Applications of Solid-Phase Microextraction and Gas Chromatography/Mass Spectrometry (SPME-GC/MS) in the Study of Grape and Wine Volatile Compounds
Abstract
:1. Introduction
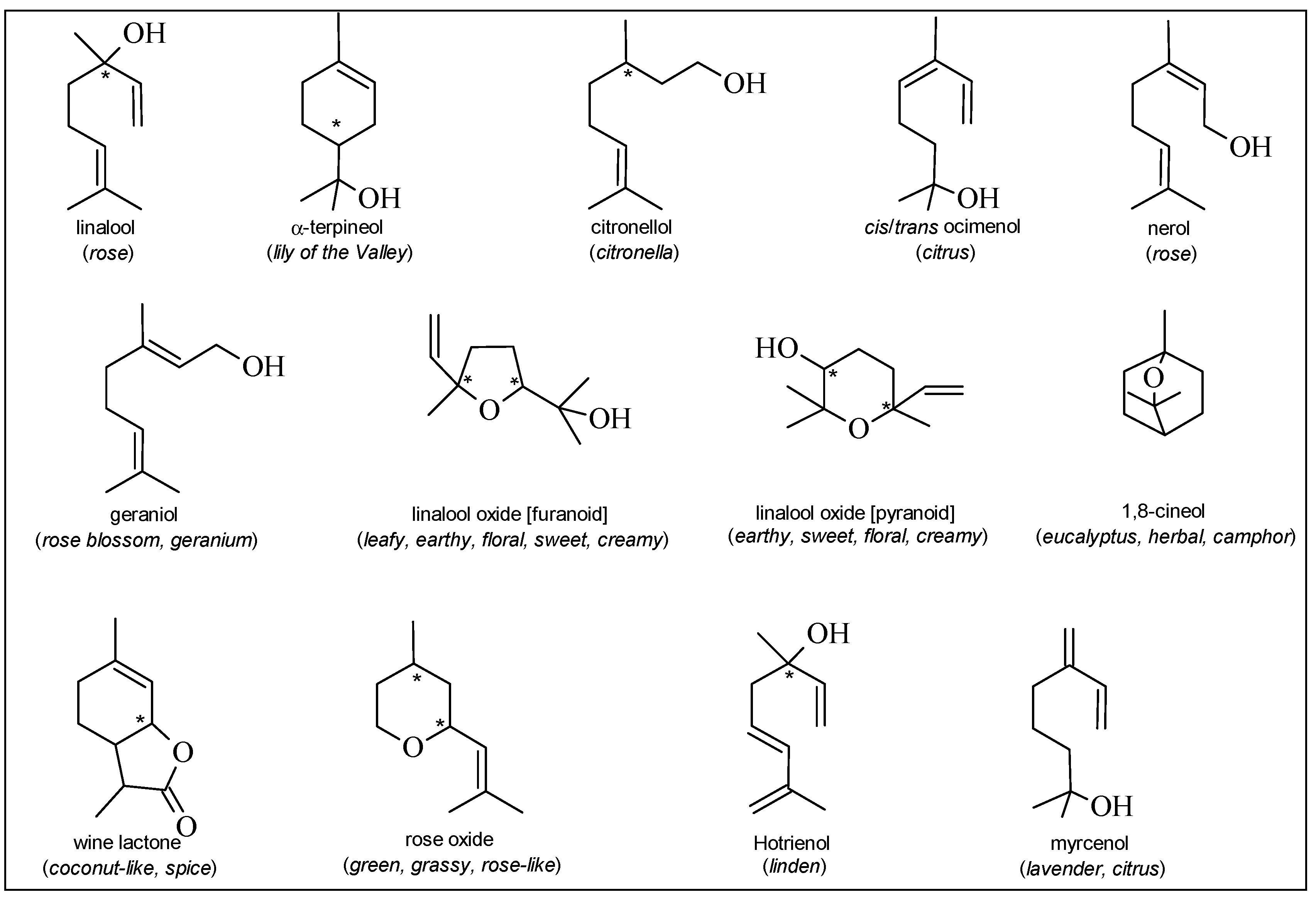
Grape and Wine Volatile Compounds
| Terpenoids | Benzenoids | Sulfur compounds |
| linalool | zingerone | methyl mercaptan |
| nerol | zingerol | ethyl mercaptan |
| geraniol | acetophenone | dimethyl sulfide |
| citronellol | vanillin | diethyl sulfide |
| α-terpineol | methyl salicylate | dimethyl disulfide |
| cis/trans ocimenol | eugenol | diethyl disulfide |
| cis/trans linalooloxide (furanic form) | cis/trans isoeugenol | methyl thioacetate |
| cis/trans linalooloxide (pyranic form) | 2-phenylethanol | ethyl thioacetate |
| hydroxycitronellol | benzyl alcohol | 2-mercaptoethanol |
| 8-hydroxydihydrolinalool | acetovanillone | 2-(methylthio)-1-ethanol |
| 7-hydroxygeraniol | benzaldehyde | 3-(methylthio)-1-propanol |
| 7-hydroxynerol | 4-hydroxybenzaldehyde | 4-(methylthio)-1-butanol |
| cis/trans 8-hydroxylinalool | 2,4-dimethylbenzaldehyde | 2-furanmethanethiol |
| diendiol I | phenylacetaldehyde | benzothiazole |
| endiol | syringaldehyde | thiazole |
| diendiol II | coniferaldehyde | 5-(2-hydroxyethyl)-4-methylthiazole |
| neroloxide | sinapaldehyde | 4-methyl-4-mercaptopentan-2-one |
| 2-exo-hydroxy-1,8-cineol | propriosyringone | 3-mercaptohexanol acetate |
| 1,8-cineol | propriovanillone | cis/trans 2-methylthiophan-3-ol |
| cis/trans 1,8-terpine | syringol | 2-methyltetrahydrothiophen-3-one |
| p-menthenediol I | coniferyl alcohol | cis/trans 2-methyltetrahydrothiophen-3-ol |
| (E)-geranic acid | vanillic alcohol | 3-mercaptohexan-1-ol |
| (E)-2,6-dimethyl -6-hydroxyocta-2,7-dienoic acid | sinapic alcohol | 3-mercaptohexyl acetate |
| (E)- and (Z)-sobrerol | o-cymene | 4-mercapto-4-methylpentan-2-ol |
| cis/trans rose oxide | p-cymene | 3-mercapto-3-methylbutan-1-ol |
| lilac alcohols | guaiacol | |
| triol | 4-ethylguaiacol | |
| hotrienol | 4-vinylguaiacol | |
| myrcenol | 4-ethylphenol | |
| limonene | 4-vinylphenol | |
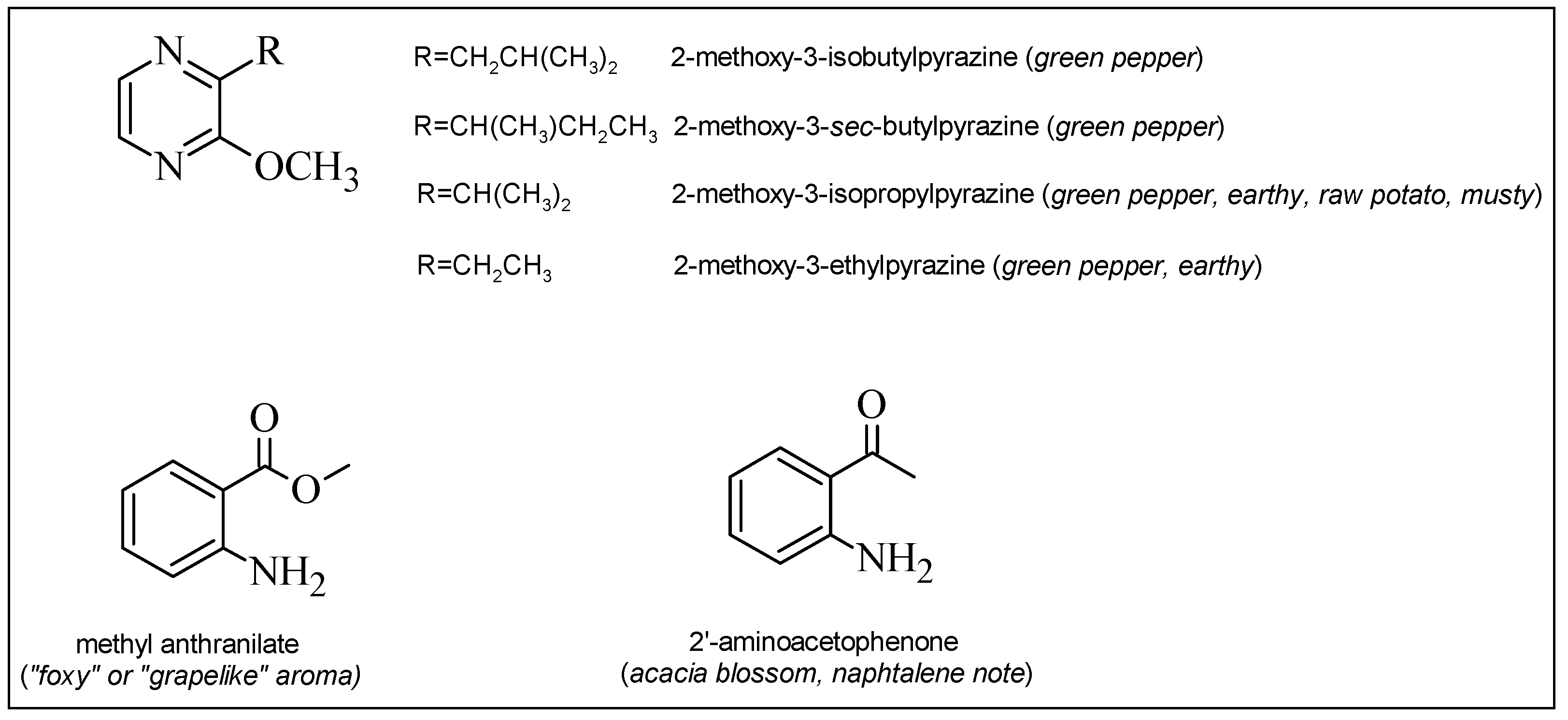
| β-phellandrene | methyl anthranilate | |
| β-ocimene | 2'-aminoacetophenone | |
| wine lactone | ||
| Aliphatic alcohols | Acids | Sesquiterpenes |
| 1-butanol | isobutyric acid | rotundone |
| 2-nonanol | isovaleric acid | farnesol |
| 3-methyl-1-butanol | acetic acid | germacrene D |
| 2-methyl-1-butanol | butyric acid | γ-cadinene |
| isobutanol | hexanoic acid | α-ylangene |
| 1-pentanol | octanoic acid | α-farnesene |
| 1-hexanol | decanoic acid | β-farnesene |
| 1-octanol | hexadecanoic acid | nerolidol |
| (E)-3-hexen-1-ol | octadecanoic acid | |
| (Z)-3-hexen-1-ol | ||
| 4-methyl-3-penten-1-ol | ||
| (E)-2-hexen-1-ol | ||
| 1-octen-3-ol | ||
| 2-ethyl-1-hexanol | ||
| furfuryl alcohol | ||
| 6-methyl-5-hepten-2-ol | ||
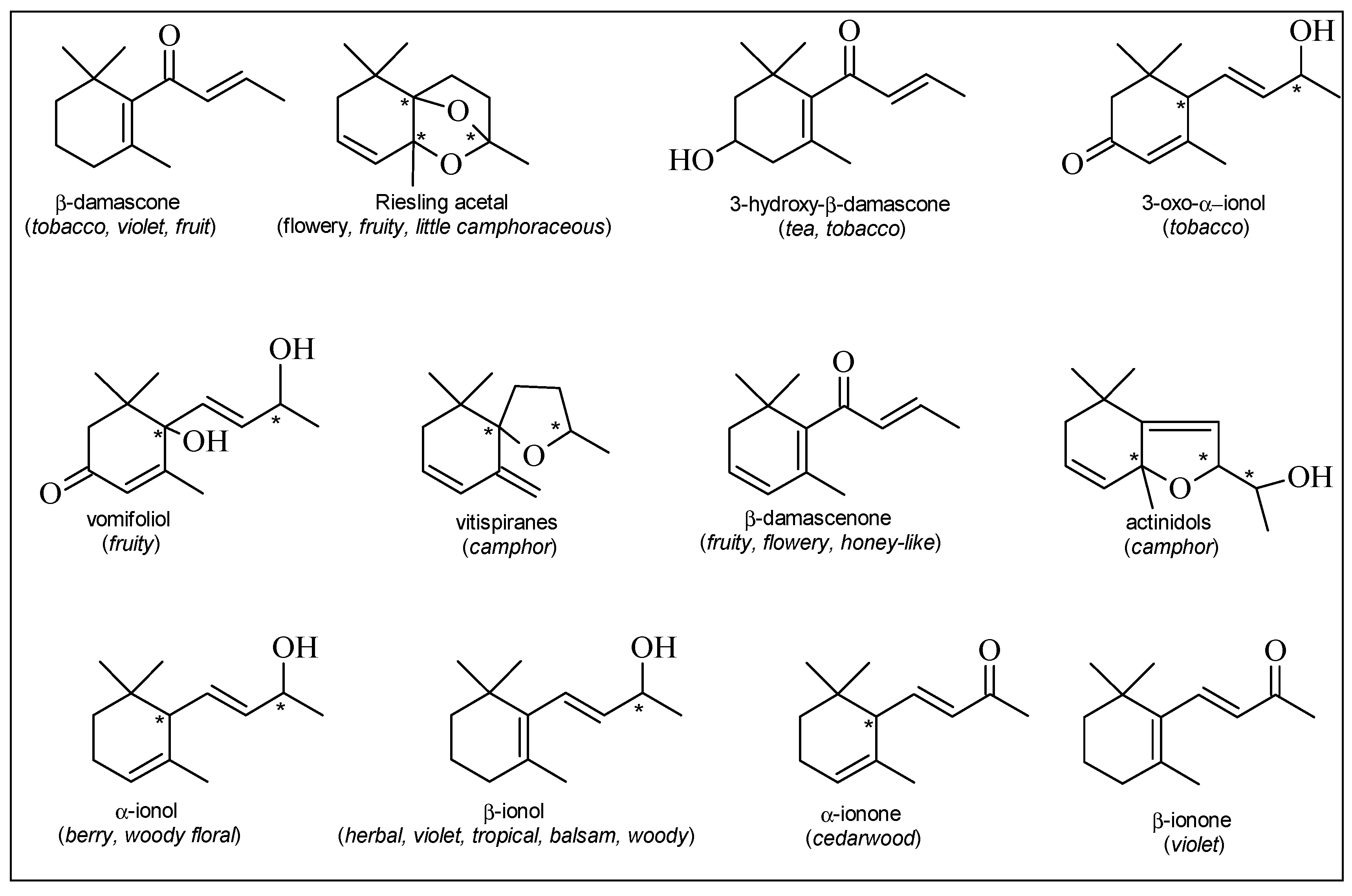
| Carbonyl compounds | Esters | Norisoprenoids |
| acetaldehyde | ethyl 2-methylpropanoate | TDN (1,1,6-trimethyl-1,2-dihydronaphthalene) |
| isobutyraldehyde | ethyl 2-methylbutanoate | β-damascone |
| 2-methylbutanal | ethyl 3-methylbutanoate | β-damascenone |
| isovaleraldehyde | ethyl 2-hydroxypropanoate | vomifoliol |
| 1-octen-3-one | ethyl 3-hydroxybutanoate | dihydrovomifoliol |
| (E)-2-heptenal | ethyl 4-hydroxybutanoate | 3-hydroxy-β-damascone |
| methional | diethyl succinate | 3-oxo-α-ionol |
| (E)-2-octenal | diethyl malate | 3-hydroxy-7,8-dihydro-β-ionol |
| hexanal | ethyl butanoate | α-ionol |
| (E)-2-hexenal | ethyl hexanoate | β-ionol |
| (Z)-3-hexenal | ethyl octanoate | α-ionone |
| (Z)-2-nonenal | ethyl decanoate | β-ionone |
| furfural | ethyl benzoate | actinidols |
| 5-methylfurfural | isoamyl octanoate | vitispiranes |
| 1H-pyrrole-2-carboxyaldehyde | ethyl furoate | Riesling acetal |
| geranial | ethyl dihydrocinnamate | hydroxy-megastigmen-2-one |
| neral | ethyl cinnamate | hydroxy-megastigmen-3-one |
| acetoin | methyl vanillate | 4-oxo-isophorone |
| diacetyl | ethyl vanillate | β-isophorone |
