 | 0.030 | 50.88 | 1696 |
| 12 | 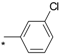 | 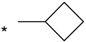 | 6.55 | 0.060 | >64 | 1067 |
| 13 | 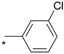 | 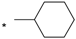 | 7.67 | 0.057 | 53.26 | 934 |
| 14 | 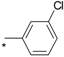 | 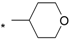 | 5.27 | 0.025 | 23.40 | 936 |
| 15 | 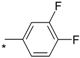 | 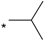 | 5.98 | 0.066 | >64 | 970 |
| 16 | 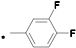 |  | 5.50 | 0.014 | 25.51 | 1822 |
| 17 | 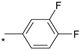 | 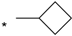 | 6.06 | 0.040 | >64 | 1600 |
| 18 | 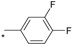 | 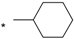 | 7.17 | 0.030 | >64 | 2133 |
| 19 | 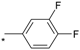 | 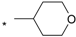 | 4.77 | 0.067 | >64 | 955 |
| 20 | 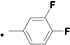 |  | 5.80 | 0.056 | >64 | 1143 |
| 21 | 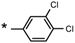 | 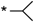 | 7.07 | 0.099 | >64 | 646 |
| 22 | 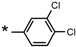 | 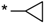 | 6.59 | 0.025 | >64 | 2560 |
| 23 | 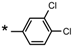 | 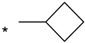 | 7.15 | 0.06 | >64 | 1067 |
| 24 |  | 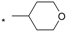 | 5.86 | 0.080 | >64 | 800 |
| 25 | 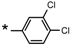 |  | 6.89 | 0.059 | >64 | 1085 |
| Compound | Number of animals that survived/Total number of animals |
|---|---|
| 15 | 5/5 |
| 22 | 6/6 |
| Groups | Dose (mg/kg) | logCFU/lung |
|---|---|---|
| Control (D3) | 1.94 ± 028 | |
| Control (D10) | 3.72 ± 0.46 | |
| Control (D30) | 8.32 ± 0.19 | |
| CFZ | 20 | 4.29 ± 0.58 |
| 15 | 20 | 4.56 ± 0.38 |
| 22 | 20 | 8.06 ± 0.13 |
| Compound | T1/2 (h) | Tmax (h) | Cmax (mg/L) | AUC0~24 h (mg/L*h) |
|---|---|---|---|---|
| CFZ | 27.11 | 2 | 0.47 | 8.22 |
| 15 | 13.80 | 2 | 1.835 | 21.156 |
3. Experimental
3.1. General Information
3.1.1. Chemistry
3.1.2. Minimum Inhibitory Concentration and Cytotoxicity Assays
3.1.3. In vivo Acute M. tuberculosis H37Rv Infection Assay and Mouse Pharmacokinetic Study
3.2. General Procedure for Preparation of Compounds 2a–d
3.3. General Procedure for Preparation of Compounds 4a–d
3.4. General Procedure for Preparation of Compounds 5a–d
3.5. General Procedure for Preparation of Compounds 7a–d
3.6. General Procedure for Preparation of Compounds 8–25
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Health Organization. Global Tuberculosis Report 2013. Available online: Http://www.who.int/tb/publications/global_report/en/ (accessed on 31 March 2014).
- Koul, A.; Arnoult, E.; Lounis, N.; Guillemont, J.; Andries, K. The challenge of new drug discovery for tuberculosis. Nature 2011, 469, 483–490. [Google Scholar] [CrossRef]
- Meeting report: World Tuberculosis Day Symposium 2012. Tuberculosis 2013, 93, 108–114. [CrossRef]
- Cohen, J. Approval of novel TB drug celebrated-with restraint. Science 2013, 339, 130. [Google Scholar] [CrossRef]
- Barry, V.C.; Belton, J.G.; Conalty, M.L.; Denneny, J.M.; Edward, D.W.; O’Sullivan, J.F.; Twomey, D.; Winder, F. A new series of phenazines (rimino-compounds) with high antituberculosis activity. Nature 1957, 179, 1013–1015. [Google Scholar] [CrossRef]
- Xu, H.B.; Jiang, R.H.; Xiao, H.P. Clofazimine in the treatment of multidrug-resistant tuberculosis. Clin. Microbiol. Infect. 2011, 18, 1104–1110. [Google Scholar]
