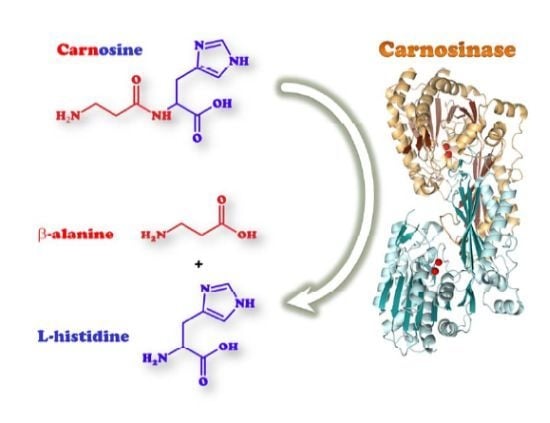
Carnosinases, Their Substrates and Diseases
Abstract
:1. Introduction
2. Carnosinase Substrates
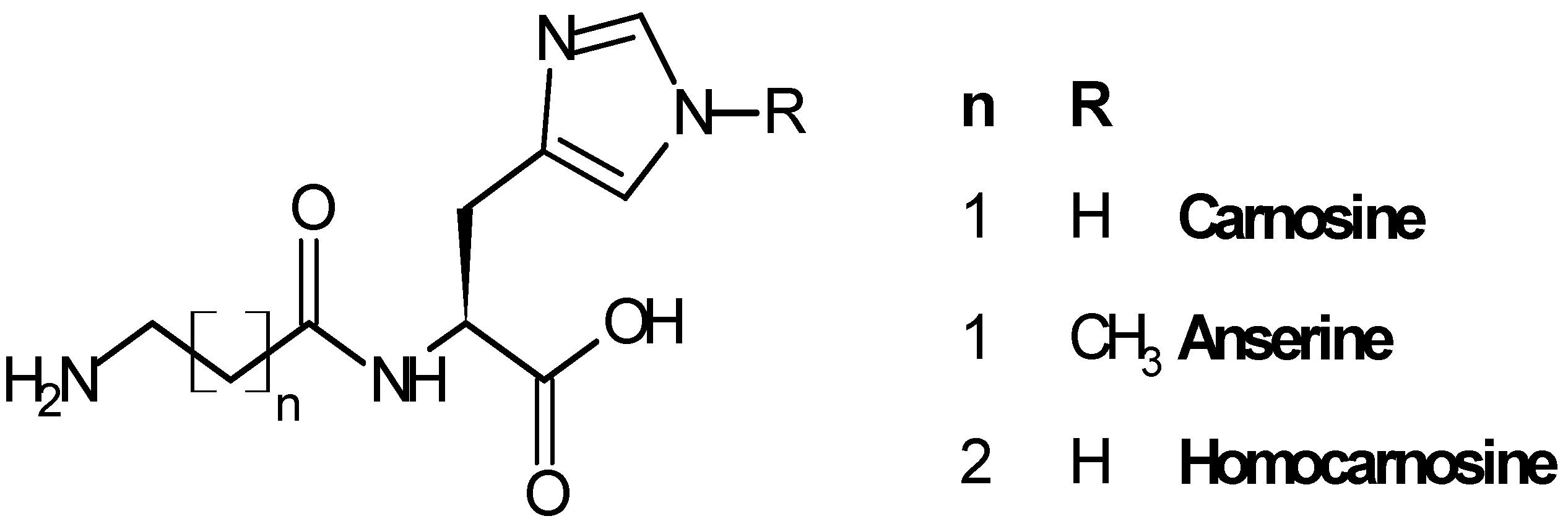
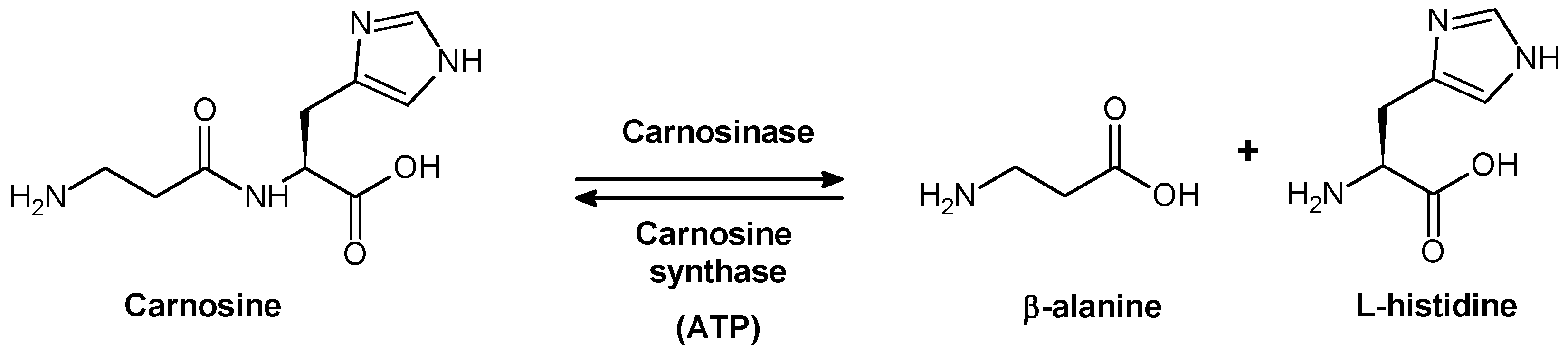
2.1. Biological Role of Carnosinase Substrates
3. Human Carnosinases
3.1. Biological Activities of Human Carnosinases

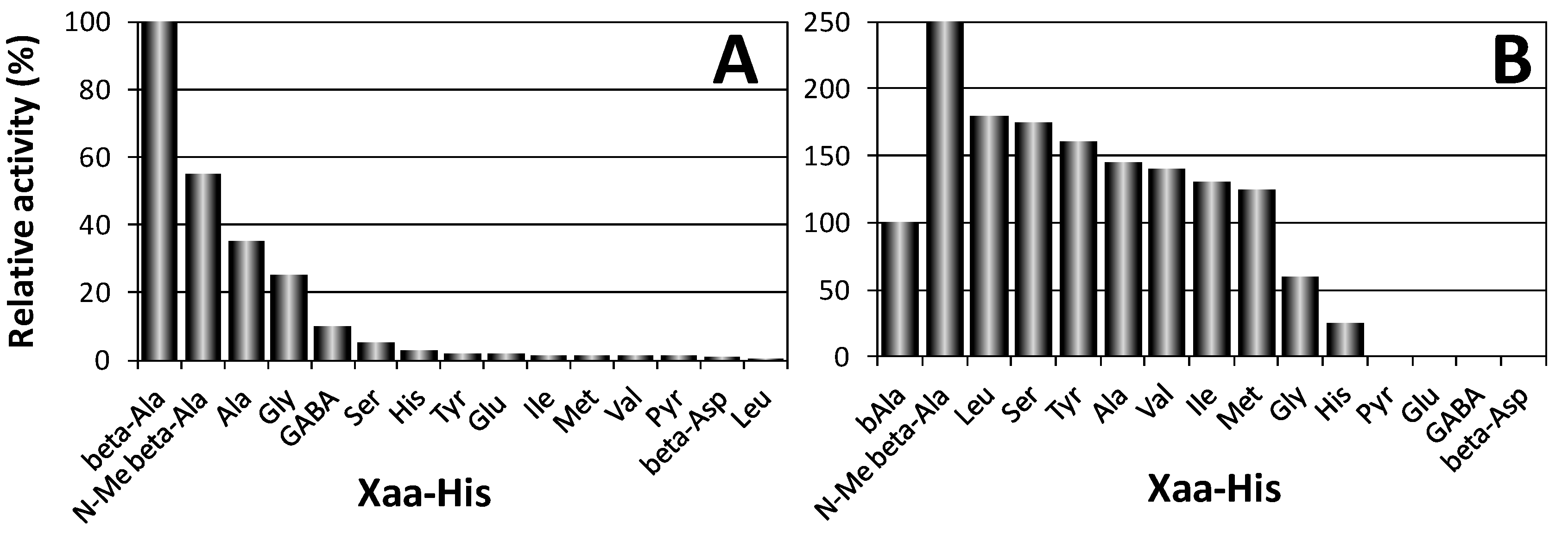
4. Other Aminoacyl-Histidine Dipeptidases

5. Structural Features of Carnosinases
| Full name | Organism | Tissue | FW (KDa) | Activation | Optimal pH | Main substrate | Main Inhibitors | |
|---|---|---|---|---|---|---|---|---|
| CN1 | Serum carnosinase | Homo sapiens | Brain, serum, liver | 56.8 | Cd2+ citrate | 7.5 | Carnosine | Phenantroline |
| CN2 | Cytosolic carnosinase | Homo sapiens | Ubiquitous | 52.9 | Mn2+ | 9.5 | Xaa-His | Bestatin |
| ANSN | Anserinase | Oncorhynchus mykiss | Brain, eyes | 55.0 | Co2+, Zn2+ | Anserine | Bestatin | |
| PepV | Peptidase V | Lactobacillus delbrueckii | Cytoplasm | 52.0 | Carnosine | Phenantroline EDTA | ||
| PepD | Peptidase D | E. Coli | Cytoplasm | 52.9 | Co2+ | 9.0 | Xaa-His | Metal chelators |




6. Carnosinase-Related Diseases
- i)
- ii)
- damage to carnosinase-producing cells, though no relationship was found between size of the infarct and carnosinase activity [135];
- iii)
- genetic factors, as suggested by the fact that deletion distal to 18q21.3 was found in a child with serum carnosinase deficiency [143] (the gene for serum carnosinase is located on 18q22.3 [5]); moreover a locus on chromosome 18 was identified for familial AD in Caribbean Hispanics [144] and an association between the allelic variation of this gene and carnosinase activity has been shown [67]. In this study, patients with MD may have always had low carnosinase activity and then susceptible to dementia.