| glyoxal | ethyl acetate | 4-oxo-2,3-dehydro-β-ionol |
| methylglyoxal | isobutyl acetate | β-cyclocitral |
| glycolaldehyde | isoamyl acetate | |
| hydroxypropandial | ethyl 2-phenylacetate | |
| 2,4-nonadienal | hexyl acetate | |
| 2,6-nonadienal | ||
| Lactones | Nitrogen compounds | |
| γ-butyrolactone | 3-isobutyl-2-methoxypyrazine | |
| γ-hexalactone | 3-sec-butyl-2-methoxypyrazine | |
| γ-nonalactone | 3-isopropyl-2-methoxypyrazine | |
| γ-decalactone | 3-ethyl-2-methoxypyrazine | |
| cis/trans oak lactone | ||
| sotolon |
2. SPME-GC/MS Methods
2.1. Analysis of PFBOA-Derivatives
| Sample volume | 100 μL |
| Vial volume | 4 mL |
| Derivatization conditions | 200 μL IS o-chlorobenzaldehyde, 3.4 mg/L in ethanol/water solution; 1 mL of PFBOA 2 g/L aqueous solution, volume adjusted to 2 mL with water |
| SPME fiber | 65-μm poly(ethylene glycol)/divinylbenzene (PEG/DVB) |
| Addition to the sample | 50 mg NaCl |
| Sample heating | 50 °C for 20 min under stirring |
| Extraction temperature and time | 50 °C for 5 min |
| Desorption temperature and time | 240 °C for 1 min |
| Fiber cleaning | 250 °C for 5 min |
| GC column | HP-INNowax (30 m × 0.25 mm i.d; 0.25-μm film thickness) |
| Carrier gas | Helium, column headpressure 16 psi |
| Injector | T = 240 °C, sample volume 0.5 μL, splitless injection |
| Oven program | 60 °C for 5 min, 3 °C/min to 210 °C, held 5 min |
| MS-IT conditions | PICI mode using methane as reagent gas (flow 1 mL/min), ion source at 200 °C, damping gas 0.3 mL/min, simultaneous SCAN (range m/z 40–660, 1.67 scan/s) and MS/MS |
| CID experiments | Collision gas He, excitation voltage 225 mV |
| Quantitative | Recorded signals at m/z 240 for acetaldehyde, m/z 282 for diacetyl, m/z 266 for acetoin, m/z 336 for o-chlorobenzaldehyde (I.S.) |
2.2. Aroma Compounds and Wine Aging
| SPME fiber | 65-μm carbowax/divinylbenzene (CAR/DVB) |
| Sample volume | 10 mL |
| Vial volume | 20 mL |
| Addition to the sample | 3 g NaCl |
| Sample heating | 70 °C for 10 min |
| Extraction temperature and time | 70 °C for 30 min |
| Desorption temperature and time | 230 °C fo 5 min |
| Fiber cleaning | 10 min |
| GC column | HP-INNowax (30 m × 0.25 mm i.d; 0.25 μm film thickness) |
| Injection | Splitless |
| Oven program | 40 °C for 5 min, 3 °C/min to 230 °C, held 10 min |
| MS conditions | ionization energy 70 eV, acquisition SIM mode |
| Barrel | Months of Aging | Compounds mg/L | |||||
|---|---|---|---|---|---|---|---|
| Furfural | 5-Methylfurfural | 4-Ethylguaiacol | Eugenol | 4-Ethylphenol | Vanillin | ||
| Acacia | 3 | 0.02 ± 0.01 | 0.03 ± 0.01 | 2.24 ± 0.21 | 0.009 ± 0.001 | 0.67 ± 0.07 | 0.09 ± 0.03 |
| 6 | 0.04 ± 0.01 | 0.03 ± 0.01 | 2.94 ± 0.14 | 0.015 ± 0.001 | 0.92 ± 0.08 | 0.16 ± 0.01 | |
| 9 | 0.03 ± 0.01 | 0.03 ± 0.01 | 3.25 ± 0.67 | 0.021 ± 0.005 | 1.29 ± 0.41 | 0.31 ± 0.07 | |
| Cherry | 3 | Nd | nd | 3.01 ± 1.13 | 0.008 ± 0.004 | 1.00 ± 0.44 | 0.08 ± 0.04 |
| 6 | Tr | nd | 3.13 ± 0.26 | 0.009 ± 0.001 | 1.04 ± 0.06 | 0.10 ± 0.01 | |
| 9 | Nd | nd | 2.79 ± 0.51 | 0.007 ± 0.001 | 0.86 ± 0.18 | 0.12 ± 0.03 | |
| Chestnut | 3 | 0.04 ± 0.02 | 0.03 ± 0.01 | 2.53 ± 0.43 | 0.024 ± 0.004 | 0.84 ± 0.20 | 0.45 ± 0.06 |
| 6 | 0.04 ± 0.01 | 0.02 ± 0.02 | 2.30 ± 0.12 | 0.035 ± 0.003 | 0.74 ± 0.08 | 0.60 ± 0.02 | |
| 9 | 0.07 ± 0.01 | 0.04 ± 0.01 | 1.84 ± 0.18 | 0.026 ± 0.002 | 0.64 ± 0.04 | 0.43 ± 0.03 | |
| Mulberry | 3 | Tr | nd | 2.69 ± 0.75 | 0.004 ± 0.001 | 1.06 ± 0.26 | 0.09 ± 0.03 |
| 6 | Tr | nd | 2.72 ± 0.44 | 0.006 ± 0.001 | 1.27 ± 0.26 | 0.08 ± 0.02 | |
| 9 | Tr | tr | 1.84 ± 0.20 | 0.006 ± 0.001 | 1.19 ± 0.07 | 0.08 ± 0.01 | |
| Oak | 3 | 0.18 ± 0.08 | 0.14 ± 0.04 | 2.51 ± 0.14 | 0.009 ± 0.001 | 0.90 ± 0.07 | 0.27 ± 0.04 |
| 6 | 0.56 ± 0.16 | 0.19 ± 0.05 | 2.08 ± 0.02 | 0.012 ± 0.003 | 0.75 ± 0.05 | 0.34 ± 0.08 | |
| 9 | 0.60 ± 0.06 | 0.32 ± 0.04 | 2.90 ± 0.75 | 0.018 ± 0.005 | 1.06 ± 0.36 | 0.36 ± 0.09 | |
2.3. “Foxy Smelling Compounds” and 3-Alkyl-2-Methoxypyrazines in Grape Juice
| SPME fiber | 50/30 μm divinylbenzene/CarboxenTM/polydimethylsiloxane (DVB/CAR/PDMS) |
