Chemical Composition and Biological Activities of Gerbera anandria
Abstract
:1. Introduction
2. Results and Discussion
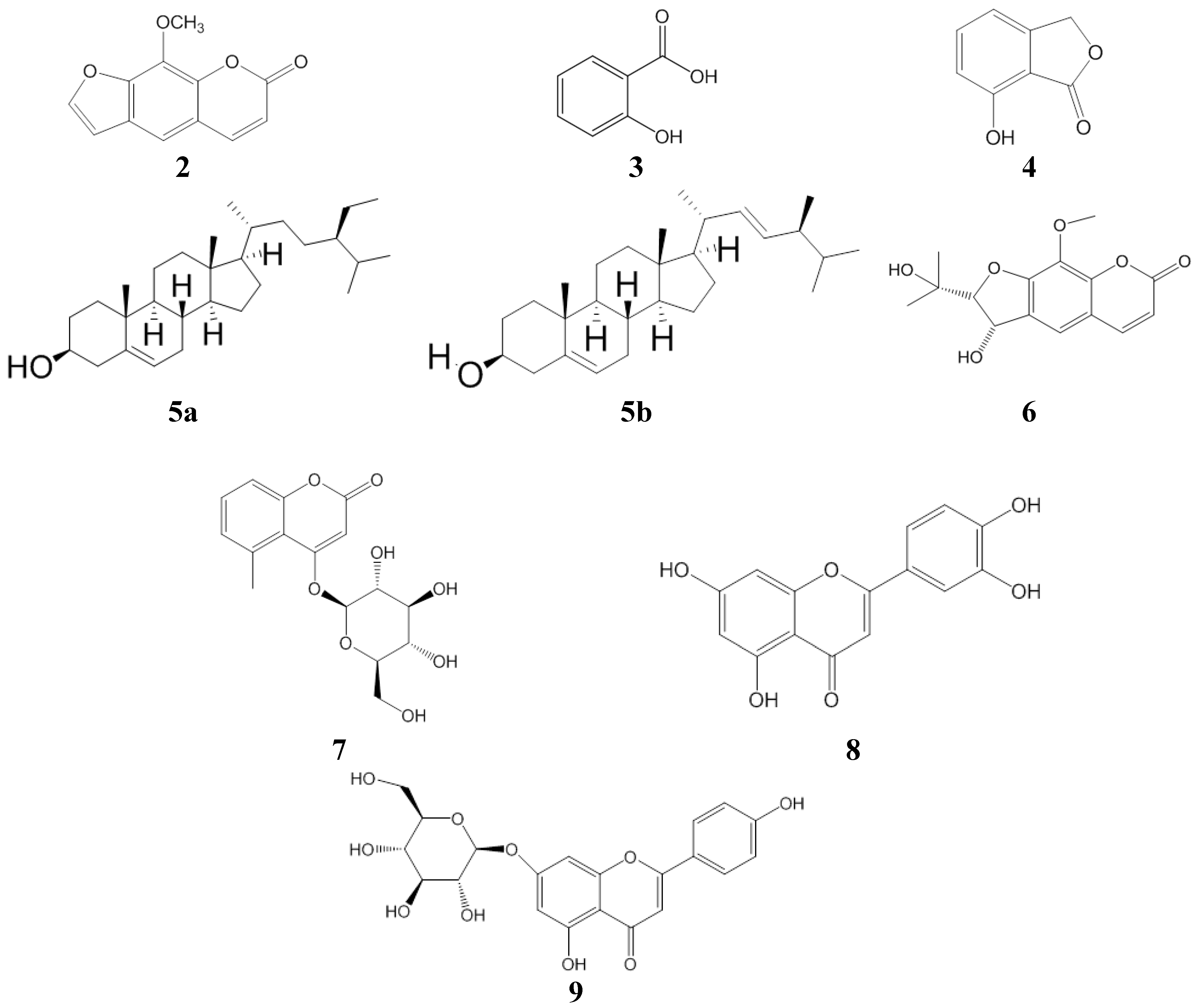

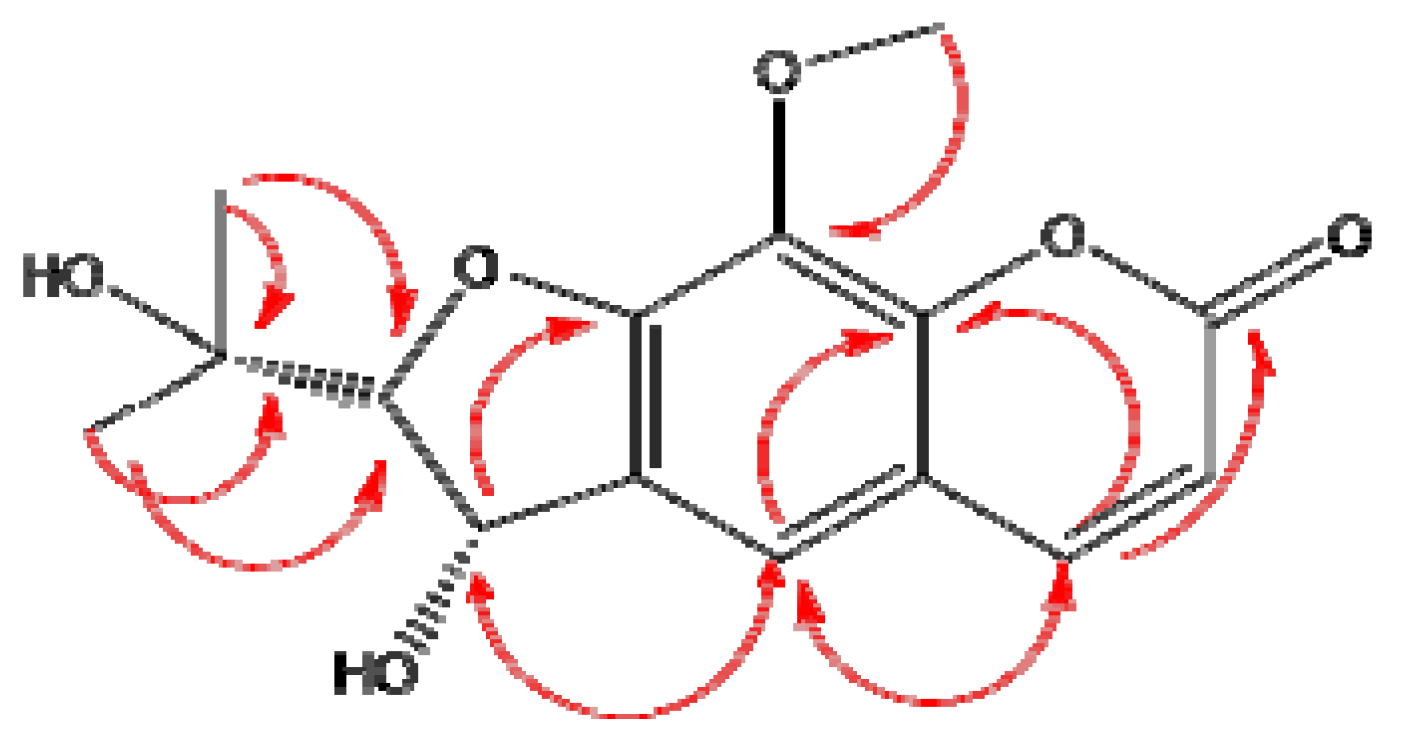
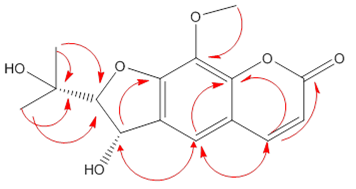
2.1. Antioxidant Activity
| Fractions | IC50 (μg·mL−1) |
|---|---|
| Total extract | 30.7 ± 0.4 |
| Petroleum ether fraction | 178.8 ± 0.2 |
| Chloroform fraction | 66.7 ± 0.3 |
| ethyl acetate fraction | 16.0 ± 0.4 |
| Isolated compounds | |
| xanthotoxin | 462.1 ± 0.4 |
| 2-hydroxy-6-methylbenzoic acid | >500 |
| 7-hydroxy-1 (3H)-isobenzofuranone | >500 |
| gerberinside | >500 |
| 8-methoxysmyrindiol | >500 |
| quercetin | 3.5 ± 0.2 |
| Positive control | |
| Ascorbic acid | 1.8 ± 0.2 |
| Rutin | 2.1 ± 0.3 |
2.2. Cytotoxicity
| Cell Line | IC50 (μg·mL−1) | |
|---|---|---|
| Chloroform Fraction 8-Methoxysmyrindiol | ||
| HeLa (human cervical cancer) | 60.0 ± 0.2 | >100 |
| MCF–7 (human breast cancer) | >100 | 50.1 ± 0.4 |
| HepG2 (Heptocellular carcinoma) | 18.3 ± 0.3 | 5.3 ± 0.2 |
| HCT116 (human colon cancer) | >100 | 53.2 ± 0.3 |
| A549 ( human lung adenocarcinoma) | >100 | 25.1 ± 0.5 |
| A375-S2 (Human melanoma) | 33.9 ± 04 | >100 |
| HT1080 (Human fibrosarcoma) | 40.4 ± 0.2 | >100 |
| HL60 (Myeloid leukemia) | 28.6 ± 0.3 | >100 |
3. Experimental
3.1. Plant Material
3.2. Chemicals
3.3. Extraction Procedure
3.4. Isolation of the Compounds
3.4.1. Compound 1
3.4.2. Compounds 2–6
3.4.3. Compounds 7–9
3.5. Structure Identification
3.6. Antioxidant Activity
3.7. Cytotoxicity
3.7.1. Cell Culture
3.7.2. MTT Assay
3.7.2.1. Adherent Cells
3.7.2.2. Suspension Cells
3.7.3. Evaluation of Results