- iv)
- down-regulation of carnosinase mRNA, as shown for the AD marker neural thread protein (AD7CNTP). It is up regulated in the brains and CSF of patients with AD compared to controls [145].
- v)
- regular physical activity, associated with increased carnosinase activity and with a decreased risk of vascular dementia in women [146].
Carnosinase as a Biomarker
7. Substrate Derivatives of Carnosine and Its Related Dipeptides
8. Concluding Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, S.L.; Marino, T.; Fang, W.H.; Russo, N.; Himo, F. Peptide hydrolysis by the binuclear zinc enzyme aminopeptidase from Aeromonas proteolytica: A density functional theory study. J. Phys. Chem. B 2008, 112, 2494–2500. [Google Scholar] [CrossRef]
- Hanson, H.T.; Smith, E.L. Carnosinase: An enzyme of swine kidney. J. Biol. Chem. 1949, 179, 789–801. [Google Scholar]
- Lenney, J.F.; Peppers, S.C.; Kucera-Orallo, C.M.; George, R.P. Characterization of human tissue carnosinase. Biochem. J. 1985, 228, 653–660. [Google Scholar]
- Jackson, M.C.; Kucera, C.M.; Lenney, J.F. Purification and properties of human serum carnosinase. Clin. Chim. Acta 1991, 196, 193–205. [Google Scholar] [CrossRef]
- Teufel, M.; Saudek, V.; Ledig, J.P.; Bernhardt, A.; Boularand, S.; Carreau, A.; Cairns, N.J.; Carter, C.; Cowley, D.J.; Duverger, D.; et al. Sequence identification and characterization of human carnosinase and a closely related non-specific dipeptidase. J. Biol. Chem. 2003, 278, 6521–6531. [Google Scholar] [CrossRef]
- Gulewitsch, W.; Amiradzibi, S. Carnosine, a new organic base from meat extracts. Ber. Dtsch. Chem. Ges. 1900, 33, 1902–1903. [Google Scholar] [CrossRef]
- Bellia, F.; Vecchio, G.; Cuzzocrea, S.; Calabrese, V.; Rizzarelli, E. Neuroprotective features of carnosine in oxidative driven diseases. Mol. Aspects Med. 2011, 32, 258–266. [Google Scholar] [CrossRef]
- Boldyrev, A.A.; Aldini, G.; Derave, W. Physiology and pathophysiology of carnosine. Physiol. Rev. 2013, 93, 1803–1845. [Google Scholar]
- Bellia, F.; Calabrese, V.; Guarino, F.; Cavallaro, M.; Cornelius, C.; de Pinto, V.; Rizzarelli, E. Carnosinase levels in aging brain: Redox state induction and cellular stress response. Antioxid. Redox Signal. 2009, 11, 2759–2775. [Google Scholar] [CrossRef]
- Gautam, P.; Nair, S.C.; Gupta, M.K.; Sharma, R.; Polisetty, R.V.; Uppin, M.S.; Sundaram, C.; Puligopu, A.K.; Ankathi, P.; Purohit, A.K.; et al. Proteins with altered levels in plasma from glioblastoma patients as revealed by iTRAQ-based quantitative proteomic analysis. PLoS One 2012, 7, e46153. [Google Scholar] [CrossRef]
- Boldyrev, A.A.; Severin, S.E. The histidine-containing dipeptides, carnosine and anserine: Distribution, properties and biological significance. Adv. Enzym. Regul. 1990, 30, 175–194. [Google Scholar] [CrossRef]
- Neidle, A.; Kandera, J. Carnosine--an olfactory bulb peptide. Brain Res. 1974, 80, 359–364. [Google Scholar] [CrossRef]
- Horinishi, H.; Grillo, M.; Margolis, F.L. Purification and characterization of carnosine synthetase from mouse olfactory bulbs. J. Neurochem. 1978, 31, 909–919. [Google Scholar] [CrossRef]
- Kalyankar, G.D.; Meister, A. Enzymatic synthesis of carnosine and related beta-alanyl and gamma-aminobutyryl peptides. J. Biol. Chem. 1959, 234, 3210–3218. [Google Scholar]
- Hoffmann, A.M.; Bakardjiev, A.; Bauer, K. Carnosine-synthesis in cultures of rat glial cells is restricted to oligodendrocytes and carnosine uptake to astrocytes. Neurosci. Lett. 1996, 215, 29–32. [Google Scholar] [CrossRef]
- Drozak, J.; Veiga-da-Cunha, M.; Vertommen, D.; Stroobant, V.; van Schaftingen, E. Molecular identification of carnosine synthase as ATP-grasp domain-containing protein 1 (ATPGD1). J. Biol. Chem. 2010, 285, 9346–9356. [Google Scholar]
- Yamada, S.; Tanaka, Y.; Furuichi, M. Partial purification and characterization of histidine acetyltransferase in brain of Nile tilapia (Oreochromis niloticus). Biochim. Biophys. Acta 1995, 1245, 239–247. [Google Scholar] [CrossRef]
- Baslow, M.H.; Lenney, J.F. Alpha-N-acetyl-l-histidine amidohydrolase activity from the brain of the skipjack tuna Katsuwonus pelamis. Can. J. Biochem. 1967, 45, 337–340. [Google Scholar] [CrossRef]
- Lenney, J.F.; Baslow, M.H.; Sugiyama, G.H. Similarity of tuna N-acetylhistidine deacetylase and cod fish anserinase. Comp. Biochem. Physiol. B: Biochem. Mol. Biol. 1978, 61, 253–258. [Google Scholar] [CrossRef]
- Drozak, J.; Chrobok, L.; Poleszak, O.; Jagielski, A.K.; Derlacz, R. Molecular identification of carnosine N-methyltransferase as chicken histamine N-methyltransferase-like protein (HNMT-Like). PLoS One 2013, 8, e64805. [Google Scholar]
- Gjessing, L.R.; Lunde, H.A.; Mørkrid, L.; Lenney, J.F.; Sjaastad, O. Inborn errors of carnosine and homocarnosine metabolism. J. Neural Transm. Suppl. 1990, 29, 91–106. [Google Scholar]
- Petroff, O.A.C. GABA and glutamate in the human brain. Neuroscientist 2002, 8, 562–573. [Google Scholar] [CrossRef]
- Petroff, O.A.C.; Hyder, F.; Rothman, D.L.; Mattson, R.H. Homocarnosine and seizure control in juvenile myoclonic epilepsy and complex partial seizures. Neurology 2001, 56, 709–715. [Google Scholar]
- Gil-Agusti, M.; Esteve-Romero, J.; Carda-Broch, S. Anserine and carnosine determination in meat samples by pure micellar liquid chromatography. J. Chromatogr. A 2008, 1189, 444–450. [Google Scholar]
- Baguet, A.; Reyngoudt, H.; Pottier, A.; Everaert, I.; Callens, S.; Achten, E.; Derave, W. Carnosine loading and washout in human skeletal muscles. J. Appl. Physiol. 2009, 106, 837–842. [Google Scholar] [CrossRef]
- Kim, H.J. Comparison of the carnosine and taurine contents of vastus lateralis of elderly Korean males, with impaired glucose tolerance, and young elite Korean swimmers. Amino Acids 2009, 36, 359–363. [Google Scholar] [CrossRef]
- Stuerenburg, H.J.; Kunze, K. Concentrations of free carnosine (a putative membrane-protective antioxidant) in human muscle biopsies and rat muscles. Arch. Gerontol. Geriatr. 1999, 29, 107–113. [Google Scholar] [CrossRef]
- Tallon, M.J.; Harris, R.C.; Maffulli, N.; Tarnopolsky, M.A. Carnosine, taurine and enzyme activities of human skeletal muscle fibres from elderly subjects with osteoarthritis and young moderately active subjects. Biogerontology 2007, 8, 129–137. [Google Scholar] [CrossRef]
- Harris, R.C.; Tallon, M.J.; Dunnett, M.; Boobis, L.; Coakley, J.; Kim, H.J.; Fallowfield, J.L.; Hill, C.A.; Sale, C.; Wise, J.A. The absorption of orally supplied beta-alanine and its effect on muscle carnosine synthesis in human vastus lateralis. Amino Acids 2006, 30, 279–289. [Google Scholar] [CrossRef]
