Extract from Armoracia rusticana and Its Flavonoid Components Protect Human Lymphocytes against Oxidative Damage Induced by Hydrogen Peroxide
Abstract
:1. Introduction
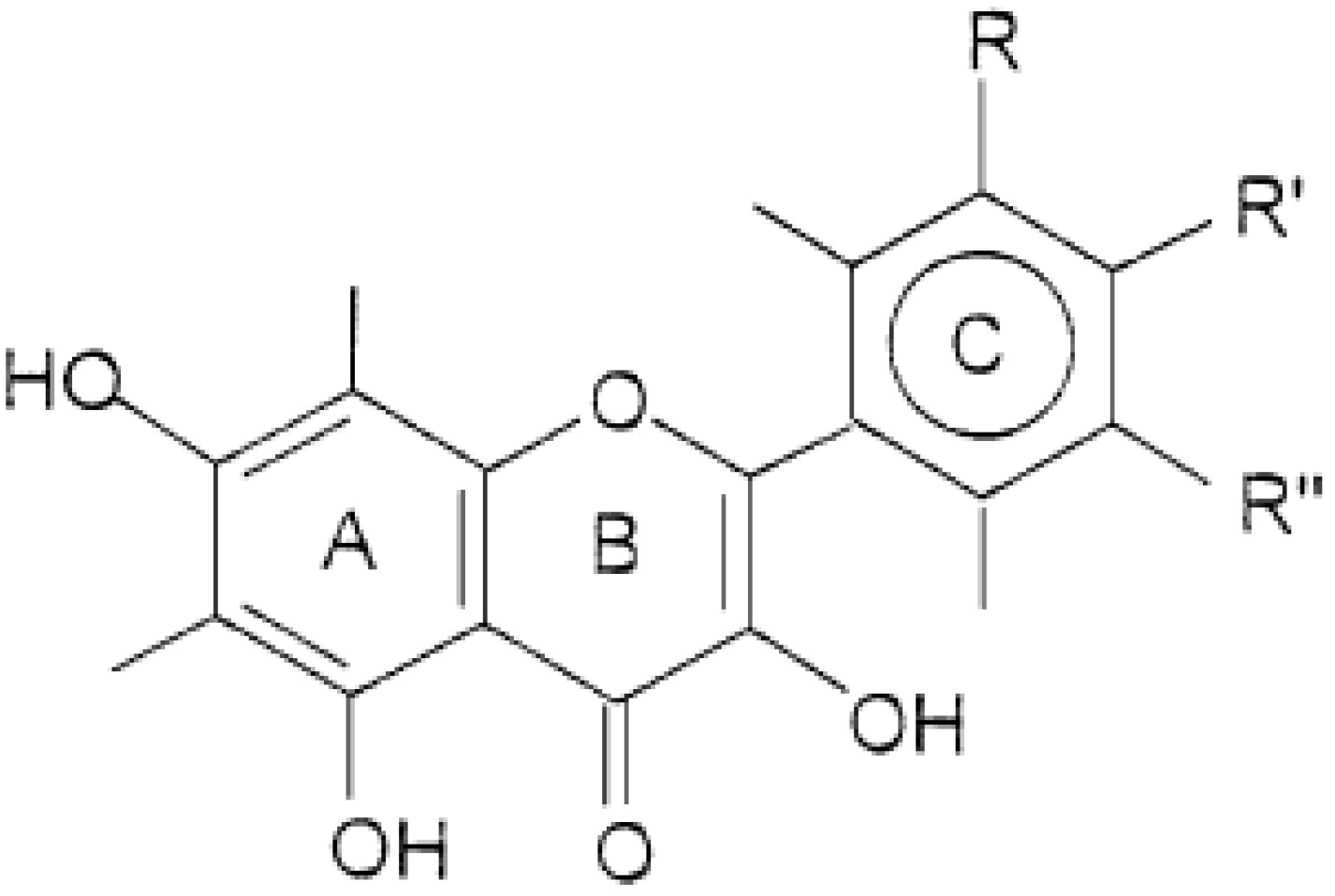
2. Results and Discussion
2.1. Non-Genotoxic Concentration of A. rusticana Extract and Flavonoids
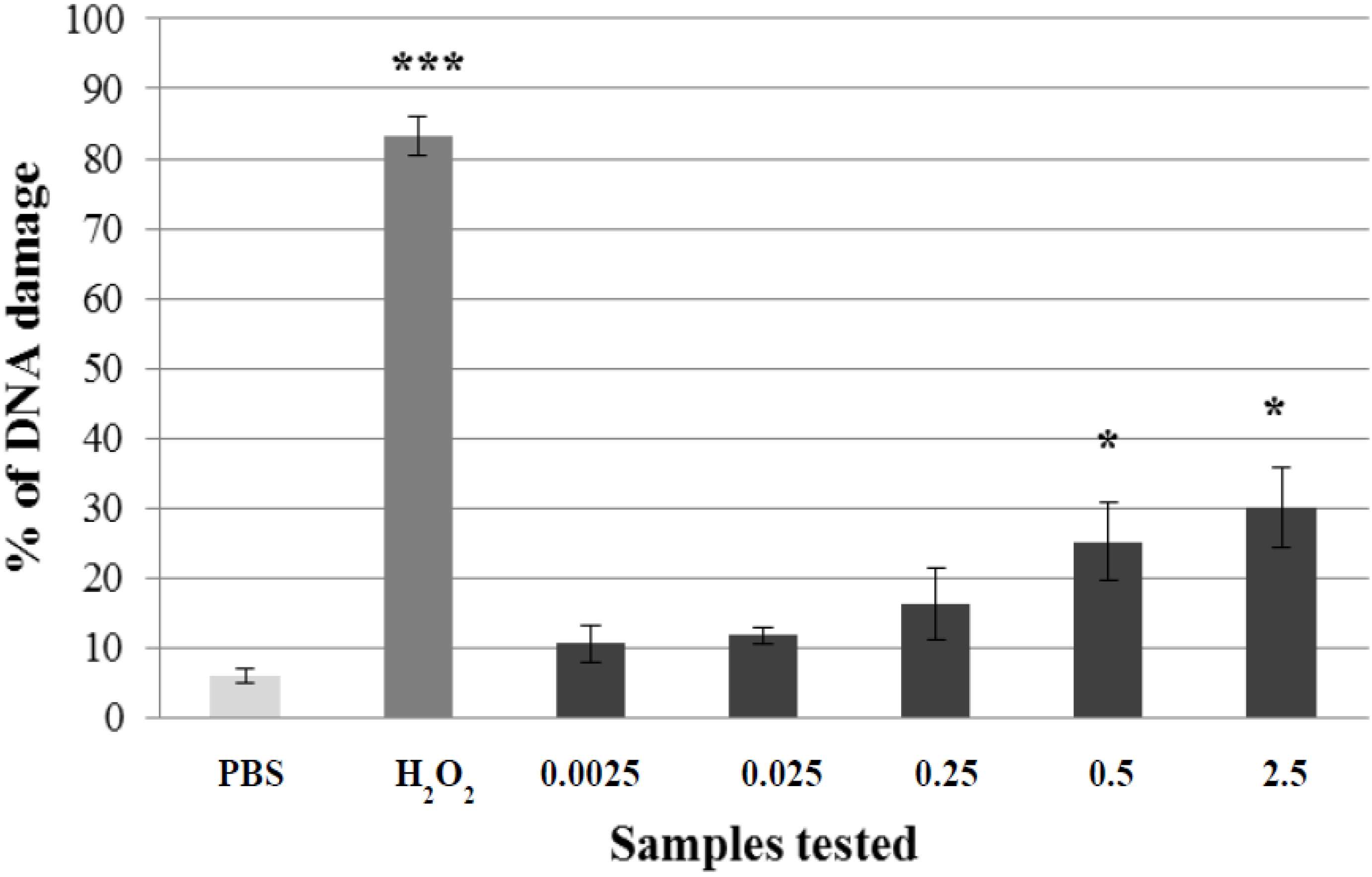
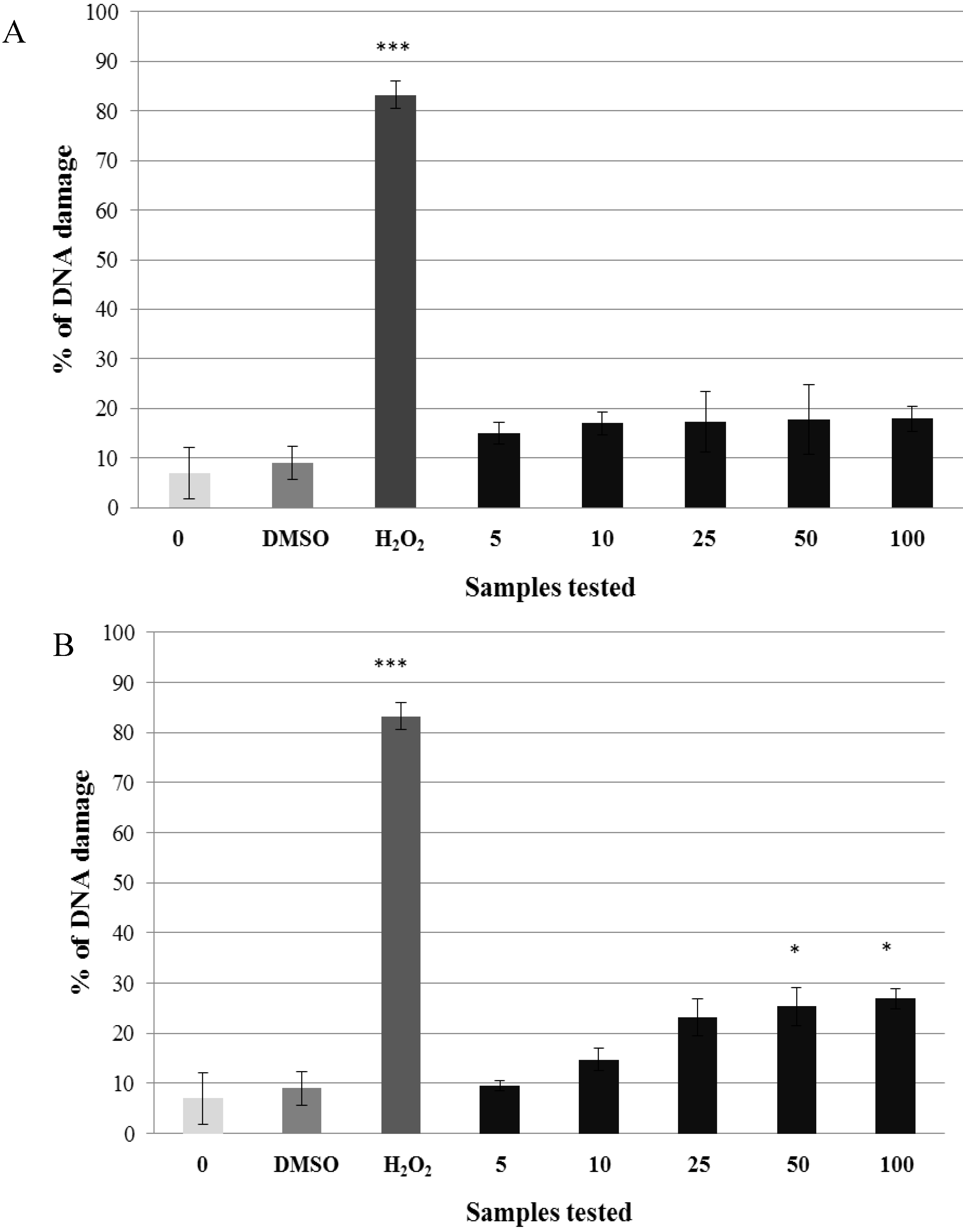
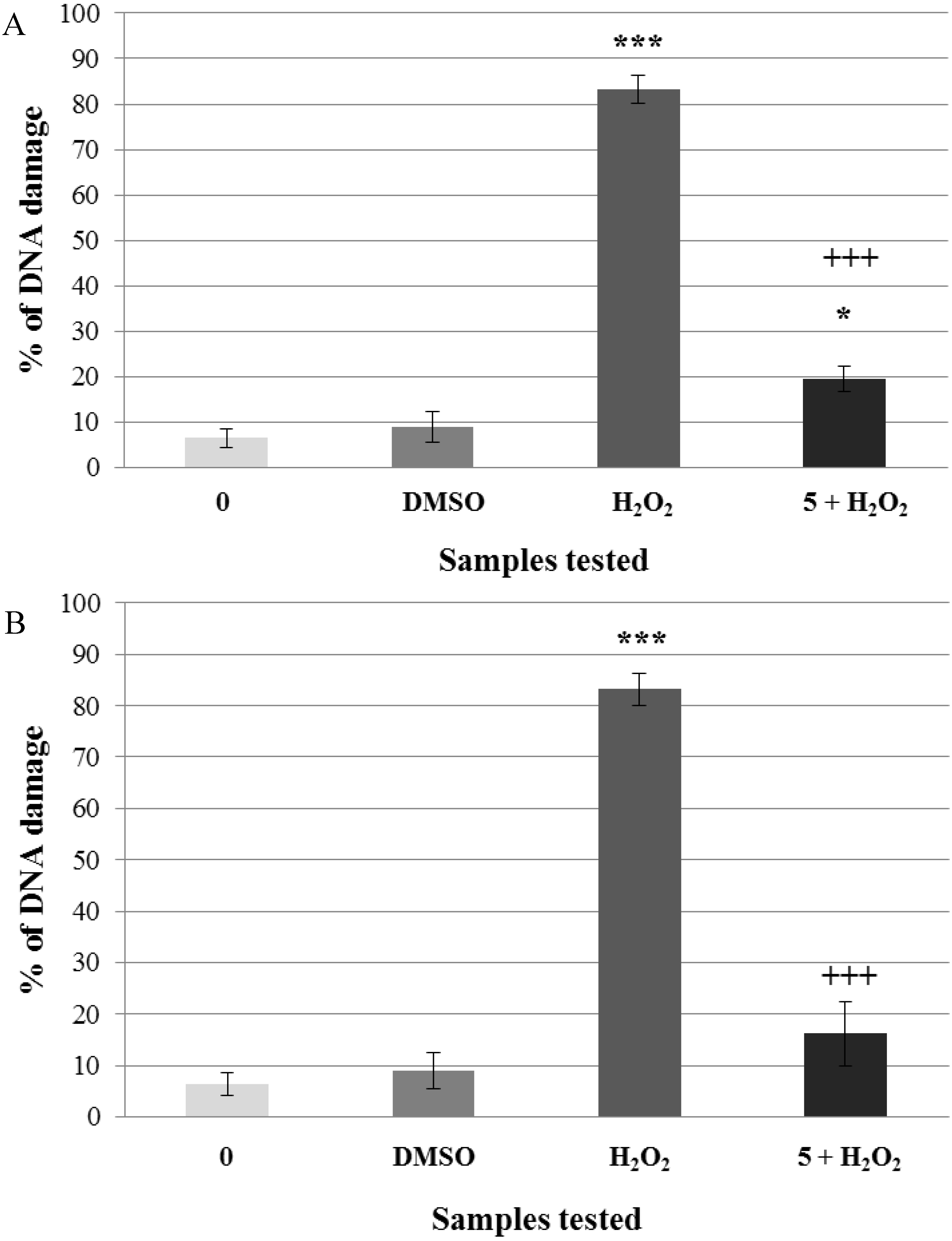
2.2. Pre-Incubation of Lymphocytes with Non-Genotoxic Concentrations of A. rusticana Extract and Flavonoids Decreased the DNA Damage Induced by Hydrogen Peroxide

3. Experimental
3.1. Preparation of Armoracia rusticana Plant Extract
3.2. Flavonoids Preparation
3.3. Lymphocytes
3.4. Comet Assay
3.5. H2O2 Challenge Assay
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflictts of Interest
References