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gu, L.; Wang, X.; Li, X.; Zhu, T. Study on the antibacterial components in Gerbera anandria. Acta Pharm. Sin. 1987, 22, 272–277. [Google Scholar]
- Bohlmann, F.; Zdero, C.; Franke, H. Naturally occurring coumarin derivatives. IX. The constituents of the Genus Gerbera. Chem Ber. 1973, 106, 382–387. [Google Scholar] [CrossRef]
- Guha, P.K.; Bhattacharyya, A. Coumarin constituents of Gerbera-lanuginosa Benth. Indian J. Chem. B Org. 1991, 30, 714–714. [Google Scholar]
- Bohlmann, F. New geranylecumarin derivatives and further constituents of the tribe Mutisieae. Phytochemistry. 1979, 18, 99–102. [Google Scholar]
- Bass, S.C. Chemical constituents of Gerbera jamesonii. J. Nat. Prod. 1988, 2, 14. [Google Scholar]
- Sen, M.; Dey, M.; Karuri, P. Chemical investigation of Gerbera lanuginose Benth (whole plant) and leaves of Ipomoen fistulosa Mart. J. Indian Chem. Soc. 1979, 6, 326–327. [Google Scholar]
- Chochkova, M.; Stoykova, B.; Lvanova, G.; Ranz, A.; Guo, X.; Lankmayr, E.; Milkova, T. N-Hydroxycinnamoyl amides of fluorinated amino acids: Synthesis, anti-tyrosinase and DPPH scavenging activities. J. Fluor. Chem. 2013, 156, 203–208. [Google Scholar] [CrossRef]
- Abderrahim, F.; Arribas, S.M.; Gonzalez, M.C.; Condezo-Hoyos, L. Rapid high-throughput assay to assess scavenging capacity index using DPPH. Food Chem. 2013, 141, 788–794. [Google Scholar] [CrossRef]
- Kumar, G.S.S.; Seethalakshmi, P.G.; Bhuvanesh, N.; Kumaresan, S. Studies on the syntheses, structural characterization, antimicrobial-, and DPPH radical scavenging activity of the cocrystals caffeine: Cinnamic acid and caffeine: Eosin dehydrate. J. Mol. Struct. 2013, 1050, 88–96. [Google Scholar]