| Sample volume | 10 mL |
| Vial volume | 20 mL |
| Addition to the sample | 3 g NaCl |
| Extraction temperature and time | 50 °C for 30 min |
| Desorption temperature and time | 250 °C fo 5 min |
| Fiber cleaning | 10 min |
| GC column | HP-5ms: (5%-phenyl) methylpolysiloxane (30 m × 0.25mm i.d; 0.25-μm film thickness) | |||
| Carrier gas | Helium at constant flow 1.2 mL/min | |||
| Injector | 250 °C | |||
| Oven program | 40 °C for 5 min, 5 °C/min to 230 °C, held 3 min | |||
| MSD conditions | Ionization energy 70 eV, transfer line temperature 280 °C, ion source 250 °C, ion trap in MS/MS mode | |||
| IT-MS/MS | ||||
| Precursor ion | MS/MS signal | |||
| Analyte | MW | GC retention time (min) | m/z | |
| 3-ethyl-2-methoxypyrazine | 138.17 | 15.10 | 138 | 119 |
| 3-isopropyl-2-methoxypyrazine | 152.20 | 16.46 | 137 | 109 |
| 3-isobutyl-2-methoxypyrazine | 166.22 | 18.90 | 124 | 81 |
| 3-sec-butyl-2-methoxypyrazine | 166.22 | 19.14 | 138 | 81 |
| 2-ethoxy-3-isopropylpyrazine (IS) | 166.22 | 18.45 | 166 | 123 |
| methyl anthranilate | 151.16 | 23.71 | 151 | TIC |
| 2'-aminoacetophenone | 135.16 | 22.59 | 135 | TIC |
| 2,4-dichloroaniline (IS) | 162.02 | 23.35 | 161 | TIC |
2.4. Volatile Phenols in Wine
2.5. Higher Alcohols and Esters in Wine
2.6. Wine Volatile Sulfur Compounds
3. Conclusions
Author Contributions
Conflicts of Interest
References
- Weldegergis, B.T.; Croucha, A.M.; Górecki, T.; de Villiers, A. Solid phase extraction in combination with comprehensive two-dimensional gas chromatography coupled to time-of-flight mass spectrometry for the detailed investigation of volatiles in South African red wines. Anal. Chim. Acta 2011, 701, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Sagratini, G.; Maggi, F.; Caprioli, G.; Cristalli, G.; Ricciutelli, M.; Torregiani, E.; Vittori, S. Comparative study of aroma profile and phenolic content of Montepulciano monovarietal red wines from the Marches and Abruzzo regions of Italy using HS-SPME-GC-MS and HPLC-MS. Food Chem. 2012, 132, 1592–1599. [Google Scholar] [CrossRef]
- Blanch, G.P.; Reglero, G.; Herraiz, M. Rapid extraction of wine aroma compounds using a new simultaneous distillation-solvent extraction device. Food Chem. 1996, 56, 439–444. [Google Scholar] [CrossRef]
- Bosch-Fusté, J.; Riu-Aumatell, M.; Guadayol, J.M.; Caixach, J.; López-Tamames, E.; Buxaderas, S. Volatile profiles of sparkling wines obtained by three extraction methods and gas chromatography-mass spectrometry (GC-MS) analysis. Food Chem. 2007, 105, 428–435. [Google Scholar] [CrossRef]
- Mayr, C.M.; Geue, J.P.; Holt, H.E.; Pearson, W.P.; Jeffery, D.W.; Francis, I.L. Characterization of the key aroma compounds in Shiraz wine by quantitation, aroma reconstitution, and omission studies. J. Agric. Food Chem. 2014, 62, 4528–4536. [Google Scholar] [CrossRef] [PubMed]
- Mamede, M.; Pastore, G.M. Study of methods for the extraction of volatile compounds from fermented grape must. Food Chem. 2006, 96, 586–590. [Google Scholar] [CrossRef]
- Mateo, J.J.; Gentilini, N.; Huerta, T.; Jiménez, M.; di Stefano, R. Fractionation of glycoside precursors of aroma in grape and wine. J. Chromatogr. A 1997, 778, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Cabrita, M.J.; Costa Freitas, A.M.; Laureano, O.; Borsa, D.; di Stefano, R. Aroma compounds in varietal wines from Alentejo, Portugal. J. Food Comp. Anal. 2007, 20, 375–390. [Google Scholar] [CrossRef]
- Rosillo, L.; Salinas, M.R.; Garijo, J.; Alonso, G.L. Study of volatiles in grapes by dynamic headspace analysis: Application to the differentiation of some Vitis vinifera varieties. J. Chromatogr. A 1999, 847, 155–159. [Google Scholar] [CrossRef]
- Marengo, E.; Aceto, M.; Maurino, V. Classification of Nebbiolo-based wines from Piedmont (Italy) by means of solid-phase microextraction-gaschromatography-mass spectrometry of volatile compounds. J. Chromatogr. A 2001, 943, 123–137. [Google Scholar] [CrossRef]