- Rhee, S.G.; Yang, K.S.; Kang, S.W.; Woo, H.A.; Chang, T.S. Controlled elimination of intracellular H2O2: Regulation of peroxiredoxin, catalase, and glutathione peroxidase via post-translational modification. Antioxid. Redox. Signal. 2005, 7, 619–626. [Google Scholar] [CrossRef]
- Szatrowski, T.P.; Nathan, C.F. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991, 51, 794–798. [Google Scholar]
- Ames, B.N.; Shigenaga, M.K.; Gold, L.S. DNA lesions, inducible DNA repair, and cell division: Three key factors in mutagenesis and carcinogenesis. Environ. Health Perspect. 1993, 101, 35–44. [Google Scholar]
- Tiwari, S. Plants: A rich source of herbal medicine. J. Nat. Prod. 2008, 1, 27–35. [Google Scholar] [CrossRef]
- Veitch, N.C. Horseradish peroxidase: A modern view of a classic enzyme. Phytochemistry 2004, 65, 249–259. [Google Scholar] [CrossRef]
- Jiang, Z.T.; Li, R.; Yu, J.C. Pungent components from thioglucosides in Armoracia rusticana grown in China, obtained by enzymatic hydrolysis. Food Technol. Biotechnol. 2006, 44, 41–45. [Google Scholar]
- Jurikova, T.; Rop, O.; Mlcek, J.; Sochor, J.; Balla, S.; Szekeres, L.; Hegedusova, A.; Hubalek, J.; Adam, V.; Kizek, R. Phenolic profile of edible honeysuckle berries (genus Lonicera.) and their biological effects. Molecules 2012, 17, 61–79. [Google Scholar]
- Smith, A.T.; Santama, N.; Dacey, S.; Edwards, M.; Bray, R.C.; Thorneley, R.N.F.; Burke, J.F. Expression of a synthetic gene for horseradish peroxidase C in Escherichia coli and folding and activation of recombinant enzyme with Ca2+ and heme. J. Biol. Chem. 1990, 265, 13335–13343. [Google Scholar]
- Li, X.; Kushad, M.M. Purification and characterization of myrosinase from horseradish (Armoracia rusticana) roots. Plant Physiol. Biochem. 2005, 43, 503–511. [Google Scholar] [CrossRef]