- Saxena, M.; Faridi, U.; Srivastava, S.K.; Darokar, M.P. A cytotoxic and hepatoprotective agent from Withania somnifera and biological evaluation of its ester derivatives. Nat. Prod. Commun. 2007, 2, 775–778. [Google Scholar]
- Cai, T.; Qi, W.; Yang, L.; Tu, G.; Yang, R.; Xie, K.; Fu, H. Chemical constituents of Pseudolarix kaempferi gord. J. Chin. Pharm. Sci. 2012, 21, 428–435. [Google Scholar]
- Weller, D.D.; Haber, A.; Rinehart, K.L.; Wiley, P.F. Carbon-13 nuclear magnetic resonance assignments of pactamycin and related compounds. J. Antibiot. 1978, 31, 997–1006. [Google Scholar] [CrossRef]
- Yang, Z.; Li, Y. Isolation and identification of the chemical constituent of Hemerocallis fulva (L.)L. Zhong Guo Yao Wu Hua Xue Za Zhi 2003, 13, 34–37. [Google Scholar]
- Chen, S.-C.; Hong, L.-L.; Chang, C.-Y.; Chen, C.-J.; Hsu, M.-H.; Huang, Y.-C.; Huang, T.-H.; Kuo, S.-C. Antiproliferative Constituents from Gynura divaricata subsp. formosana. Chin. Pharmaceut. J. 2003, 55, 109–119. [Google Scholar]
- Chen, J.; Wei, J.H.; Cai, S.F.; Zhang, X.H.; Liang, W.J.; Wu, S.G. Chemical constituents in whole herb of Bidens pilosa var. radiata. J. Chin. Med. Mater. 2013, 36, 410–413. [Google Scholar]
- Chakthong, S.; Weaaryee, P.; Puangphet, P.; Mahabusarakam, W.; Plodpai, P.; Voravuthikunchai, S.P.; Kanjana-Opas, A. Alkaloid and coumarins from the green fruits of Aegle marmelos. Phytochemistry 2012, 75, 108–113. [Google Scholar] [CrossRef]
- Berhanu, A.M.; Keige, A.W.; Diaz, J.D.; Herz, W. Sesquiterpene lactones and other constituents of Vernonia species from Ethopia. Phytochemistry 1994, 37, 191–196. [Google Scholar] [CrossRef]
- Jia, L.; Sun, Q.; Huang, S. Isolation and identification of flavonoids from Chrysanthemum morifolium Ramat. J. Chin. Med. Chem. 2003, 13, 159–161. [Google Scholar]