- Liu, B.; Liu, K.; Lu, Y.; Zhang, D.; Yang, T.; Li, X.; Ma, C.; Zheng, M.; Wang, B.; Zhang, G.; et al. Systematic evaluation of structure-activity relationships of the riminophenazine class and discovery of a c2 pyridylamino series for the treatment of multidrug-resistant tuberculosis. Molecules 2012, 17, 4545–4559. [Google Scholar] [CrossRef]
- Lu, Y.; Zheng, M.Q.; Wang, B.; Fu, L.; Zhao, W.; Li, P.; Xu, J.; Zhu, H.; Jin, H.; Yin, D.; et al. Clofazimine analogs with efficacy against experimental tuberculosis and reduced potential for accumulation. Antimicrob. Agents Chemother. 2011, 55, 5185–5193. [Google Scholar] [CrossRef]
- Zhang, D.; Lu, Y.; Liu, K.; Liu, B.; Wang, J.; Zhang, G.; Zhang, H.; Liu, Y.; Wang, B.; Zheng, M.; et al. Identification of less lipophilic riminophenazine derivatives for the treatment of drug-resistant tuberculosis. J. Med. Chem. 2012, 55, 8409–8417. [Google Scholar] [CrossRef]
- Van Rensburg, C.E.; Joone, G.K.; Sirgel, F.A.; Matlola, N.M.; O’Sullivan, J.F. In vitro investigation of the antimicrobial activities of novel tetramethylpiperidine-substituted phenazines against mycobacterium tuberculosis. Chemotherapy 2000, 46, 43–48. [Google Scholar] [CrossRef]
- Cholo, M.C.; Steel, H.C.; Fourie, P.B.; Germishuizen, W.A.; Anderson, R. Clofazimine: Current status and future prospects. J. Antimicrob. Chemother. 2012, 67, 290–298. [Google Scholar] [CrossRef]
- Liu, K.; Cooper, C.B.; Huang, H.; Li, C.; Liu, B.; Liu, Y.; Ma, Z.; Wang, J.; Yin, D.; Zhang, D.; et al. Riminophenazines with 2-(Heteroaryl)amino Substituents and Their Anti-Microbial Activity. WO2012003190, 8 May 2013. [Google Scholar]
- Feng, P.C.; Fenselau, C.C.; Jacobson, R.R. Metabolism of clofazimine in leprosy patients. Drug Metab. Dispos. 1981, 9, 521–524. [Google Scholar]
- O’Connor, R.; O’Sullivan, J.F.; O’Kennedy, R. The pharmacology, metabolism, and chemistry of clofazimine. Drug Metab. Rev. 1995, 27, 591–614. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, C.; Zhang, D.; Lu, Y.; Wang, B.; Zheng, M.; Li, C.; Yin, D.; Huang, H. Synthesis and anti-tubercular activity of novel alkyl substituted riminophenazine derivatives. Acta Pharm. Sin. 2012, 47, 745–754. [Google Scholar]
- Van Landingham, R.M.; Walker, L.L.; O’Sullivan, J.F.; Shinnick, T.M. Activity of phenazine analogs against Mycobacterium leprae infections in mice. Int. J. Lepr. 1993, 61, 406–414. [Google Scholar]
- Sample Availability: Not available.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhang, D.; Liu, Y.; Zhang, C.; Zhang, H.; Wang, B.; Xu, J.; Fu, L.; Yin, D.; Cooper, C.B.; Ma, Z.; et al. Synthesis and Biological Evaluation of Novel 2-Methoxypyridylamino-Substituted Riminophenazine Derivatives as Antituberculosis Agents. Molecules 2014, 19, 4380-4394. https://doi.org/10.3390/molecules19044380
Zhang D, Liu Y, Zhang C, Zhang H, Wang B, Xu J, Fu L, Yin D, Cooper CB, Ma Z, et al. Synthesis and Biological Evaluation of Novel 2-Methoxypyridylamino-Substituted Riminophenazine Derivatives as Antituberculosis Agents. Molecules. 2014; 19(4):4380-4394. https://doi.org/10.3390/molecules19044380
Chicago/Turabian StyleZhang, Dongfeng, Yang Liu, Chunlin Zhang, Hao Zhang, Bin Wang, Jian Xu, Lei Fu, Dali Yin, Christopher B. Cooper, Zhenkun Ma, and et al. 2014. "Synthesis and Biological Evaluation of Novel 2-Methoxypyridylamino-Substituted Riminophenazine Derivatives as Antituberculosis Agents" Molecules 19, no. 4: 4380-4394. https://doi.org/10.3390/molecules19044380
APA StyleZhang, D., Liu, Y., Zhang, C., Zhang, H., Wang, B., Xu, J., Fu, L., Yin, D., Cooper, C. B., Ma, Z., Lu, Y., & Huang, H. (2014). Synthesis and Biological Evaluation of Novel 2-Methoxypyridylamino-Substituted Riminophenazine Derivatives as Antituberculosis Agents. Molecules, 19(4), 4380-4394. https://doi.org/10.3390/molecules19044380