- Li, X.; Kushad, M.M. Correlation of glucosinolates content to myrosinase activity in horseradish (Armoracia rusticana). J. Agric. Food Chem. 2004, 52, 6950–6955. [Google Scholar] [CrossRef]
- Prockop, D.J.; Kivirikko, K.I. Collagens: Molecular biology, diseases, and potentials for therapy. Ann. Rev. Biochem. 1995, 64, 403–434. [Google Scholar] [CrossRef]
- Padayatty, S.J.; Katz, A.; Wang, Y.; Eck, P.; Kwon, O.; Lee, J.H.; Chen, S.; Corpe, C.; Dutta, A.; Dutta, S.K.; et al. Vitamin C as an antioxidant: Evaluation of its role in disease prevention. J. Am. Coll. Nutr. 2003, 22, 18–35. [Google Scholar] [CrossRef]
- Drouin, G.; Godin, J.R.; Pagé, B. The genetics of vitamin C loss in vertebrates. Curr. Genomics 2011, 12, 371–378. [Google Scholar] [CrossRef]
- Fursa, N.S.; Litvinenko, V.I.; Krivenchuk, P.E. Flavonoids of Armoracia rusticana and Barbarea arcuata. Chem. Nat. Compd. 1969, 5, 270–271. [Google Scholar]
- Harborne, J.B.; Baxter, H. Chemical Dictionary of Economic Plants; John Wiley & Sons Ltd: New York, NY, USA, 2001; p. 114. [Google Scholar]
- Cho, E.J.; Yokozawa, T.; Rhyu, D.Y.; Kim, H.Y.; Shibahara, N. The inhibitory effects of 12 medicinal plants and their component compounds on lipid peroxidation. Am. J. Chin. Med. 2003, 31, 907–917. [Google Scholar] [CrossRef]
- Bhagwat, S.; Haytowitz, D.B.; Holden, J.M. USDA Database for the Flavonoid Content of Selected Foods. Department of Agriculture, Agricultural Research Service, Beltsville Human Nutrition Research Center. Available online: http://www.ars.usda.gov/SP2UserFiles/Place/12354500/Data/Flav/Flav3-1.pdf (accessed on 24 February 2014).