- Sanchéz-Palomo, E.; Díaz-Maroto, M.C.; Pérez-Coello, S. Rapid determination of volatile compounds in grapes by HS-SPME coupled with GC-MS. Talanta 2005, 66, 1152–1157. [Google Scholar] [CrossRef] [PubMed]
- Coelho, E.; Rocha, S.M.; Delgadillo, I.; Coimbra, M.A. Headspace-SPME applied to varietal volatile components evolution during Vitis vinifera L. cv. “Baga” ripening. Anal. Chim. Acta 2006, 563, 204–214. [Google Scholar] [CrossRef]
- Verzera, A.; Ziino, M.; Scacco, A.; Lanza, C.M.; Mazzaglia, A.; Romeo, V.; Condurso, C. Volatile compound and sensory analysis for the characterization of an Italian white wine from “Inzolia” grapes. Food Anal. Methods 2008, 1, 144–151. [Google Scholar] [CrossRef]
- Arthur, C.L.; Pawliszyn, J. Solid-phase microextraction with thermal desorption using silica optical fibers. Anal. Chem. 1990, 62, 2145–2148. [Google Scholar] [CrossRef]
- Bojko, B.; Cudjoe, E.; Gómez-Ríos, G.A.; Gorynski, K.; Jiang, R.; Reyes-Garcés, N.; Risticevic, S.; Silva, É.A.S.; Togunde, O.; Vuckovic, D.; et al. SPME-Quo vadis? Anal. Chim. Acta 2012, 750, 132–151. [Google Scholar] [CrossRef]
- Harmon, A.D. Solid-phase microextraction for the analysis of flavors. In Techniques for Analyzing Food Aroma; Marsili, R., Ed.; Marcel Decker, Inc.: New York, NY, USA, 1997; pp. 81–112. [Google Scholar]
- Yu, Y.-J.; Lu, Z.-M.; Yu, N.-H.; Xu, W.; Li, G.-Q.; Shi, J.-S.; Xu, Z.-H. HS-SPME/GC-MS and chemometrics for volatile composition of Chinese traditional aromatic vinegar in the Zhenjiang region. J. Inst. Brew. 2012, 118, 133–141. [Google Scholar] [CrossRef]
- Vas, G.; Vékey, K. Solid-phase microextraction: A powerful sample preparation tool prior to mass spectrometric analysis. J. Mass Spectrom. 2004, 39, 233–254. [Google Scholar] [CrossRef] [PubMed]
- Castro, R.; Natera, R.; Durán, E.; García-Barroso, C. Application of solid phase extraction techniques to analyse volatile compounds in wines and other enological products. Eur. Food Res. Technol. 2008, 228, 1–18. [Google Scholar] [CrossRef]
- Flamini, R. Volatile and aroma compounds in wines. In Mass Spectrometry in Grape and Wine Chemistry; Flamini, R., Traldi, P., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; pp. 117–162. [Google Scholar]
- Garcóa, E; Chacón, J.L.; Martínez, J.; Izquierdo, P.M. Changes in volatile compounds during ripening in grapes of Airén, Macabeo and Chardonnay white varieties grown in La Mancha Region (Spain). Food Sci. Technol. Int. 2003, 9, 33–41. [Google Scholar] [CrossRef]
- Noguerol-Pato, R.; González-Barreiro, C.; Cancho-Grande, B.; Santiago, J.L.; Martínez, M.C.; Simal-Gándara, J. Aroma potential of Brancellao grapes from different cluster positions. Food Chem. 2012, 132, 112–124. [Google Scholar] [CrossRef]
- Marais, J. Terpenes in the aroma of grapes and wines: A review. S. Afr. J. Enol. Vitic. 1983, 4, 49–58. [Google Scholar]
- Flamini, R. Grape aroma compounds: Terpenes, C13-norisoprenoids, benzene compounds, and 3-alkyl-2-methoxypyrazines. In Mass Spectrometry in Grape and Wine Chemistry; Flamini, R., Traldi, P., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; pp. 97–116. [Google Scholar]
- Riberéau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Varietal Aroma. In Handbook of Enology: The Chemistry of Wine. Stabilization and Treatments, 2nd ed.; John Wiley & Sons Ltd: Chichester, UK, 2006; Volume 2, pp. 205–230. [Google Scholar]
- Wang, Y.; Kays, S.J. Contribution of volatile compounds to the characteristic aroma of baked Jewel sweetpotatos. J. Am. Soc. Hortic. Sci. 2000, 125, 638–643. [Google Scholar]
- Robinson, A.L.; Boss, P.K.; Solomon, P.S.; Trengove, R.D.; Heymann, H.; Ebeler, S.E. Origins of grape and wine aroma. Part 1. Chemical Components and Viticultural Impacts. Am. J. Enol. Vitic. 2014, 65, 1–24. [Google Scholar] [CrossRef]
- Riberéau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Alcohols and other volatile compounds. In Handbook of Enology: The Chemistry of Wine. Stabilization and Treatments, 2nd ed.; John Wiley & Sons Ltd: Chichester, UK, 2006; Volume 2, pp. 51–64. [Google Scholar]