- Smith, E.C. The buffering of muscle in rigor; protein, phosphate and carnosine. J. Physiol. 1938, 92, 336–343. [Google Scholar]
- Aruoma, O.I.; Laughton, M.J.; Halliwell, B. Carnosine, homocarnosine and anserine: Could they act as antioxidants in vivo? Biochem. J. 1989, 264, 863–869. [Google Scholar]
- Kohen, R.; Misgav, R.; Ginsburg, I. The SOD like activity of copper:carnosine, copper:anserine and copper:homocarnosine complexes. Free Radic. Res. Commun. 1991, 12, 179–185. [Google Scholar] [CrossRef]
- Baran, E.J. Metal complexes of carnosine. Biochemistry (Moscow) 2000, 65, 789–797. [Google Scholar]
- Bonomo, R.P.; Bruno, V.; Conte, E.; de Guidi, G.; la Mendola, D.; Maccarrone, G.; Nicoletti, F.; Rizzarelli, E.; Sortino, S.; Vecchio, G. Potentiometric, spectroscopic and antioxidant activity studies of SOD mimics containing carnosine. Dalton Trans. 2003, 4406–4415. [Google Scholar] [CrossRef]
- Cuzzocrea, S.; Genovese, T.; Failla, M.; Vecchio, G.; Fruciano, M.; Mazzon, E.; di Paola, R.; Muia, C.; la Rosa, C.; Crimi, N.; et al. Protective effect of orally administered carnosine on bleomycin-induced lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007, 292, L1095–L1104. [Google Scholar]
- Chuang, C.H.; Hu, M.L. L-carnosine inhibits metastasis of SK-Hep-1 cells by inhibition of matrix metaoproteinase-9 expression and induction of an antimetastatic gene, nm23-H1. Nutr. Cancer 2008, 60, 526–533. [Google Scholar] [CrossRef]
- Hipkiss, A.R.; Michaelis, J.; Syrris, P. Non-enzymatic glycosylation of the dipeptide L-carnosine, a potential anti-protein-cross-linking agent. FEBS Lett. 1995, 371, 81–85. [Google Scholar]
- Hipkiss, A.R. Carnosine and protein carbonyl groups: A possible relationship. Biochemistry (Moscow) 2000, 65, 771–778. [Google Scholar]
- Lee, Y.T.; Hsu, C.C.; Lin, M.H.; Liu, K.S.; Yin, M.C. Histidine and carnosine delay diabetic deterioration in mice and protect human low density lipoprotein against oxidation and glycation. Eur. J. Pharmacol. 2005, 513, 145–150. [Google Scholar]
- Sauerhofer, S.; Yuan, G.; Braun, G.S.; Deinzer, M.; Neumaier, M.; Gretz, N.; Floege, J.; Kriz, W.; van der Woude, F.; Moeller, M.J. L-carnosine, a substrate of carnosinase-1, influences glucose metabolism. Diabetes 2007, 56, 2425–2432. [Google Scholar]
- Biffo, S.; DeLucia, R.; Mulatero, B.; Margolis, F.; Fasolo, A. Carnosine-, calcitonin gene-related peptide- and tyrosine hydroxylase-immunoreactivity in the mouse olfactory bulb following peripheral denervation. Brain Res. 1990, 528, 353–357. [Google Scholar]
- Bonfanti, L.; Peretto, P.; de Marchis, S.; Fasolo, A. Carnosine-related dipeptides in the mammalian brain. Prog. Neurobiol. 1999, 59, 333–353. [Google Scholar] [CrossRef]
- Xiang, J.; Hu, Y.; Smith, D.E.; Keep, R.F. PEPT2-mediated transport of 5-aminolevulinic acid and carnosine in astrocytes. Brain Res. 2006, 1122, 18–23. [Google Scholar] [CrossRef]
- Hipkiss, A.R. Glycation, ageing and carnosine: Are carnivorous diets beneficial? Mech. Ageing Dev. 2005, 126, 1034–1039. [Google Scholar] [CrossRef]
- Huang, Y.; Duan, J.; Chen, H.; Chen, M.; Chen, G. Separation and determination of carnosine-related peptides using capillary electrophoresis with laser-induced fluorescence detection. Electrophoresis 2005, 26, 593–599. [Google Scholar] [CrossRef]
- Hipkiss, A.R. Could carnosine suppress zinc-mediated proteasome inhibition and neurodegeneration? Therapeutic potential of a non-toxic but non-patentable dipeptide. Biogerontology 2005, 6, 147–149. [Google Scholar] [CrossRef]
- Hipkiss, A.R. Could carnosine or related structures suppress Alzheimer’s disease? J. Alzheimers Dis. 2007, 11, 229–240. [Google Scholar]
- La Mendola, D.; Sortino, S.; Vecchio, G.; Rizzarelli, E. Synthesis of new carnosine derivatives of b-cyclodextrin and their hydroxyl radical scavenger ability. Helv. Chim. Acta 2002, 85, 1633–1643. [Google Scholar] [CrossRef]
- Fontana, M.; Pinnen, F.; Lucente, G.; Pecci, L. Prevention of peroxynitrite-dependent damage by carnosine and related sulphonamido pseudodipeptides. Cell. Mol. Life Sci. 2002, 59, 546–551. [Google Scholar] [CrossRef]
- Severina, I.S.; Bussygina, O.G.; Pyatakova, N.V. Carnosine as a regulator of soluble guanylate cyclase. Biochemistry (Moscow) 2000, 65, 783–788. [Google Scholar]
- Dukic-Stefanovic, S.; Schinzel, R.; Riederer, P.; Munch, G. AGES in brain ageing: AGE-inhibitors as neuroprotective and anti-dementia drugs? Biogerontology 2001, 2, 19–34. [Google Scholar] [CrossRef]
- Pubill, D.; Verdaguer, E.; Sureda, F.X.; Camins, A.; Pallas, M.; Camarasa, J.; Escubedo, E. Carnosine prevents methamphetamine-induced gliosis but not dopamine terminal loss in rats. Eur. J. Pharmacol. 2002, 448, 165–168. [Google Scholar] [CrossRef]
- Dobrota, D.; Fedorova, T.; Stvolinsky, S.; Babusikova, E.; Likavcanova, K.; Drgova, A.; Strapkova, A.; Boldyrev, A. Carnosine protects the brain of rats and Mongolian gerbils against ischemic injury: After-stroke-effect. Neurochem. Res. 2005, 30, 1283–1288. [Google Scholar]
- Stvolinsky, S.; Kukley, M.; Dobrota, D.; Mezesova, V.; Boldyrev, A. Carnosine protects rats under global ischemia. Brain Res. Bull. 2000, 53, 445–448. [Google Scholar] [CrossRef]
- Tang, S.C.; Arumugam, T.V.; Cutler, R.G.; Jo, D.G.; Magnus, T.; Chan, S.L.; Mughal, M.R.; Telljohann, R.S.; Nassar, M.; Ouyang, X.; et al. Neuroprotective actions of a histidine analogue in models of ischemic stroke. J. Neurochem. 2007, 101, 729–736. [Google Scholar] [CrossRef]
- Calabrese, V.; Colombrita, C.; Guagliano, E.; Sapienza, M.; Ravagna, A.; Cardile, V.; Scapagnini, G.; Santoro, A.M.; Mangiameli, A.; Butterfield, D.A.; et al. Protective effect of carnosine during nitrosative stress in astroglial cell cultures. Neurochem. Res. 2005, 30, 797–807. [Google Scholar]
- Calabrese, V.; Mancuso, C.; Calvani, M.; Rizzarelli, E.; Butterfield, D.A.; Stella, A.M. Nitric oxide in the central nervous system: Neuroprotection versus neurotoxicity. Nat. Rev. Neurosci. 2007, 8, 766–775. [Google Scholar] [CrossRef]
- Nicoletti, V.G.; Santoro, A.M.; Grasso, G.; Vagliasindi, L.I.; Giuffrida, M.L.; Cuppari, C.; Purrello, V.S.; Stella, A.M.; Rizzarelli, E. Carnosine interaction with nitric oxide and astroglial cell protection. J. Neurosci. Res. 2007, 85, 2239–2245. [Google Scholar]
