Determination of Bioactive Components in Chinese Herbal Formulae and Pharmacokinetics of Rhein in Rats by UPLC-MS/MS
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of LC-MS/MS Conditions
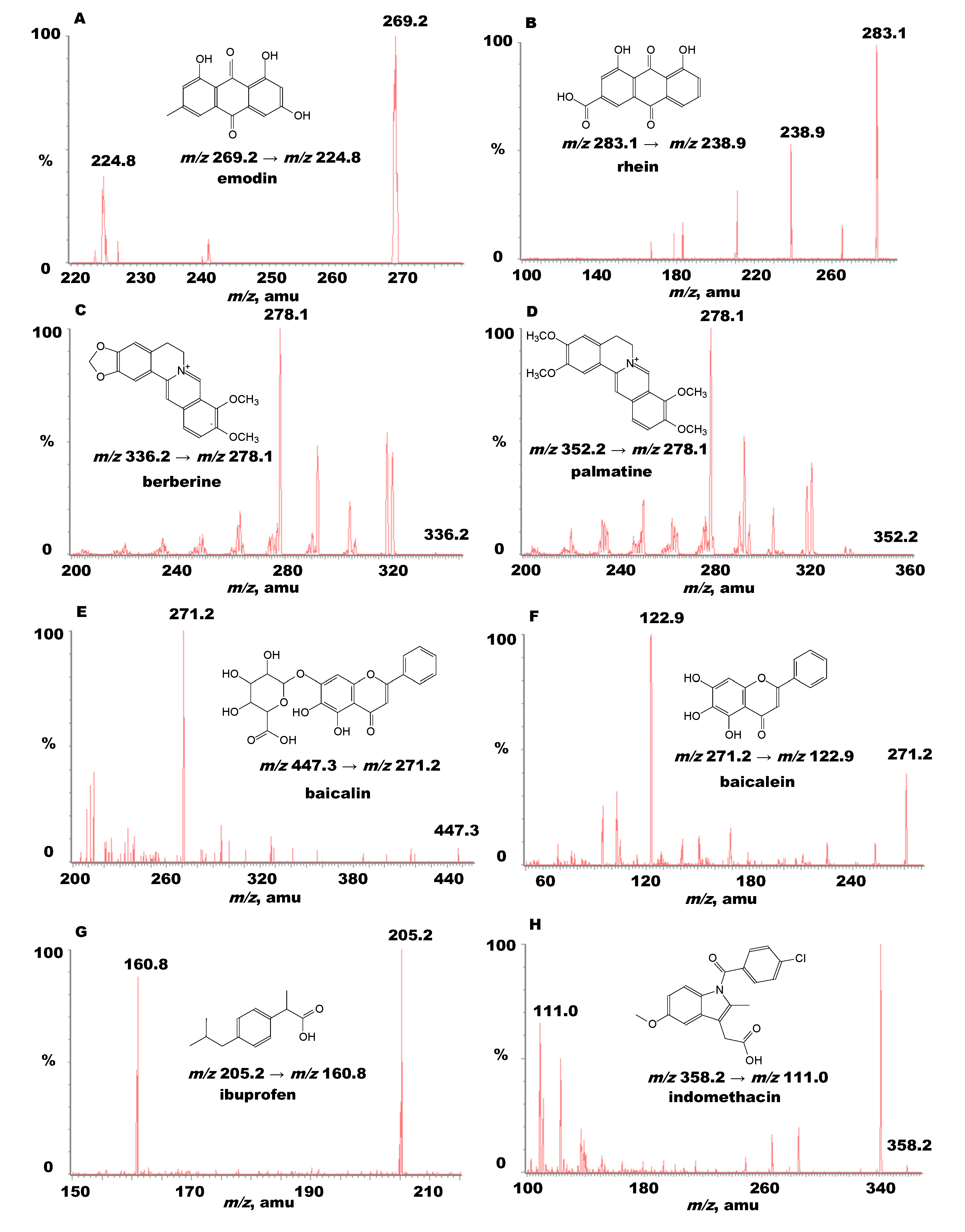

2.2. Determination of Bioactive Components in Commercial Pharmaceutical Herbal Products
| Intra-day | Inter-day | |||||
|---|---|---|---|---|---|---|
| Nominal concentration (ng/mL) | Observed concentration (ng/mL) | Precision (% RSD) | Accuracy (% Bias) | Observed concentration (ng/mL) | Precision (% RSD) | Accuracy (% Bias) |
| Emodin | ||||||
| 25 | 25.6 ± 3.84 | 2.53 | 14.9 | 25.7 ± 3.73 | 2.87 | 14.5 |
| 50 | 56.2 ± 4.87 | 12.4 | 8.67 | 55.4 ± 4.26 | 10.8 | 7.69 |
| 100 | 97.8 ± 2.01 | −2.21 | 2.06 | 98.4 ± 2.27 | −1.64 | 2.31 |
| 250 | 239 ± 5.31 | −4.10 | 2.22 | 241 ± 5.63 | −3.62 | 2.33 |
| 500 | 503 ± 3.09 | 0.65 | 0.61 | 504 ± 2.97 | 0.71 | 0.59 |
| Rhein | ||||||
| 100 | 94.1 ± 10.7 | −5.86 | 11.3 | 103 ± 10.2 | 3.11 | 9.11 |
| 250 | 229 ± 24.9 | −8.37 | 10.9 | 226 ± 25.7 | −9.74 | 11.4 |
| 500 | 492 ± 64.8 | −1.56 | 13.2 | 506 ± 70.4 | 1.23 | 13.9 |
| 1000 | 1017 ± 99.6 | 1.72 | 9.79 | 992 ± 76.3 | −0.70 | 7.68 |
| 2500 | 2480 ± 144 | −0.77 | 5.82 | 2504 ± 138 | 0.19 | 5.51 |
| Berberine | ||||||
| 100 | 94.3 ± 1.59 | 1.69 | −5.70 | 97.4 ± 7.26 | 7.46 | −2.60 |
| 250 | 259 ± 2.75 | 1.06 | 3.65 | 252 ± 17.5 | 6.96 | 0.67 |
| 500 | 497 ± 0.92 | 0.19 | −0.68 | 502 ± 13.2 | 0.62 | 0.40 |
| Palmatine | ||||||
| 25 | 28.4 ± 3.48 | 12.3 | 13.5 | 26.2 ± 1.94 | 7.41 | 4.88 |
| 50 | 48.5 ± 2.36 | 4.86 | −3.01 | 48.5 ± 3.06 | 6.30 | −3.07 |
| 100 | 88.1 ± 1.52 | 1.73 | −11.9 | 92.3 ± 5.69 | 6.17 | −7.74 |
| 250 | 265 ± 8.49 | 3.20 | 6.16 | 265 ± 9.21 | 3.47 | 6.00 |
| 500 | 495 ± 3.80 | 0.77 | −1.06 | 495 ± 3.80 | 0.77 | −1.06 |
| Baicalin | ||||||
| 50 | 56.3 ± 4.94 | 8.79 | 12.6 | 55.4 ± 4.20 | 7.59 | 10.8 |
| 100 | 95.4 ± 4.61 | 4.83 | −4.61 | 94.3 ± 5.27 | 5.59 | −5.69 |