- Bakker, J.; Clarke, R.J. Volatile components. In Wine Flavour Chemistry, 2nd ed.; John Wiley & Sons, Ltd: Chichester, UK, 2012; pp. 155–238. [Google Scholar]
- Flamini, R.; de Luca, G.; di Stefano, R. Changes in carbonyl compounds in Chardonnay and Cabernet Sauvignon wines as a consequence of malolactic fermentation. Vitis 2002, 41, 107–112. [Google Scholar]
- Flamini, R.; Dalla Vedova, A. Glyoxal/glycolaldehyde: A redox system involved in malolactic fermentation of wine. J. Agric. Food Chem. 2003, 51, 2300–2303. [Google Scholar] [CrossRef] [PubMed]
- Baumes, R. Wine Aroma Precursors. In Wine Chemistry and Biochemistry; Victoria Moreno-Arribas, M., Carmen Polo, M., Eds.; Springer Science + Business Media, LLC: New York, NY, USA, 2009; pp. 251–274. [Google Scholar]
- Gambetta, J.M.; Bastian, S.E. P.; Cozzolino, D.; Jeffery, D.W. Factors influencing the aroma composition of Chardonnay wines. J. Agric. Food Chem. 2014, 62, 6512–6534. [Google Scholar] [CrossRef] [PubMed]
- Bell, S.J.; Henschke, P.A. Implications of nitrogen nutrition for grapes, fermentation and wine. Aust. J. Grape Wine Res. 2005, 11, 242–295. [Google Scholar] [CrossRef]
- Swiegers, J.H.; Bartowsky, E.J; Henschke, P.A.; Pretorius, I.S. Yeast and bacterial modulation of wine aroma and flavour. Aust. J. Grape Wine Res. 2005, 11, 139–173. [Google Scholar] [CrossRef]
- Rapp, A.; Versini, G. Influence of nitrogen compounds in grapes on aroma compounds in wine. In Proceedings of the International Symposium on Nitrogen in Grapes and Wine, Seattle, DC, USA, 18–19 June 1991; American Society of Enology and Viticulture: Davis, CA, USA, 1991; pp. 156–164. [Google Scholar]
- Riberéau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Phenolic compounds. In Handbook of Enology: The Chemistry of Wine. Stabilization and Treatments, 2nd ed.; John Wiley & Sons Ltd: Chichester, UK, 2006; Volume 2, pp. 141–203. [Google Scholar]
- Cancilla, D.A.; Que Hee, S.S. O-(2,3,4,5,6-Pentafluorophenyl)methylhydroxylamine hydrochloride: A versatile reagent for the determination of carbonyl-containing compounds. J. Chromatogr. A 1992, 627, 1–16. [Google Scholar] [CrossRef]
- Vanderlinde, R.; Bertrand, A.; Segur, M.C. Dosage des aldehydes dans les eaux-de-vie. In Proceedings of the 1er Symposium Scientifique International du Cognac, “Elaboration et connaissance des spiritueux”, Cognac, France, 11–15 May 1992; Lavoiser TEC & DOC: Paris, France, 1992; pp. 506–511. [Google Scholar]
- Vidal, J.P.; Mazerolles, G.; Estreguil, S.; Cantagrel, R. Analyse quantitative de la fraction carbonylée volatile des eaux-de-vie de Cognac. In Proceedings of the 1er Symposium Scientifique International du Cognac, “Elaboration et connaissance des spiritueux”, Cognac, France, 11–15 May 1992; Lavoiser TEC & DOC: Paris, France, 1992; pp. 529–537. [Google Scholar]
- De Revel, G.; Bertrand, A. A method for the detection of carbonyl compounds in wine: Glyoxal and methylglyoxal. J. Sci. Food Agric. 1993, 61, 267–272. [Google Scholar] [CrossRef]
- De Revel, G.; Bertrand, A. Dicarbonyl compounds and their reduction products in wine. Identification of wine aldehydes. In Trends in Flavour Research; Maarse, H., van der Heij, D.G., Eds.; Elsevier Science B.V.: Amsterdam, The Netherlands, 1994; pp. 353–361. [Google Scholar]
- Guillou, I.; Bertrand, A.; de Revel, G.; Barbe, J.C. Occurrence of hydroxypropanedial in certain musts and wines. J. Agric. Food Chem. 1997, 45, 3382–3386. [Google Scholar] [CrossRef]
- Flamini, R.; Tonus, T.; Dalla Vedova, A. A GC-MS method for determining acetaldehyde in wines. Riv. Vitic. Enol. 2002, 2/3, 15–21. [Google Scholar]
- Flamini, R.; Dalla Vedova, A.; Panighel, A. Study of carbonyl compounds in some Italian marc distillate (grappa) samples by synthesis of O-(2,3,4,5,6-pentafluorobenzyl)-hydroxylamine derivatives. Riv. Vitic. Enol. 2005, 1, 51–63. [Google Scholar]
- Flamini, R.; Dalla Vedova, A.; Panighel, A.; Perchiazzi, N.; Ongarato, S. Monitoring of the principal carbonyl compounds involved in malolactic fermentation of wine by solid-phase microextraction and positive ion chemical ionization GC/MS analysis. J. Mass Spectrom. 2005, 40, 1558–1564. [Google Scholar] [CrossRef] [PubMed]