- Preston, J.E.; Hipkiss, A.R.; Himsworth, D.T.; Romero, I.A.; Abbott, J.N. Toxic effects of beta-amyloid(25–35) on immortalised rat brain endothelial cell: Protection by carnosine, homocarnosine and beta-alanine. Neurosci. Lett. 1998, 242, 105–108. [Google Scholar] [CrossRef]
- Attanasio, F.; Convertino, M.; Magno, A.; Caflisch, A.; Corazza, A.; Haridas, H.; Esposito, G.; Cataldo, S.; Pignataro, B.; Milardi, D.; et al. Carnosine inhibits Abeta(42) aggregation by perturbing the H-bond network in and around the central hydrophobic cluster. ChemBioChem 2013, 14, 583–592. [Google Scholar] [CrossRef]
- Hipkiss, A.R.; Preston, J.E.; Himsworth, D.T.; Worthington, V.C.; Keown, M.; Michaelis, J.; Lawrence, J.; Mateen, A.; Allende, L.; Eagles, P.A.; et al. Pluripotent protective effects of carnosine, a naturally occurring dipeptide. Ann. N. Y. Acad. Sci. 1998, 854, 37–53. [Google Scholar] [CrossRef]
- Fonteh, A.N.; Harrington, R.J.; Tsai, A.; Liao, P.; Harrington, M.G. Free amino acid and dipeptide changes in the body fluids from Alzheimer’s disease subjects. Amino Acids 2007, 32, 213–224. [Google Scholar] [CrossRef]
- Jin, C.L.; Yang, L.X.; Wu, X.H.; Li, Q.; Ding, M.P.; Fan, Y.Y.; Zhang, W.P.; Luo, J.H.; Chen, Z. Effects of carnosine on amygdaloid-kindled seizures in Sprague-Dawley rats. Neuroscience 2005, 135, 939–947. [Google Scholar] [CrossRef]
- Pegova, A.; Abe, H.; Boldyrev, A. Hydrolysis of carnosine and related compounds by mammalian carnosinases. Comp. Biochem. Physiol. B: Biochem. Mol. Biol. 2000, 127, 443–446. [Google Scholar] [CrossRef]
- Hou, W.C.; Chen, H.J.; Lin, Y.H. Antioxidant peptides with Angiotensin converting enzyme inhibitory activities and applications for Angiotensin converting enzyme purification. J. Agric. Food Inf. 2003, 51, 1706–1709. [Google Scholar] [CrossRef]
- Aydogan, S.; Yapislar, H.; Artis, S.; Aydogan, B. Impaired erythrocytes deformability in H(2)O(2)-induced oxidative stress: Protective effect of L-carnosine. Clin. Hemorheol. Microcirc. 2008, 39, 93–98. [Google Scholar]
- Janssen, B.; Hohenadel, D.; Brinkkoetter, P.; Peters, V.; Rind, N.; Fischer, C.; Rychlik, I.; Cerna, M.; Romzova, M.; de Heer, E.; et al. Carnosine as a protective factor in diabetic nephropathy: Association with a leucine repeat of the carnosinase gene CNDP1. Diabetes 2005, 54, 2320–2327. [Google Scholar] [CrossRef]
- Tanida, M.; Shen, J.; Kubomura, D.; Nagai, K. Effects of anserine on the renal sympathetic nerve activity and blood pressure in urethane-anesthetized rats. Physiol. Res. 2010, 59, 177–185. [Google Scholar]
- Kubomura, D.; Matahira, Y.; Nagai, K.; Niijima, A. Effect of anserine ingestion on hyperglycemia and the autonomic nerves in rats and humans. Nutr. Neurosci. 2010, 13, 183–188. [Google Scholar] [CrossRef]
- Suzuki, Y.; Nakao, T.; Maemura, H.; Sato, M.; Kamahara, K.; Morimatsu, F.; Takamatsu, K. Carnosine and anserine ingestion enhances contribution of nonbicarbonate buffering. Med. Sci. Sports Exercise 2006, 38, 334–338. [Google Scholar]
- Szwergold, B.S. Carnosine and anserine act as effective transglycating agents in decomposition of aldose-derived Schiff bases. Biochem. Biophys. Res. Commun. 2005, 336, 36–41. [Google Scholar] [CrossRef]
- Boldyrev, A.; Bulygina, E.; Leinsoo, T.; Petrushanko, I.; Tsubone, S.; Abe, H. Protection of neuronal cells against reactive oxygen species by carnosine and related compounds. Comp. Biochem. Physiol. B: Biochem. Mol. Biol. 2004, 137, 81–88. [Google Scholar] [CrossRef]
- Kang, J.H.; Kim, K.S.; Choi, S.Y.; Kwon, H.Y.; Won, M.H.; Kang, T.C. Protective effects of carnosine, homocarnosine and anserine against peroxyl radical-mediated Cu,Zn-superoxide dismutase modification. Biochim. Biophys. Acta 2002, 1570, 89–96. [Google Scholar]
- Aldini, G.; Facino, R.M.; Beretta, G.; Carini, M. Carnosine and related dipeptides as quenchers of reactive carbonyl species: From structural studies to therapeutic perspectives. BioFactors 2005, 24, 77–87. [Google Scholar] [CrossRef]
- Min, J.; Senut, M.C.; Rajanikant, K.; Greenberg, E.; Bandagi, R.; Zemke, D.; Mousa, A.; Kassab, M.; Farooq, M.U.; Gupta, R.; et al. Differential neuroprotective effects of carnosine, anserine, and N-acetyl carnosine against permanent focal ischemia. J. Neurosci. Res. 2008, 86, 2984–2991. [Google Scholar]
- Sadzuka, Y.; Sonobe, T. Anserine induced advantage effects on the antitumor activity of doxorubicin. Food Chem. Toxicol. 2007, 45, 985–989. [Google Scholar] [CrossRef]
- Shao, L.; Li, Q.H.; Tan, Z. L-carnosine reduces telomere damage and shortening rate in cultured normal fibroblasts. Biochem. Biophys. Res. Commun. 2004, 324, 931–936. [Google Scholar] [CrossRef]
- Boldyrev, A.A.; Dupin, A.M.; Bunin, A.; Babizhaev, M.A.; Severin, S.E. The antioxidative properties of carnosine, a natural histidine containing dipeptide. Biochem. Int. 1987, 15, 1105–1113. [Google Scholar]
- Babizhayev, M.A.; Kasus-Jacobi, A. State of the art clinical efficacy and safety evaluation of N-acetylcarnosine dipeptide ophthalmic prodrug. Principles for the delivery, self-bioactivation, molecular targets and interaction with a highly evolved histidyl-hydrazide structure in the treatment and therapeutic management of a group of sight-threatening eye diseases. Curr. Clin. Pharmacol. 2009, 4, 4–37. [Google Scholar] [CrossRef]
- Babizhayev, M.A.; Yegorov, Y.E. Telomere attrition in lens epithelial cells - a target for N-acetylcarnosine therapy. Front. Biosci. 2010, 15, 934–956. [Google Scholar] [CrossRef]
- Katayama, S.; Nishizawa, K.; Hirano, M.; Yamamura, S.; Momose, Y. Effect of polaprezinc on healing of acetic acid-induced stomatitis in hamsters. J. Pharm. Pharm. Sci. 2000, 3, 114–117. [Google Scholar]
- Matsukura, T.; Tanaka, H. Applicability of zinc complex of L-carnosine for medical use. Biochemistry (Moscow) 2000, 65, 817–823. [Google Scholar]
- Ueda, K.; Ueyama, T.; Oka, M.; Ito, T.; Tsuruo, Y.; Ichinose, M. Polaprezinc (Zinc L-carnosine) is a potent inducer of anti-oxidative stress enzyme, heme oxygenase (HO)-1 - a new mechanism of gastric mucosal protection. J. Pharmacol. Sci. 2009, 110, 285–294. [Google Scholar] [CrossRef]
- Odashima, M.; Otaka, M.; Jin, M.; Konishi, N.; Sato, T.; Kato, S.; Matsuhashi, T.; Nakamura, C.; Watanabe, S. Induction of a 72-kDa heat-shock protein in cultured rat gastric mucosal cells and rat gastric mucosa by zinc L-carnosine. Dig. Dis. Sci. 2002, 47, 2799–2804. [Google Scholar] [CrossRef]