| 250 | 247 ± 10.6 | 4.30 | −1.40 | 252 ± 5.05 | 2.01 | 0.75 |
| 500 | 503 ± 10.5 | 2.09 | 0.51 | 503 ± 10.4 | 2.07 | 0.58 |
| Baicalein | ||||||
| 25 | 25.0 ± 1.40 | 5.61 | −0.17 | 25.9 ± 0.99 | 3.83 | 3.85 |
| 50 | 53.7 ± 4.62 | 8.61 | 7.43 | 54.8 ± 2.92 | 5.33 | 9.61 |
| 100 | 91.3 ± 6.68 | 7.32 | −8.70 | 92.8 ± 8.48 | 9.14 | −7.17 |
| 250 | 257 ± 18.9 | 7.40 | 2.69 | 249 ± 6.66 | 2.68 | −0.55 |
| 500 | 497 ± 19.9 | 4.01 | −0.52 | 506 ± 20.9 | 4.15 | 1.11 |
| 1000 | 1004 ± 13.7 | 1.36 | 0.41 | 1000 ± 16.6 | 1.66 | −0.01 |
| Brand | Emodin(mg/g) | Rhein(mg/g) | Berberine(mg/g) | Palmatine(mg/g) | Baicalin(mg/g) | Baicalein(mg/g) |
|---|---|---|---|---|---|---|
| A | 0.23 ± 0.01 | 1.04 ± 0.04 | 29.2 ± 1.53 | 2.48 ± 0.08 | 56.0 ± 6.36 | 5.26 ± 0.32 |
| B | 0.71 ± 0.02 | 2.38 ± 0.10 | 26.2 ± 1.60 | 2.13 ± 0.04 | 84.1 ± 3.48 | 16.6 ± 1.00 |
| C | 0.39 ± 0.03 | 0.64 ± 0.06 | 35.8 ± 2.98 | 2.72 ± 0.16 | 36.9 ± 3.48 | 30.5 ± 1.63 |
| D | 0.26 ± 0.01 | 0.49 ± 0.01 | 24.1 ± 1.06 | 2.29 ± 0.08 | 69.8 ± 4.04 | 3.69 ± 0.29 |
| E | 0.55 ± 0.04 | 1.07 ± 0.11 | 28.5 ± 2.20 | 2.32 ± 0.09 | 66.4 ± 2.26 | 5.70 ± 0.46 |
| F | 0.07 ± 0.00 | 0.06 ± 0.02 | 14.5 ± 0.65 | 1.11 ± 0.10 | 32.4 ± 0.81 | 6.53 ± 0.34 |
| G | 0.17 ± 0.01 | ND | 21.0 ± 2.41 | 2.05 ± 0.08 | 18.7 ± 2.25 | 9.76 ± 2.65 |
| H | 1.04 ± 0.04 | 2.29 ± 0.13 | 0.01 ± 0.02 | ND | 0.10 ± 0.00 | ND |
2.3. Method Validation of Rhein in Rat Plasma

| Nominal concentration (ng/mL) | Set 1 | Set 2 | Set 3 | Matrix effect (%) | Recovery (%) |
|---|---|---|---|---|---|
| Rhein | |||||
| 100 | 201 ± 25 | 222 ± 34 | 211 ± 26 | 111 ± 17 | 95 ± 12 |
| 500 | 1464 ± 76 | 1497 ± 81 | 1322 ± 105 | 102 ± 6 | 88 ± 7 |
| 1000 | 2390 ± 158 | 2286 ± 157 | 2085 ± 98 | 96 ± 7 | 91 ± 4 |
| Mean ± SD | 102.9 ± 7.6 | 91.6 ± 3.4 | |||
| Ibuprofen (IS) | |||||
| 2500 | 3380 ± 143 | 2626 ± 147 | 2646 ± 184 | 77.7 ± 4.4 | 100.7 ± 7.0 |