- López-Vázquez, C.; Orriols, I.; Perelló, M.-C.; de Revel, G. Determination of aldehydes as pentafluorobenzyl derivatives in grape pomace distillates by HS-SPME-GC/MS. Food Chem. 2012, 130, 1127–1133. [Google Scholar] [CrossRef]
- Bauer, R.; Dicks, L.M.T. Control of Malolactic Fermentation in Wine. A Review. S. Afr. J. Enol. Vitic. 2004, 25, 74–88. [Google Scholar]
- Lerm, E.; Engelbrecht, L.; du Toit, M. Malolactic Fermentation: The ABC’s of MLF. S. Afr. J. Enol. Vitic. 2010, 31, 186–212. [Google Scholar]
- Zapata, J.; Mateo-Vivaracho, L.; Cacho, J.; Ferreira, V. Comparison of extraction techniques and mass spectrometric ionization modes in the analysis of wine volatile carbonyls. Anal. Chim. Acta 2010, 660, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Coello, M.S.; Sanz, J.; Cabezudo, M.D. Determination of volatile compounds in hydroalcoholic extracts of French and American oak wood. Am. J. Enol. Vitic. 1999, 50, 162–165. [Google Scholar]
- Garde-Cedàn, T.; Lorenzo, C.; Carot, J.M.; Jabaloyes, J.M.; Esteve, M.D.; Salinas, M.R. Statistical differentiation of wines of different geographic origin and aged in barrel according to some volatile components and ethylphenols. Food Chem. 2008, 111, 1025–1031. [Google Scholar] [CrossRef]
- Dìaz-Maroto, M.C.; Sànchez-Palomo, E.; Pérez-Coello, M.S. Fast screening method for volatile compounds of oak wood used for aging wines by headspace SPME-GC-MS (SIM). J. Agric. Food Chem. 2004, 52, 6857–6861. [Google Scholar] [CrossRef] [PubMed]
- Carillo, J.D.; Garrido-Lòpez, A.; Tena, M.T. Determination of oak volatile compounds in wine by headspace solid-phase microextraction and gas chromatography-mass spectrometry. J. Chromatogr. A 2006, 1102, 25–36. [Google Scholar] [CrossRef] [PubMed]
- De Rosso, M.; Panighel, A.; Dalla Vedova, A.; Stella, L.; Flamini, R. Changes in chemical composition of a red wine aged in acacia, cherry, chestnut, mulberry, and oak wood barrels. J. Agric. Food Chem. 2009, 57, 1915–1920. [Google Scholar] [CrossRef] [PubMed]
- Rapp, A.; Versini, G.; Ullemeyer, H. 2-aminoacetophenone: causal component of “untypical aging flavor” (“naphtalene note”, “hybrid note”) of wine. Vitis 1993, 32, 61–62. [Google Scholar]
- Hoenicke, K.; Borchert, O.; Grüning, K.; Simat, T.J. “Untypical aging off-flavor” in wine: synthesis of potential degradation compounds of indole-3-acetic acid and kynurenine and their evaluation as precursors of 2-aminoacetophenone. J. Agric. Food Chem. 2002, 50, 4303–4309. [Google Scholar] [CrossRef] [PubMed]
- Hoenicke, K.; Simat, T.J.; Steinhart, H.; Christoph, N.; Geßner, M.; Köhler, H.-J. “Untypical aging off-flavor” in wine: formation of 2-aminoacetophenone and evaluation of its influencing factors. Anal. Chim. Acta 2002, 458, 29–37. [Google Scholar] [CrossRef]
- Rapp, A.; Versini, G. Methylanthranilate (“foxy taint”) concentrations of hybrid and Vitis vinifera wines. Vitis 1996, 35, 215–216. [Google Scholar]
- Fan, W.; Tsai, I.-M.; Qian, M.C. Analysis of 2-aminoacetophenone by direct-immersion solid-phase microextraction and gas chromatography-mass spectrometry and its sensory impact in Chardonnay and Pinot gris wines. Food Chem. 2007, 105, 1144–1150. [Google Scholar] [CrossRef]
- Massa, M.J.; Robacker, D.C.; Patt, J. Identification of grape juice aroma volatiles and attractiveness to the Mexican fruit fly (Diptera: Tephritidae). Florida Entomologist 2008, 91, 266–276. [Google Scholar] [CrossRef]
- Lacey, M.; Allen, M.S.; Harris, R.L.N.; Brown, W.V. Methoxypyrazines in Sauvignon blanc grapes and wines. Am. J. Enol. Vitic. 1991, 42, 103–108. [Google Scholar]
- Allen, M.S.; Lacey, M.J.; Harris, R.L.N.; Brown, W.V. Contribution of Methoxypyrazines to Sauvignon blanc Wine Aroma. Am. J. Enol. Vitic. 1991, 42, 109–112. [Google Scholar]
- Allen, M.S.; Lacey, M.J. Methoxypyrazine grape flavour: Influence of climate, cultivar and viticulture. Die Wein-Wiss. 1993, 48, 211–213. [Google Scholar]