- Ohata, S.; Moriyama, C.; Yamashita, A.; Nishida, T.; Kusumoto, C.; Mochida, S.; Minami, Y.; Nakada, J.; Shomori, K.; Inagaki, Y.; et al. Polaprezinc Protects Mice against Endotoxin Shock. J. Clin. Biochem. Nutr. 2010, 46, 234–243. [Google Scholar] [CrossRef]
- Kalmar, B.; Greensmith, L. Induction of heat shock proteins for protection against oxidative stress. Adv. Drug Del. Rev. 2009, 61, 310–318. [Google Scholar] [CrossRef]
- Corona, C.; Frazzini, V.; Silvestri, E.; Lattanzio, R.; la Sorda, R.; Piantelli, M.; Canzoniero, L.M.; Ciavardelli, D.; Rizzarelli, E.; Sensi, S.L. Effects of dietary supplementation of carnosine on mitochondrial dysfunction, amyloid pathology, and cognitive deficits in 3xTg-AD mice. PLoS One 2011, 6, e17971. [Google Scholar] [CrossRef]
- Di Paola, R.; Impellizzeri, D.; Salinaro, A.T.; Mazzon, E.; Bellia, F.; Cavallaro, M.; Cornelius, C.; Vecchio, G.; Calabrese, V.; Rizzarelli, E.; et al. Administration of carnosine in the treatment of acute spinal cord injury. Biochem. Pharmacol. 2011, 82, 1478–1489. [Google Scholar] [CrossRef]
- Barnham, K.J.; Bush, A.I. Metals in Alzheimer’s and Parkinson’s diseases. Curr. Opin. Chem. Biol. 2008, 12, 222–228. [Google Scholar] [CrossRef]
- Di Donato, M.; Hsu, H.F.; Narindrasorasak, S.; Que, L., Jr.; Sarkar, B. Copper-induced conformational changes in the N-terminal domain of the Wilson disease copper-transporting ATPase. Biochemistry (Moscow) 2000, 39, 1890–1896. [Google Scholar] [CrossRef]
- Fu, Q.; Dai, H.; Hu, W.; Fan, Y.; Shen, Y.; Zhang, W.; Chen, Z. Carnosine protects against Abeta42-induced neurotoxicity in differentiated rat PC12 cells. Cell. Mol. Neurobiol. 2008, 28, 307–316. [Google Scholar] [CrossRef]
- Trombley, P.Q.; Horning, M.S.; Blakemore, L.J. Interactions between carnosine and zinc and copper: Implications for neuromodulation and neuroprotection. Biochemistry (Moscow) 2000, 65, 807–816. [Google Scholar]
- Lenney, J.F. Specificity and distribution of mammalian carnosinase. Biochim. Biophys. Acta 1976, 429, 214–219. [Google Scholar] [CrossRef]
- Lenney, J.F.; Kan, S.C.; Siu, K.; Sugiyama, G.H. Homocarnosinase: A hog kidney dipeptidase with a broader specificity than carnosinase. Arch. Biochem. Biophys. 1977, 184, 257–266. [Google Scholar] [CrossRef]
- Rosenberg, A. The activation of carnosinase by divalent metal ions. Biochim. Biophys. Acta 1960, 45, 297–316. [Google Scholar] [CrossRef]
- Rosenberg, A. Purification and some properties of carnosinase of swine kidney. Arch. Biochem. Biophys. 1960, 88, 83–93. [Google Scholar] [CrossRef]
- Wolos, A.; Piekarska, K.; Glogowski, J.; Konieczka, I. Two molecular forms of swine kidney carnosinase. Int. J. Biochem. 1978, 9, 57–62. [Google Scholar] [CrossRef]
- Margolis, F.L.; Grillo, M.; Brown, C.E. Enzymatic and immunological evidence for two forms of carnosinase in the mouse. Biochim. Biophys. Acta 1979, 570, 311–323. [Google Scholar] [CrossRef]
- Margolis, F.L.; Grillo, M.; Grannot Reisfeld, N.; Farbman, A.I. Purification, characterization and immunocytochemical localization of mouse kidney carnosinase. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 1983, 744, 237–248. [Google Scholar] [CrossRef]
- Kunze, N.; Kleinkauf, H.; Bauer, K. Characterization of two carnosine-degrading enzymes from rat brain. Partial purification and characterization of a carnosinase and a beta-alanyl-arginine hydrolase. Eur. J. Biochem. 1986, 160, 605–613. [Google Scholar] [CrossRef]
- Lenney, J.F. Human cytosolic carnosinase: Evidence of identity with prolinase, a non-specific dipeptidase. Biol. Chem. Hoppe-Seyler 1990, 371, 167–171. [Google Scholar] [CrossRef]
- Perry, T.L.; Hansen, S.; Love, D.L. Serum-carnosinase deficiency in carnosinaemia. Lancet 1968, 1, 1229–1230. [Google Scholar] [CrossRef]
- Zoch, E.; Muller, H. Demonstration and determination of earnosinase activity of the human placenta. Enzymologia 1971, 40, 199–208. [Google Scholar]
- Lenney, J.F.; George, R.P.; Weiss, A.M.; Kucera, C.M.; Chan, P.W.; Rinzler, G.S. Human serum carnosinase: Characterization, distinction from cellular carnosinase, and activation by cadmium. Clin. Chim. Acta 1982, 123, 221–231. [Google Scholar] [CrossRef]
- Otani, H.; Okumura, N.; Hashida-Okumura, A.; Nagai, K. Identification and characterization of a mouse dipeptidase that hydrolyzes L-carnosine. J. Biochem. 2005, 137, 167–175. [Google Scholar] [CrossRef]
- Murphey, W.H.; Patchen, L.; Lindmark, D.G. Carnosinase: A fluorometric assay and demonstration of two electrophoretic forms in human tissue extracts. Clin. Chim. Acta 1972, 42, 309–314. [Google Scholar] [CrossRef]
- Pandya, V.; Ekka, M.K.; Dutta, R.K.; Kumaran, S. Mass spectrometry assay for studying kinetic properties of dipeptidases: Characterization of human and yeast dipeptidases. Anal. Biochem. 2011, 418, 134–142. [Google Scholar] [CrossRef]
- Bando, K.; Shimotsuji, T.; Toyoshima, H. Fluorometric assay of human serum carnosinase activity in normal children, adults and patients with myopathy. Ann. Clin. Biochem. 1984, 21, 510–514. [Google Scholar] [CrossRef]
- Jones, N.R. The free amino acids of fish; 1-methylhistidine and beta-alanine liberation by skeletal muscle anserinase of codling (Gadus callarias). Biochem. J. 1955, 60, 81–87. [Google Scholar]
- Yamada, S.; Tanaka, Y.; Sameshima, M.; Furuichi, M. Properties of Nα-acetylhistidine deacetylase in brain of rainbow trout Oncorhynchus mykiss. Comp. Biochem. Physiol. B: Biochem. Mol. Biol. 1993, 106, 309–315. [Google Scholar] [CrossRef]
- Yamada, S.; Tanaka, Y.; Sameshima, M.; Furuichi, M. Effects of starvation and feeding on tissue Nα -acetylhistidine levels in Nile tilapia Oreochromis niloticus. Comp. Biochem. Physiol. A: Physiol. 1994, 109, 277–283. [Google Scholar] [CrossRef]
- Yamada, S.; Tanaka, Y.; Ando, S. Purification and sequence identification of anserinase. FEBS J. 2005, 272, 6001–6013. [Google Scholar] [CrossRef]
- Chang, C.Y.; Hsieh, Y.C.; Wang, T.Y.; Chen, Y.C.; Wang, Y.K.; Chiang, T.W.; Chen, Y.J.; Chang, C.H.; Chen, C.J.; Wu, T.K. Crystal structure and mutational analysis of aminoacylhistidine dipeptidase from Vibrio alginolyticus reveal a new architecture of M20 metallopeptidases. J. Biol. Chem. 2010, 285, 39500–39510. [Google Scholar] [CrossRef]
- Jozic, D.; Bourenkow, G.; Bartunik, H.; Scholze, H.; Dive, V.; Henrich, B.; Huber, R.; Bode, W.; Maskos, K. Crystal structure of the dinuclear zinc aminopeptidase PepV from Lactobacillus delbrueckii unravels its preference for dipeptides. Structure 2002, 10, 1097–1106. [Google Scholar] [CrossRef]