| Intra-day | Inter-day | |||||
|---|---|---|---|---|---|---|
| Nominal concentration (ng/mL) | Observed concentration (ng/mL) | Precision (% RSD) | Accuracy (% Bias) | Observed concentration (ng/mL) | Precision (% RSD) | Accuracy (% Bias) |
| Rhein | ||||||
| 100 | 96.2 ± 9.48 | 9.86 | −3.83 | 104.4 ± 8.46 | 8.10 | 4.39 |
| 250 | 241 ± 20.5 | 8.51 | −3.70 | 229 ± 20.4 | 8.93 | −8.49 |
| 500 | 567 ± 81.4 | 14.34 | 13.43 | 499 ± 24.1 | 4.83 | −0.20 |
| 1000 | 1057 ± 143 | 13.50 | 5.67 | 1035 ± 129 | 12.5 | 3.47 |
| 2500 | 2491 ± 49.7 | 2.00 | −0.36 | 2505 ± 63.4 | 2.53 | 0.20 |
2.4. Comparative Pharmacokinetics of Rhein in Rats
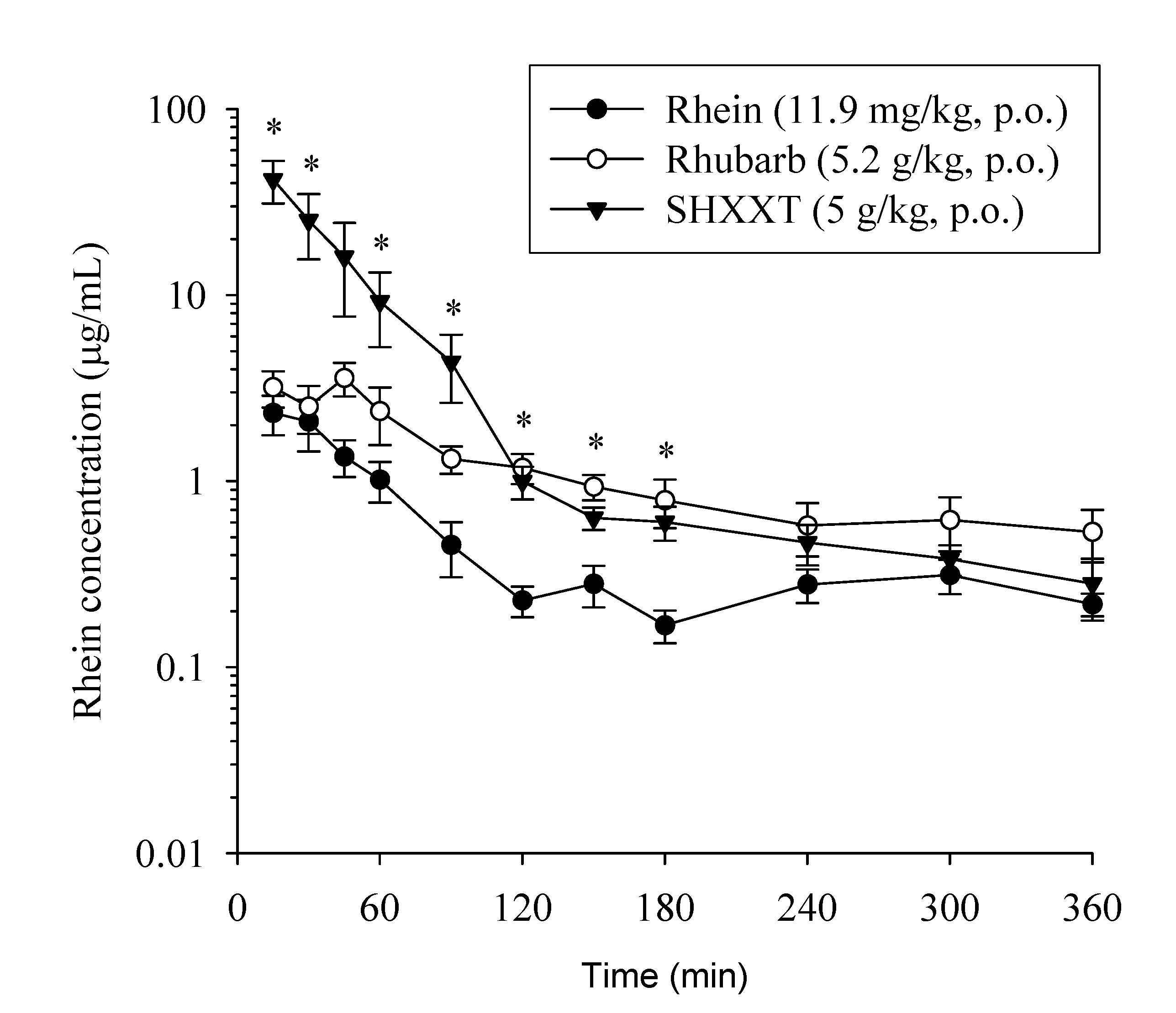
| PK parameters | Rhein | Rhubarb | SHXXT |
|---|---|---|---|
| 11.9 mg/kg | 5.2 g/kg | 5 g/kg | |
| Cmax (µg/mL) | 2.45 ± 0.55 | 4.34 ± 0.65 | 41.8 ± 10.7 * |
| Tmax (min) | 25 ± 7.42 | 47.5 ± 7.2 * | 15 ± 0 |
| T1/2 (min) | 144 ± 17.2 | 179 ± 25.9 | 50.2 ± 2.73 * |
| AUC (min µg/mL) | 188 ± 36.4 | 419 ± 80.5 | 1712 ± 575 * |
| CL (mL/min/kg) | 58 ± 7.89 | 25.7 ± 4.85 * | 9.22 ± 1.44 * |
| MRT (min) | 112 ± 4.29 | 120 ± 9.12 | 50.2 ± 4.14 * |
| Vss (L/kg) | 12.2 ± 2.10 | 6.03 ± 0.97 * | 0.69 ± 0.12 * |
3. Experimental
3.1. Chemicals and Reagents
3.2. LC-MS/MS
3.3. Quantitative Determination of Bioactive Components in Commercial Pharmaceutical Chinese Herbal Formulae, Sample Preparation, Method Validation and Sample Analysis
3.4. Method Validation of Rhein in Rat Plasma
RE (%) = C/B × 100
3.5. Pharmacokinetic Study of Rhein in Rats
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dinning, P.G.; Smith, T.K.; Scott, S.M. Pathophysiology of colonic causes of chronic constipation. Neurogastroenterol. Motil. 2009, 21, 20–30. [Google Scholar] [CrossRef]
- Emmanuel, A.V.; Tack, J.; Quigley, E.M.; Talley, N.J. Pharmacological management of constipation. Neurogastroenterol. Motil. 2009, 21, 41–54. [Google Scholar] [CrossRef]