- Hashizume, K.; Umeda, N. Methoxypyrazine content of Japanese red wines. Biosci. Biotechnol. Biochem. 1996, 60, 802–805. [Google Scholar] [CrossRef]
- Hashizume, K.; Samuta, T.J. Green odorants of grape cluster stem and their ability to cause a wine stemmy flavor. J. Agric. Food Chem. 1997, 45, 1333–1337. [Google Scholar] [CrossRef]
- Hashizume, K.; Samuta, T. Grape maturity and light exposure affect berry methoxypyrazine concentration. Am. J. Enol. Vitic. 1999, 50, 194–198. [Google Scholar]
- Roujou de Boubee, D.; Cumsille, A.M.; Pons, D.; Dubordieu, D. Location of 2-methoxy-3-isobutylpyrazine in Cabernet Sauvignon grape bunches and its extractability during vinification. Am. J. Enol. Vitic. 2002, 53, 1–5. [Google Scholar]
- Kotseridis, Y.S.; Spink, M.; Brindle, I.D.; Blake, A.J.; Sears, M.; Chen, X.; Soleas, G.; Inglis, D.; Pickering, G.J. Quantitative analysis of 3-alkyl-2-methoxypyrazines in juice and wine using stable isotope labelled internal standard assay. J. Chromatogr. A 2008, 1190, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Sala, C.; Mestres, M.; Martì, M.P.; Busto, O.; Guasch, J. Headspace solid-phase microextraction analysis of 3-alkyl-2- methoxypyrazines in wines. J. Chromatogr. A 2002, 953, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Galvan, T.L.; Kells, S.; Hutchison, W.D. Determination of 3-alkyl-2-methoxypyrazines in Lady beetle-Infested wine by Solid-Phase Microextraction headspace sampling. J. Agric. Food Chem. 2008, 56, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Ryan, D.; Watkins, P.; Smith, J.; Allen, M.; Marriott, P. Analysis of methoxypyrazines in wine using headspace solid phase microextraction with isotope dilution and comprehensive two-dimensional gas chromatography. J. Sep. Sci. 2005, 28, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Panighel, A.; Dalla Vedova, A.; de Rosso, M.; Gardiman, M.; Flamini, R. A solid-phase microextraction gas chromatography/ion trap tandem mass spectrometry method for simultaneous determination of “foxy smelling compounds” and 3-alkyl-2-methoxypyrazines in grape juice. Rapid Commun. Mass Spectrom. 2010, 24, 2023–2029. [Google Scholar] [CrossRef] [PubMed]
- Chatonnet, P.; Boidron, J.-N.; Pons, M. Elevage des vins rouges en fûts de chêne: Évolution de certains composés volatils et de leur impact aromatique. Sci. Aliment. 1990, 10, 565–587. [Google Scholar]
- Chatonnet, P.; Dubourdieu, D.; Boidron, J.-N.; Pons, M. The origin of ethylphenols in wines. J. Sci. Food Agric. 1992, 60, 165–178. [Google Scholar] [CrossRef]
- Rodrigues, N.; Gonçalves, G.; Pereira-da-Silva, S.; Malfeito-Ferreira, M.; Loureiro, V. Development and use of a new medium to detect yeast of the genera Dekkera/Brettanomyces sp. J. Appl. Microbiol. 2001, 90, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, J.D.; Tena, M.T. Determination of ethylphenols in wine by in situ derivatisation and headspace solid-phase microextraction gas chromatography mass spectrometry. Anal. Bioanal. Chem. 2007, 387, 2547–2558. [Google Scholar] [CrossRef] [PubMed]
- Monje, M.-C.; Privat, C.; Gastine, V.; Nepveu, F. Determination of ethylphenol compound in wine by headspace solid-phase microextraction in conjunction with gas chromatography and flame ionization detection. Anal. Chim. Acta 2002, 458, 111–117. [Google Scholar] [CrossRef]
- Castro Mejías, R.; Natera Marín, R.; García Moreno, M.d.V.; García Barroso, C. Optimisation of headspace solid-phase microextraction for the analysis of volatile phenols in wine. J. Chromatogr. A 2003, 995, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Martorell, N.; Martì, M.P.; Mestres, M.; Busto, O.; Guasch, J. Determination of 4-ethylguaiacol and 4-ethylphenol in red wines using headspace-solid-phase microextraction-gas chromatography. J. Chromatogr. A 2002, 975, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Pizarro, C.; Pérez-del-Notario, N.; Gonzàlez-Sàiz, J.M. Determination of Brett character responsible compounds in wines by using multiple headspace solid-phase microextraction. J. Chromatogr. A 2007, 1143, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Francioli, S.; Guerra, M.; López-Tamames, E.; Guadayoi, J.M.; Caixach, J. Aroma of sparkling wines by headspace/solid phase microextraction and gas chromatography/mass spectrometry. Am. J. Enol. Vitic. 1999, 50, 404–408. [Google Scholar]