- Rowsell, S.; Pauptit, R.A.; Tucker, A.D.; Melton, R.G.; Blow, D.M.; Brick, P. Crystal structure of carboxypeptidase G2, a bacterial enzyme with applications in cancer therapy. Structure 1997, 5, 337–347. [Google Scholar] [CrossRef]
- Unno, H.; Yamashita, T.; Ujita, S.; Okumura, N.; Otani, H.; Okumura, A.; Nagai, K.; Kusunoki, M. Structural basis for substrate recognition and hydrolysis by mouse carnosinase CN2. J. Biol. Chem. 2008, 283, 27289–27299. [Google Scholar]
- Partmann, W. Eine Carnosine spaltende Enzymaktivität im Skelettmuskel des Aales. Arch. Fisch Wiss. 1976, 27, 55–62. [Google Scholar]
- Perez, B.S.; Jones, N.R. Effects of tetracycline antibiotics on the products of anserinase action in chill stored haddock (Gadus aeglefinus) muscle. J. Food Sci. 1962, 27, 69–72. [Google Scholar] [CrossRef]
- Oku, T.; Ando, S.; Tsai, H.C.; Yamashita, Y.; Ueno, H.; Shiozaki, K.; Nishi, R.; Yamada, S. Purification and identification of two carnosine-cleaving enzymes, carnosine dipeptidase I and Xaa-methyl-His dipeptidase, from Japanese eel (Anguilla japonica). Biochimie 2012, 94, 1281–1290. [Google Scholar] [CrossRef]
- Vongerichten, K.F.; Klein, J.R.; Matern, H.; Plapp, R. Cloning and nucleotide sequence analysis of pepV, a carnosinase gene from Lactobacillus delbrueckii subsp. lactis DSM 7290, and partial characterization of the enzyme. Microbiology 1994, 140, 2591–2600. [Google Scholar] [CrossRef]
- Chang, C.Y.; Hsieh, Y.C.; Wang, T.Y.; Chen, C.J.; Wu, T.K. Purification, crystallization and preliminary X-ray analysis of an aminoacylhistidine dipeptidase (PepD) from Vibrio alginolyticus. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2009, 65, 216–218. [Google Scholar] [CrossRef]
- Chevrier, B.; Schalk, C.; D’Orchymont, H.; Rondeau, J.M.; Moras, D.; Tarnus, C. Crystal structure of Aeromonas proteolytica aminopeptidase: A prototypical member of the co-catalytic zinc enzyme family. Structure 1994, 2, 283–291. [Google Scholar] [CrossRef]
- Greenblatt, H.M.; Almog, O.; Maras, B.; Spungin-Bialik, A.; Barra, D.; Blumberg, S.; Shoham, G. Streptomyces griseus aminopeptidase: X-ray crystallographic structure at 1.75 A resolution. J. Mol. Biol. 1997, 265, 620–636. [Google Scholar] [CrossRef]
- Hakansson, K.; Miller, C.G. Structure of peptidase T from Salmonella typhimurium. Eur. J. Biochem. 2002, 269, 443–450. [Google Scholar] [CrossRef]
- Lindner, H.A.; Lunin, V.V.; Alary, A.; Hecker, R.; Cygler, M.; Menard, R. Essential roles of zinc ligation and enzyme dimerization for catalysis in the aminoacylase-1/M20 family. J. Biol. Chem. 2003, 278, 44496–44504. [Google Scholar]
- Lundgren, S.; Gojkovic, Z.; Piskur, J.; Dobritzsch, D. Yeast beta-alanine synthase shares a structural scaffold and origin with dizinc-dependent exopeptidases. J. Biol. Chem. 2003, 278, 51851–51862. [Google Scholar]
- Wang, T.Y.; Chen, Y.C.; Kao, L.W.; Chang, C.Y.; Wang, Y.K.; Liu, Y.H.; Feng, J.M.; Wu, T.K. Expression and characterization of the biofilm-related and carnosine-hydrolyzing aminoacylhistidine dipeptidase from Vibrio alginolyticus. FEBS J. 2008, 275, 5007–5020. [Google Scholar] [CrossRef]
- Rawlings, N.D.; Barrett, A.J. Evolutionary families of metallopeptidases. Methods Enzymol. 1995, 248, 183–228. [Google Scholar] [CrossRef]
- Bayliss, M.E.; Prescott, J.M. Modified activity of Aeromonas aminopeptidase: Metal ion substitutions and role of substrates. Biochemistry (Moscow) 1986, 25, 8113–8117. [Google Scholar] [CrossRef]
- Vistoli, G.; Carini, M.; Aldini, G. Transforming dietary peptides in promising lead compounds: The case of bioavailable carnosine analogs. Amino Acids 2012, 43, 111–126. [Google Scholar] [CrossRef]
- Vistoli, G.; Pedretti, A.; Cattaneo, M.; Aldini, G.; Testa, B. Homology modeling of human serum carnosinase, a potential medicinal target, and MD simulations of its allosteric activation by citrate. J. Med. Chem. 2006, 49, 3269–3277. [Google Scholar] [CrossRef]
- Cohen, M.; Hartlage, P.L.; Krawiecki, N. Serum carnosinase deficiency: A non-disabling phenotype? J. Ment. Defic. Res. 1985, 29, 383–389. [Google Scholar]
- Lunde, H.A.; Gjessing, L.R.; Sjaastad, O. Homocarnosinosis: Influence of dietary restriction of histidine. Neurochem. Res. 1986, 11, 825–838. [Google Scholar] [CrossRef]
- Balion, C.M.; Benson, C.; Raina, P.S.; Papaioannou, A.; Patterson, C.; Ismaila, A.S. Brain type carnosinase in dementia: A pilot study. BMC Neurol. 2007, 7, 38. [Google Scholar] [CrossRef]
- Butterworth, R.J.; Wassif, W.S.; Sherwood, R.A.; Gerges, A.; Poyser, K.H.; Garthwaite, J.; Peters, T.J.; Bath, P.M.W. Serum neuron-specific enolase, carnosinase, and their ratio in acute stroke: An enzymatic test for predicting outcome? Stroke 1996, 27, 2064–2068. [Google Scholar] [CrossRef]
- Wassif, W.S.; Sherwood, R.A.; Amir, A.; Idowu, B.; Summers, B.; Leigh, N.; Peters, T.J. Serum carnosinase activities in central nervous system disorders. Clin. Chim. Acta 1994, 225, 57–64. [Google Scholar] [CrossRef]
- Licker, V.; Côte, M.; Lobrinus, J.A.; Rodrigo, N.; Kövari, E.; Hochstrasser, D.F.; Turck, N.; Sanchez, J.C.; Burkhard, P.R. Proteomic profiling of the substantia nigra demonstrates CNDP2 overexpression in Parkinson’s disease. J. Proteomics 2012, 75, 4656–4667. [Google Scholar] [CrossRef]
- Schoen, P.; Everts, H.; de Boer, T.; van Oeveren, W. Serum carnosinase activity in plasma and serum: Validation of a method and values in cardiopulmonary bypass surgery. Clin. Chem. 2003, 49, 1930–1932. [Google Scholar] [CrossRef]
- Holdenrieder, S.; Lütjohann, D.; Geiger, S.; von Bergmann, K.; Stieber, P.; Hamann, G.F. Does brain specific 24S-hydroxycholesterol in plasma indicate the disruption of the blood-brain barrier in patients with ischemic stroke? Neurosci. Lett. 2004, 368, 201–204. [Google Scholar] [CrossRef]
- Kirk, J.; Plumb, J.; Mirakhur, M.; McQuaid, S. Tight junctional abnormality in multiple sclerosis white matter affects all calibres of vessel and is associated with blood-brain barrier leakage and active demyelination. J. Pathol. 2003, 201, 319–327. [Google Scholar] [CrossRef]
- Skoog, I.; Wallin, A.; Fredman, P.; Hesse, C.; Aevarsson, O.; Karlsson, I.; Gottfries, C.G.; Blennow, K. A population study on blood-brain barrier function in 85-year-olds: Relation to Alzheimer’s disease and vascular dementia. Neurology 1998, 50, 966–971. [Google Scholar] [CrossRef]