- Foxx-Orenstein, A.E.; McNally, M.A.; Odunsi, S.T. Update on constipation: One treatment does not fit all. Clevel. Clin. J. Med. 2008, 75, 813–824. [Google Scholar] [CrossRef]
- Chey, W.D.; Maneerattaporn, M.; Saad, R. Pharmacologic and complementary and alternative medicine therapies for irritable bowel syndrome. Gut Liver 2011, 5, 253–266. [Google Scholar] [CrossRef]
- Jong, M.S.; Hwang, S.J.; Chen, Y.C.; Chen, T.J.; Chen, F.J.; Chen, F.P. Prescriptions of Chinese herbal medicine for constipation under the national health insurance in Taiwan. J. Chin. Med. Assoc. 2010, 73, 375–383. [Google Scholar] [CrossRef]
- Camilleri, M.; Bharucha, A.E. Behavioural and new pharmacological treatments for constipation: Getting the balance right. Gut 2010, 59, 1288–1296. [Google Scholar] [CrossRef] [Green Version]
- Candy, B.; Jones, L.; Goodman, M.L.; Drake, R.; Tookman, A. Laxatives or methylnaltrexone for the management of constipation in palliative care patients. Cochr. Datab. Syst. Rev. (Online) 2011. [Google Scholar] [CrossRef]
- Lai, J.N.; Wu, C.T.; Wang, J.D. Prescription pattern of chinese herbal products for breast cancer in taiwan: A population-based study. Evid. Based Complement. Alternat. Med. 2012, 2012, 891893. [Google Scholar]
- Shih, W.T.; Yang, Y.H.; Chen, P.C. Prescription patterns of chinese herbal products for osteoporosis in taiwan: A population-based study. Evid. Based Complement. Alternat. Med. 2012, 2012, 752837. [Google Scholar]
- Trivedi, N.R.; Rajan, M.G.; Johnson, J.R.; Shukla, A.J. Pharmaceutical approaches to preparing pelletized dosage forms using the extrusion-spheronization process. Crit. Rev. Therap. Drug Carr. Syst. 2007, 24, 1–40. [Google Scholar] [CrossRef]
- Shia, C.S.; Hou, Y.C.; Juang, S.H.; Tsai, S.Y.; Hsieh, P.H.; Ho, L.C.; Chao, P.D. Metabolism and pharmacokinetics of san-huang-xie-xin-tang, a polyphenol-rich chinese medicine formula, in rats and ex vivo antioxidant activity. Evid. Based Complement. Alternat. Med. 2011, 2011, 721293. [Google Scholar]