- Cirimbei, M.R.; Dinică, R.; Gitina, L.; Vizireanu, C. Study on herbal action of horseradish (Armoracia rusticana). J. Agroaliment. Proc. Technol. 2013, 19, 111–115. [Google Scholar]
- Hollman, P.C.H.; Katan, M.B. Absorption, metabolism and health effects of dietary flavonoids in man. Biomed. Pharmacother. 1997, 51, 305–310. [Google Scholar] [CrossRef]
- Hodek, P.; Trefil, P.; Striborova, M. Flavonoids-potent and versatile biologically active compounds interacting with cytochromes P450. Chem. Biol. Interact. 2002, 139, 1–21. [Google Scholar] [CrossRef]
- Moon, Y.J.; Wang, X.; Morris, M.E. Dietary flavonoids: Effects on xenobiotic and carcinogen metabolism. Toxicol. In Vitro 2006, 20, 187–210. [Google Scholar] [CrossRef]
- Slavin, J. Whole grains and human health. Nutr. Res. Rev. 2004, 17, 99–110. [Google Scholar] [CrossRef]
- Lim, Y.H.; Kim, I.H.; Seo, J.J. In vitro activity of kaempferol isolated from the Impatiens balsamina alone and in combination with erythromycin or clindamycin against Propionibacterium acnes. J. Microbiol. 2007, 45, 473–477. [Google Scholar]
- Calderón-Montaño, J.M.; Burgos-Morón, E.; Pérez-Guerrero, C.; López-Lázaro, M. A review on the dietary flavonoid kaempferol. Mini Rev. Med. Chem. 2011, 11, 298–344. [Google Scholar] [CrossRef]
- Sharma, V.; Joseph, C.; Ghosh, S.; Agarwal, A.; Mishra, M.K.; Sen, E. Kaempferol induces apoptosis in glioblastoma cells through oxidative stress. Mol. Cancer. Ther. 2007, 6, 2544–2553. [Google Scholar]
- Lamson, D.W.; Brignall, M.S. Antioxidants and cancer III: Quercetin. Altern. Med. Rev. 2000, 5, 196–208. [Google Scholar]
- Molina, M.F.; Sanchez-Reus, I.; Iglesias, I.; Benedi, J. Quercetin, a flavonoid antioxidant, prevents and protects against ethanol-induced oxidative stress in mouse liver. Biol. Pharm. Bull. 2003, 26, 1398–1402. [Google Scholar] [CrossRef]
- Jakubowitz-Gil, J.; Rzymowska, J.; Gawron, A. Quercetin, apoptosis, heat shock. Biochem. Pharmacol. 2002, 64, 1591–1595. [Google Scholar] [CrossRef]
- Anderson, D.; Dobrzyńska, M.M.; Başaran, N.; Başaran, A.; Yu, T.-W. Flavonoids modulate comet assay responses to food mutagens in human lymphocytes and sperm. Mutat. Res. 1998, 40, 269–277. [Google Scholar]
- Hidalgo, M.; Sánchez-Moreno, C.; Pascual-Teresa, S. Flavonoid-flavonoid interaction and its effect on their antioxidant activity. Food Chem. 2010, 121, 691–696. [Google Scholar] [CrossRef]
- Hudecova, A.; Hasplova, K.; Miadokova, E.; Magdolenova, Z.; Rinna, A.; Collins, A.R.; Galova, E.; Vaculcikova, D.; Gregan, F.; Dusinska, M. Gentiana. asclepiadea protects human cells against oxidation DNA lesions. Cell Biochem. Funct. 2012, 30, 101–107. [Google Scholar] [CrossRef]
- Harwood, M.; Danielewska-Nikiel, B.; Borzelleca, J.F.; Flamm, G.W.; Williams, G.M.; Lines, T.C. A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties. Food Chem. Toxicol. 2007, 45, 2179–2205. [Google Scholar] [CrossRef]