- Kumar-Singh, S.; Pirici, D.; McGowan, E.; Serneels, S.; Ceuterick, C.; Hardy, J.; Duff, K.; Dickson, D.; van Broeckhoven, C. Dense-core plaques in Tg2576 and PSAPP mouse models of Alzheimer’s disease are centered on vessel walls. Am. J. Pathol. 2005, 167, 527–543. [Google Scholar] [CrossRef]
- Willi, S.M.; Zhang, Y.; Hill, J.B.; Phelan, M.C.; Michaelis, R.C.; Holden, K.R. A deletion in the long arm of chromosome 18 in a child with serum carnosinase deficiency. Pediatr. Res. 1997, 41, 210–213. [Google Scholar]
- Lee, J.H.; Mayeux, R.; Mayo, D.; Mo, J.; Santana, V.; Williamson, J.; Flaquer, A.; Ciappa, A.; Rondon, H.; Estevez, P.; et al. Fine mapping of 10q and 18q for familial Alzheimer’s disease in Caribbean Hispanics. Mol. Psychiatry 2004, 9, 1042–1051. [Google Scholar] [CrossRef]
- Munzar, M.; Levy, S.; Rush, R.; Averback, P. Clinical study of a urinary competitve ELISA for neural thread protein in Alzheimer disease. Neurol. Clin. Neurophysiol. 2002, 2002, 2–8. [Google Scholar]
- Larson, E.B.; Wang, L.; Bowen, J.D.; McCormick, W.C.; Teri, L.; Crane, P.; Kukull, W. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann. Intern. Med. 2006, 144, 73–81. [Google Scholar] [CrossRef]
- Vionnet, N.; Tregouet, D.; Kazeem, G.; Gut, I.; Groop, P.H.; Tarnow, L.; Parving, H.H.; Hadjadj, S.; Forsblom, C.; Farrall, M.; et al. Analysis of 14 candidate genes for diabetic nephropathy on chromosome 3q in European populations: Strongest evidence for association with a variant in the promoter region of the adiponectin gene. Diabetes 2006, 55, 3166–3174. [Google Scholar] [CrossRef]
- Freedman, B.I.; Hicks, P.J.; Sale, M.M.; Pierson, E.D.; Langefeld, C.D.; Rich, S.S.; Xu, J.; McDonough, C.; Janssen, B.; Yard, B.A.; et al. A leucine repeat in the carnosinase gene CNDP1 is associated with diabetic end-stage renal disease in European Americans. Nephrol. Dial. Transplant. 2007, 22, 1131–1135. [Google Scholar] [CrossRef]
- Riedl, E.; Koeppel, H.; Brinkkoetter, P.; Sternik, P.; Steinbeisser, H.; Sauerhoefer, S.; Janssen, B.; van der Woude, F.J.; Yard, B.A. A CTG polymorphism in the CNDP1 gene determines the secretion of serum carnosinase in Cos-7-transfected cells. Diabetes 2007, 56, 2410–2413. [Google Scholar] [CrossRef]
- Iyengar, S.K.; Freedman, B.I.; Sedor, J.R. Mining the genome for susceptibility to diabetic nephropathy: The role of large-scale studies and consortia. Semin. Nephrol. 2007, 27, 208–222. [Google Scholar] [CrossRef]
- Wanic, K.; Placha, G.; Dunn, J.; Smiles, A.; Warram, J.H.; Krolewski, A.S. Exclusion of polymorphisms in carnosinase genes (CNDP1 and CNDP2) as a cause of diabetic nephropathy in type 1 diabetes: Results of large case-control and follow-up studies. Diabetes 2008, 57, 2547–2551. [Google Scholar] [CrossRef]
- McDonough, C.W.; Hicks, P.J.; Lu, L.; Langefeld, C.D.; Freedman, B.I.; Bowden, D.W. The influence of carnosinase gene polymorphisms on diabetic nephropathy risk in African-Americans. Hum. Genet. 2009, 126, 265–275. [Google Scholar] [CrossRef]
- Mooyaart, A.L.; Zutinic, A.; Bakker, S.J.L.; Grootendorst, D.C.; Kleefstra, N.; van Valkengoed, I.G.M.; Böhringer, S.; Bilo, H.J.G.; Dekker, F.W.; Bruijn, J.A.; et al. Association between CNDP1 genotype and diabetic nephropathy is sex specific. Diabetes 2010, 59, 1555–1559. [Google Scholar] [CrossRef]
- Riedl, E.; Koeppel, H.; Pfister, F.; Peters, V.; Sauerhoefer, S.; Sternik, P.; Brinkkoetter, P.; Zentgraf, H.; Navis, G.; Henning, R.H.; et al. N-glycosylation of carnosinase influences protein secretion and enzyme activity: Implications for hyperglycemia. Diabetes 2010, 59, 1984–1990. [Google Scholar] [CrossRef]
- Ahluwalia, T.S.; Lindholm, E.; Groop, L.C. Common variants in CNDP1 and CNDP2, and risk of nephropathy in type 2 diabetes. Diabetologia 2011, 54, 2295–2302. [Google Scholar] [CrossRef]
- Chakkera, H.A.; Hanson, R.L.; Kobes, S.; Millis, M.P.; Nelson, R.G.; Knowler, W.C.; Distefano, J.K. Association of variants in the carnosine peptidase 1 gene (CNDP1) with diabetic nephropathy in American Indians. Mol. Genet. Metab. 2011, 103, 185–190. [Google Scholar] [CrossRef]
- Everaert, I.; Mooyaart, A.; Baguet, A.; Zutinic, A.; Baelde, H.; Achten, E.; Taes, Y.; de Heer, E.; Derave, W. Vegetarianism, female gender and increasing age, but not CNDP1 genotype, are associated with reduced muscle carnosine levels in humans. Amino Acids 2011, 40, 1221–1229. [Google Scholar] [CrossRef]
- Peters, V.; Jansen, E.E.W.; Jakobs, C.; Riedl, E.; Janssen, B.; Yard, B.A.; Wedel, J.; Hoffmann, G.F.; Zschocke, J.; Gotthardt, D.; et al. Anserine inhibits carnosine degradation but in human serum carnosinase (CN1) is not correlated with histidine dipeptide concentration. Clin. Chim. Acta 2011, 412, 263–267. [Google Scholar] [CrossRef]
- Peters, V.; Schmitt, C.P.; Zschocke, J.; Gross, M.L.; Brismar, K.; Forsberg, E. Carnosine treatment largely prevents alterations of renal carnosine metabolism in diabetic mice. Amino Acids 2012, 42, 2411–2416. [Google Scholar] [CrossRef]
- McManus, I.R. Enzymic synthesis of anserine in skeletal muscle by N-methylation of carnosine. J. Biol. Chem. 1962, 237, 1207–1211. [Google Scholar]
- Bossy-Wetzel, E.; Schwarzenbacher, R.; Lipton, S.A. Molecular pathways to neurodegeneration. Nat. Med. 2004, 10 (Suppl.), S2–S9. [Google Scholar]
- Blennow, K.; Hampel, H.; Weiner, M.; Zetterberg, H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat. Rev. Neurol. 2010, 6, 131–144. [Google Scholar] [CrossRef]
- Hu, Y.; Hosseini, A.; Kauwe, J.S.K.; Gross, J.; Cairns, N.J.; Goate, A.M.; Fagan, A.M.; Townsend, R.R.; Holtzman, D.M. Identification and validation of novel CSF biomarkers for early stages of Alzheimer’s disease. Proteomics Clin. Appl. 2007, 1, 1373–1384. [Google Scholar] [CrossRef]
- Perrin, R.J.; Craig-Schapiro, R.; Malone, J.P.; Shah, A.R.; Gilmore, P.; Davis, A.E.; Roe, C.M.; Peskind, E.R.; Li, G.; Galasko, D.R.; et al. Identification and Validation of Novel Cerebrospinal Fluid Biomarkers for Staging Early Alzheimer’s Disease. PLoS One 2011, 6, e16032. [Google Scholar] [CrossRef]
- Renner, C.; Zemitzsch, N.; Fuchs, B.; Geiger, K.D.; Hermes, M.; Hengstler, J.; Gebhardt, R.; Meixensberger, J.; Gaunitz, F. Carnosine retards tumor growth in vivo in an NIH3T3-HER2/neu mouse model. Mol. Cancer 2010, 9, 2. [Google Scholar] [CrossRef]
- Pahari, D.R.; Gu, Y.J.; van Oeveren, W.; El-Essawi, A.; Harringer, W.; Brouwer, R.M. Effect of minimized perfusion circuit on brain injury markers carnosinase and brain-type fatty binding protein in coronary artery bypass grafting patients. Artif. Organs 2013, 37, 128–135. [Google Scholar] [CrossRef]