- Yin, L.; Lu, B.; Qi, Y.; Xu, L.; Han, X.; Xu, Y.; Peng, J.; Sun, C. Simultaneous determination of 11 active components in two well-known traditional Chinese medicines by HPLC coupled with diode array detection for quality control. J. Pharm. Biomed. Anal. 2009, 49, 1101–1108. [Google Scholar] [CrossRef]
- Shi, Y.; Sun, C.; Gao, B.; Sun, A. Development of a liquid chromatography tandem mass spectrometry method for simultaneous determination of eight adulterants in slimming functional foods. J. Chromatogr. A 2011, 1218, 7655–7662. [Google Scholar] [CrossRef]
- Lu, C.M.; Hou, M.L.; Lin, L.C.; Tsai, T.H. Chemical and physical methods to analyze a multicomponent traditional chinese herbal prescription using LC-MS/MS, electron microscope, and Congo red staining. Evid. Based Complement. Alternat. Med. 2013, 2013, 952796. [Google Scholar]
- Shaw, L.H.; Chen, W.M.; Tsai, T.H. Identification of multiple ingredients for a Traditional Chinese Medicine preparation (bu-yang-huan-wu-tang) by liquid chromatography coupled with tandem mass spectrometry. Molecules 2013, 18, 11281–11298. [Google Scholar] [CrossRef]
- Shaw, L.H.; Lin, L.C.; Tsai, T.H. HPLC-MS/MS analysis of a traditional Chinese medical formulation of Bu-Yang-Huan-Wu-Tang and its pharmacokinetics after oral administration to rats. PLoS One 2012, 7, e43848. [Google Scholar] [CrossRef]
- Zan, B.; Shi, R.; Wang, T.; Wu, J.; Ma, Y.; Cheng, N. Simultaneous quantification of multiple active components from Xiexin decoction in rat plasma by LC-ESI-MS/MS: Application in pharmacokinetics. Biomed. Chromatogr. 2011, 25, 816–826. [Google Scholar] [CrossRef]