- Seidl, K.; Zinkernagel, A.S. The MTT assay is a rapid and reliable quantitative method to assess Staphylococcus aureus induced endothelial cell damage. J. Microbiol. Meth. 2013, 92, 307–309. [Google Scholar] [CrossRef]
- Boncler, M.; Rozalski, M.; Krajewska, U.; Podsedek, A.; Watala, C. Comparison of prestoblue and MTT assays of cellular viability in the assessment of anti-proliferative effects of plant extracts on human endothelial cells. J. Pharmacol. Toxicol. Meth. 2014, 69, 9–16. [Google Scholar] [CrossRef]
- Mozes, E.; Hunya, A.; Posa, A.; Penke, B.; Datki, Z. A novel method for the rapid determination of beta-amyloid toxicity on acute hippocampal slices using MTT and LDH assays. Brain Res. Bull. 2012, 87, 521–525. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
He, F.; Wang, M.; Gao, M.; Zhao, M.; Bai, Y.; Zhao, C. Chemical Composition and Biological Activities of Gerbera anandria. Molecules 2014, 19, 4046-4057. https://doi.org/10.3390/molecules19044046
He F, Wang M, Gao M, Zhao M, Bai Y, Zhao C. Chemical Composition and Biological Activities of Gerbera anandria. Molecules. 2014; 19(4):4046-4057. https://doi.org/10.3390/molecules19044046
Chicago/Turabian StyleHe, Fa, Miao Wang, Minghuan Gao, Min Zhao, Yuhua Bai, and Chunjie Zhao. 2014. "Chemical Composition and Biological Activities of Gerbera anandria" Molecules 19, no. 4: 4046-4057. https://doi.org/10.3390/molecules19044046
APA StyleHe, F., Wang, M., Gao, M., Zhao, M., Bai, Y., & Zhao, C. (2014). Chemical Composition and Biological Activities of Gerbera anandria. Molecules, 19(4), 4046-4057. https://doi.org/10.3390/molecules19044046