- Sale, C.; Artioli, G.G.; Gualano, B.; Saunders, B.; Hobson, R.M.; Harris, R.C. Carnosine: From exercise performance to health. Amino Acids 2013, 44, 1477–1491. [Google Scholar] [CrossRef]
- Derave, W.; Everaert, I.; Beeckman, S.; Baguet, A. Muscle carnosine metabolism and beta-alanine supplementation in relation to exercise and training. Sports Med. 2010, 40, 247–263. [Google Scholar] [CrossRef]
- Artioli, G.G.; Gualano, B.; Smith, A.; Stout, J.; Lancha, A.H., Jr. Role of beta-alanine supplementation on muscle carnosine and exercise performance. Med. Sci. Sports Exercise 2010, 42, 1162–1173. [Google Scholar]
- Bellia, F.; Vecchio, G.; Rizzarelli, E. Carnosine derivatives: New multifunctional drug-like molecules. Amino Acids 2012, 43, 153–163. [Google Scholar] [CrossRef]
- Grasso, G.I.; Bellia, F.; Arena, G.; Vecchio, G.; Rizzarelli, E. Noncovalent interaction-driven stereoselectivity of copper(II) complexes with cyclodextrin derivatives of L- and D-carnosine. Inorg. Chem. 2011, 50, 4917–4924. [Google Scholar] [CrossRef]
- Grasso, G.I.; Arena, G.; Bellia, F.; Maccarrone, G.; Parrinello, M.; Pietropaolo, A.; Vecchio, G.; Rizzarelli, E. Intramolecular weak interactions in the thermodynamic stereoselectivity of copper(II) complexes with carnosine-trehalose conjugates. Chem. Eur. J. 2011, 17, 9448–9455. [Google Scholar] [CrossRef]
- Lanza, V.; Bellia, F.; D’Agata, R.; Grasso, G.; Rizzarelli, E.; Vecchio, G. New glycoside derivatives of carnosine and analogs resistant to carnosinase hydrolysis: Synthesis and characterization of their copper(II) complexes. J. Inorg. Biochem. 2011, 105, 181–188. [Google Scholar] [CrossRef]
- Stvolinsky, S.L.; Bulygina, E.R.; Fedorova, T.N.; Meguro, K.; Sato, T.; Tyulina, O.V.; Abe, H.; Boldyrev, A.A. Biological activity of novel synthetic derivatives of carnosine. Cell. Mol. Neurobiol. 2010, 30, 395–404. [Google Scholar] [CrossRef]
- Astete, C.E.; Songe Meador, D.; Spivak, D.; Sabliov, C. Synthesis of vitamin E-carnosine (VECAR): New antioxidant molecule with potential application in atherosclerosis. Synth. Commun. 2013, 43, 1299–1313. [Google Scholar] [CrossRef]
- Sozio, P.; Iannitelli, A.; Cerasa, L.S.; Cacciatore, I.; Cornacchia, C.; Giorgioni, G.; Ricciutelli, M.; Nasuti, C.; Cantalamessa, F.; di Stefano, A. New L-dopa codrugs as potential antiparkinson agents. Arch. Pharm. 2008, 341, 412–417. [Google Scholar] [CrossRef]
- Nativi, C.; Gualdani, R.; Dragoni, E.; di Cesare Mannelli, L.; Sostegni, S.; Norcini, M.; Gabrielli, G.; la Marca, G.; Richichi, B.; Francesconi, O.; et al. A TRPA1 antagonist reverts oxaliplatin-induced neuropathic pain. Sci. Rep. 2013, 3. [Google Scholar] [CrossRef]
- Saada, M.C.; Montero, J.L.; Vullo, D.; Scozzafava, A.; Winum, J.Y.; Supuran, C.T. Carbonic anhydrase activators: Gold nanoparticles coated with derivatized histamine, histidine, and carnosine show enhanced activatory effects on several mammalian isoforms. J. Med. Chem. 2011, 54, 1170–1177. [Google Scholar]
- Bellia, F.; Oliveri, V.; Rizzarelli, E.; Vecchio, G. New derivative of carnosine for nanoparticle assemblies. Eur. J. Med. Chem. 2013, 70, 225–232. [Google Scholar] [CrossRef]
- Bertinaria, M.; Rolando, B.; Giorgis, M.; Montanaro, G.; Guglielmo, S.; Buonsanti, M.F.; Carabelli, V.; Gavello, D.; Daniele, P.G.; Fruttero, R.; et al. Synthesis, physicochemical characterization, and biological activities of new carnosine derivatives stable in human serum as potential neuroprotective agents. J. Med. Chem. 2011, 54, 611–621. [Google Scholar] [CrossRef]
- Supuran, C.T.; Briganti, F.; Tilli, S.; Chegwidden, W.R.; Scozzafava, A. Carbonic anhydrase inhibitors: Sulfonamides as antitumor agents? Biorg. Med. Chem. 2001, 9, 703–714. [Google Scholar] [CrossRef]
- Nielsen, C.U.; Supuran, C.T.; Scozzafava, A.; Frokjaer, S.; Steffansen, B.; Brodin, B. Transport characteristics of L-carnosine and the anticancer derivative 4-toluenesulfonylureido-carnosine in a human epithelial cell line. Pharm. Res. 2002, 19, 1337–1344. [Google Scholar] [CrossRef]
- Vistoli, G.; Orioli, M.; Pedretti, A.; Regazzoni, L.; Canevotti, R.; Negrisoli, G.; Carini, M.; Aldini, G. Design, synthesis, and evaluation of carnosine derivatives as selective and efficient sequestering agents of cytotoxic reactive carbonyl species. ChemMedChem 2009, 4, 967–975. [Google Scholar]
- Grasso, G.I.; Arena, G.; Bellia, F.; Rizzarelli, E.; Vecchio, G. Copper(II)-chelating homocarnosine glycoconjugate as a new multifunctional compound. J. Inorg. Biochem. 2014, 131, 56–63. [Google Scholar]
- Orioli, M.; Vistoli, G.; Regazzoni, L.; Pedretti, A.; Lapolla, A.; Rossoni, G.; Canevotti, R.; Gamberoni, L.; Previtali, M.; Carini, M.; et al. Design, synthesis, ADME properties, and pharmacological activities of beta-alanyl-d-histidine (d-carnosine) prodrugs with improved bioavailability. ChemMedChem 2011, 6, 1269–1282. [Google Scholar] [CrossRef]
- Amorini, A.M.; Bellia, F.; di Pietro, V.; Giardina, B.; la Mendola, D.; Lazzarino, G.; Sortino, S.; Tavazzi, B.; Rizzarelli, E.; Vecchio, G. Synthesis and antioxidant activity of new homocarnosine β-cyclodextrin conjugates. Eur. J. Med. Chem. 2007, 42, 910–920. [Google Scholar] [CrossRef]
- Bellia, F.; Amorini, A.M.; la Mendola, D.; Vecchio, G.; Tavazzi, B.; Giardina, B.; di Pietro, V.; Lazzarino, G.; Rizzarelli, E. New glycosidic derivatives of histidine-containing dipeptides with antioxidant properties and resistant to carnosinase activity. Eur. J. Med. Chem. 2008, 43, 373–380. [Google Scholar] [CrossRef]
- Bellia, F.; la Mendola, D.; Maccarrone, G.; Mineo, P.; Vitalini, D.; Scamporrino, E.; Sortino, S.; Vecchio, G.; Rizzarelli, E. Copper(II) complexes with β-cyclodextrin-homocarnosine conjugates and their antioxidant activity. Inorg. Chim. Acta 2007, 360, 945–954. [Google Scholar] [CrossRef]
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bellia, F.; Vecchio, G.; Rizzarelli, E. Carnosinases, Their Substrates and Diseases. Molecules 2014, 19, 2299-2329. https://doi.org/10.3390/molecules19022299
Bellia F, Vecchio G, Rizzarelli E. Carnosinases, Their Substrates and Diseases. Molecules. 2014; 19(2):2299-2329. https://doi.org/10.3390/molecules19022299
Chicago/Turabian StyleBellia, Francesco, Graziella Vecchio, and Enrico Rizzarelli. 2014. "Carnosinases, Their Substrates and Diseases" Molecules 19, no. 2: 2299-2329. https://doi.org/10.3390/molecules19022299