- Jiang, J.Y.; Yang, M.W.; Qian, W.; Lin, H.; Geng, Y.; Zhou, Z.Q.; Xiao, D.W. Quantitative determination of rhein in human plasma by liquid chromatography-negative electrospray ionization tandem mass/mass spectrometry and the application in a pharmacokinetic study. J. Pharm. Biomed. Anal. 2012, 57, 19–25. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, Y.M.; Wang, Z.T.; Wang, C.H. Differences in pharmacokinetics and anti-inflammatory effects between decoction and maceration of Sanhuang Xiexin Tang in rats and mice. Planta Med. 2013, 79, 1666–1673. [Google Scholar] [CrossRef]
- Balasundram, N.; Sundram, K.; Samir Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Bound phenolics in foods, a review. Food Chem. 2014, 152, 46–55. [Google Scholar] [CrossRef]
- Figueiredo-González, M.; Cancho-Grande, B.; Simal-Gándara, J. Garnacha Tintorera-based sweet wines: Chromatic properties and global phenolic composition by means of UV-Vis spectrophotometry. Food Chem. 2013, 140, 217–224. [Google Scholar] [CrossRef]
- Van Eeckhaut, A.; Lanckmans, K.; Sarre, S.; Smolders, I.; Michotte, Y. Validation of bioanalytical LC-MS/MS assays: Evaluation of matrix effects. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2009, 877, 2198–2207. [Google Scholar]
- Tukey, R.H.; Strassburg, C.P. Human UDP-glucuronosyltransferases: Metabolism, expression, and disease. Annu. Rev. Pharmacol. Toxicol. 2000, 40, 581–616. [Google Scholar] [CrossRef]
- Pan, J.F.; Yu, C.; Zhu, D.Y.; Zhang, H.; Zeng, J.F.; Jiang, S.H.; Ren, J.Y. Identification of three sulfate-conjugated metabolites of berberine chloride in healthy volunteers’ urine after oral administration. Acta Pharmacol. Sin. 2002, 23, 77–82. [Google Scholar]
- Zuo, F.; Nakamura, N.; Akao, T.; Hattori, M. Pharmacokinetics of berberine and its main metabolites in conventional and pseudo germ-free rats determined by liquid chromatography/ion trap mass spectrometry. Drug Metab. Dispos. 2006, 34, 2064–2072. [Google Scholar] [CrossRef]