- Hudecova, A.; Kusznierewicz, B.; Hasplova, K.; Huk, A.; Magdolenova, Z.; Miadokova, E.; Galova, E.; Dusinska, M. Gentiana asclepiadea exerts antioxidant activity and enhances DNA repair of hydrogen peroxide- and silver nanoparticles-induced DNA damage. Food Chem. Toxicol. 2012, 50, 3352–3359. [Google Scholar] [CrossRef]
- Nakamura, Y.; Matsuo, T.; Okamoto, S.; Nishikawa, A.; Imai, T.; Park, E.Y.; Sato, K. Antimutagenic and anticarcinogenic properties of Kyo-yasai, heirloom vegetables in Kyoto. Genes Environ. 2008, 30, 41–47. [Google Scholar] [CrossRef]
- Bhattacharya, S. Natural antimutagens: A review. Res. J. Med. Plant 2011, 5, 116–126. [Google Scholar] [CrossRef]
- Gáfriková, M.; Kellovská, L.; Ikréniová, M.; Miadoková, E.; Gálová, E.; Hudecová, A. Comparison of Antimutagenic Effect of Extract from Armoracia. Rusticana and Gentiana. Asclepiadea; Proceedings of the Student Scientific Conference; Bratislava, Slovakia: 25 April 2012 ISBN: 978-80-2-3213-223. , 2012; pp. 206–211.
- Kopaskova, M.; Hadjo, L.; Yankulova, B.; Jovtchev, G.; Galova, E.; Sevcovicova, A.; Mucaji, P.; Miadokova, E.; Bryant, P.; Chankova, S. Extract from Lillium candidum L. can modulate the genotoxicity of the antibiotic zeocin. Molecules 2012, 17, 80–97. [Google Scholar]
- Kozics, K.; Valovičová, Z.; Slameňová, D. Structure of flavonoids influences the degree inhibition of benzo[a]pyrene-induced DNA damage and micronuclei in HepG2 cells. Neoplasma 2011, 58, 516–524. [Google Scholar] [CrossRef]
- Khadem, S.; Marles, R.J. Chromone and flavonoid alkaloids: Occurrence and bioactivity. Molecules 2012, 17, 191–206. [Google Scholar]
- Collins, A.R.; Oscoz, A.A.; Brunborg, G.; Gaiväo, I.; Giovannelli, L.; Kruszewski, M.; Smith, C.C.; Štetina, R. The comet assay: topical issues. Mutagenesis 2008, 23, 143–151. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gafrikova, M.; Galova, E.; Sevcovicova, A.; Imreova, P.; Mucaji, P.; Miadokova, E. Extract from Armoracia rusticana and Its Flavonoid Components Protect Human Lymphocytes against Oxidative Damage Induced by Hydrogen Peroxide. Molecules 2014, 19, 3160-3172. https://doi.org/10.3390/molecules19033160
Gafrikova M, Galova E, Sevcovicova A, Imreova P, Mucaji P, Miadokova E. Extract from Armoracia rusticana and Its Flavonoid Components Protect Human Lymphocytes against Oxidative Damage Induced by Hydrogen Peroxide. Molecules. 2014; 19(3):3160-3172. https://doi.org/10.3390/molecules19033160
Chicago/Turabian StyleGafrikova, Michala, Eliska Galova, Andrea Sevcovicova, Petronela Imreova, Pavel Mucaji, and Eva Miadokova. 2014. "Extract from Armoracia rusticana and Its Flavonoid Components Protect Human Lymphocytes against Oxidative Damage Induced by Hydrogen Peroxide" Molecules 19, no. 3: 3160-3172. https://doi.org/10.3390/molecules19033160
APA StyleGafrikova, M., Galova, E., Sevcovicova, A., Imreova, P., Mucaji, P., & Miadokova, E. (2014). Extract from Armoracia rusticana and Its Flavonoid Components Protect Human Lymphocytes against Oxidative Damage Induced by Hydrogen Peroxide. Molecules, 19(3), 3160-3172. https://doi.org/10.3390/molecules19033160