- Layek, B.; Kumar, T.S.; Trivedi, R.K.; Mullangi, R.; Srinivas, N.R. Development and validation of a sensitive LC-MS/MS method with electrospray ionization for quantitation of rhein in human plasma: Application to a pharmacokinetic study. Biomed. Chromatogr. 2008, 22, 616–624. [Google Scholar] [CrossRef]
- Gong, H.L.; Tang, W.F.; Wang, H.; Xia, Q.; Huang, X. Effects of food and gender on the pharmacokinetics of rhein and emodin in rats after oral dosing with Da-Cheng-Qi decoction. Phytother. Res. 2011, 25, 74–80. [Google Scholar] [CrossRef]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef]
- Hou, M.L.; Chang, L.W.; Chiang, C.J.; Tsuang, Y.H.; Lin, C.H.; Tsai, T.H. Pharmacokinetics of di-isononyl phthalate in freely moving rats by UPLC-MS/MS. Int. J. Pharm. 2013, 450, 36–43. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds, rhein, emodin, berberine, palmatine, baicalin and baicalein, are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hou, M.-L.; Chang, L.-W.; Lin, C.-H.; Lin, L.-C.; Tsai, T.-H. Determination of Bioactive Components in Chinese Herbal Formulae and Pharmacokinetics of Rhein in Rats by UPLC-MS/MS. Molecules 2014, 19, 4058-4075. https://doi.org/10.3390/molecules19044058
Hou M-L, Chang L-W, Lin C-H, Lin L-C, Tsai T-H. Determination of Bioactive Components in Chinese Herbal Formulae and Pharmacokinetics of Rhein in Rats by UPLC-MS/MS. Molecules. 2014; 19(4):4058-4075. https://doi.org/10.3390/molecules19044058
Chicago/Turabian StyleHou, Mei-Ling, Li-Wen Chang, Chi-Hung Lin, Lie-Chwen Lin, and Tung-Hu Tsai. 2014. "Determination of Bioactive Components in Chinese Herbal Formulae and Pharmacokinetics of Rhein in Rats by UPLC-MS/MS" Molecules 19, no. 4: 4058-4075. https://doi.org/10.3390/molecules19044058
APA StyleHou, M.-L., Chang, L.-W., Lin, C.-H., Lin, L.-C., & Tsai, T.-H. (2014). Determination of Bioactive Components in Chinese Herbal Formulae and Pharmacokinetics of Rhein in Rats by UPLC-MS/MS. Molecules, 19(4), 4058-4075. https://doi.org/10.3390/molecules19044058