- Demyttenaere, J.C.R.; Dagher, C.; Sandra, P.; Kallithraka, S.; Verhé, R.; de Kimpe, N. Flavour analysis of Greek white wine by solid-phase microextraction-capillary gas chromatography-mass spectrometry. J. Chromatogr. A 2003, 985, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Bonino, M.; Schellino, R.; Rizzi, C.; Aigotti, R.; Delfini, C.; Baiocchi, C. Aroma compounds of an Italian wine (Ruchè) by HS-SPME analysis coupled with GC/IT/MS. Food Chem. 2003, 80, 125–133. [Google Scholar] [CrossRef]
- Vianna, E.; Ebeler, S. Monitoring ester formation in grape juice fermentations using solid phase microextraction coupled with gas chromatography-mass spectrometry. J. Agric. Food Chem. 2001, 49, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Martí, M.P.; Mestres, M.; Sala, C.; Busto, O.; Guasch, J. Solid-phase microextraction and gas chromatography olfactometry analysis of successively diluted samples. A new approach of the aroma extract dilution analysis applied to the characterization of wine aroma. J. Agric. Food Chem. 2003, 51, 7861–7865. [Google Scholar]
- Antalick, G.; Perello, M.-C.; de Revel, G. Development, validation and application of a specific method for the quantitative determination of wine esters by headspace-solid-phase microextraction-gas chromatography-mass spectrometry. Food Chem. 2010, 121, 1236–1245. [Google Scholar] [CrossRef]
- Welke, J.E.; Zanus, M.; Lazarotto, M.; Schmitta, K.G.; Zini, C.A. Volatile characterization by multivariate optimization of headspace-solid phase microextraction and sensorial evaluation of Chardonnay base wines. J. Braz. Chem. Soc. 2012, 23, 678–687. [Google Scholar] [CrossRef]
- Mestres, M.; Busto, O.; Guasch, J. Analysis of organic sulfur compounds in wine aroma. J. Chromatogr. A 2000, 881, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Fedrizzi, B.; Magno, F.; Moser, S.; Nicolini, G.; Versini, G. Concurrent quantification of light and heavy sulphur volatiles in wine by headspace solid-phase microextraction coupled with gas chromatography/mass spectrometry. Rapid Commun. Mass Spectrom. 2007, 21, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Flamini, R. Analysis of aroma compounds in wine. In Hyphenated Techniques in Grape and Wine Chemistry; Flamini, R., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008; pp. 173–225. [Google Scholar]
- Bouchilloux, P.; Darriet, P.; Dubourdieu, D. Identification d’un thiol fortement odorant, le 2-methyl-3-furanthiol, dans les vins. Vitis 1998, 37, 177–180. [Google Scholar]
- Fedrizzi, B.; Versini, G.; Lavagnini, I.; Nicolini, G.; Magno, F. Gas chromatography-mass spectrometry determination of 3-mercaptohexan-1-ol and 3-mercaptohexyl acetate in wine. A comparison between Solid Phase Extraction and Headspace Solid Phase Microextraction methods. Anal. Chim. Acta 2007, 596, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Mateo-Vivaracho, L.; Ferreira, V.; Cacho, J. Automated analysis of 2-methyl-3-furanthiol and 3-mercaptohexyl acetate at ng/L level by headspace solid-phase microextracion with on—Fibre derivatisation and gas chromatography-negative chemical ionization mass spectrometric determination. J. Chromatogr. A 2006, 1121, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kinton, V.R.; Collins, R.J.; Kolahgar, B.; Goodner, K.L. Fast analysis of beverages using mass spectral based chemical sensor. Gerstel AppNote 2003, 4, 1–10. [Google Scholar]
- Cozzolino, D.; Smyth, H.E.; Lattey, K.A.; Cynkar, W.; Janik, L.; Dambergs, R.G.; Francis, I.L.; Gishen, M. Combining mass spectrometry based electronic nose, visible-near infrared spectroscopy and chemometrics to assess the sensory properties of Australian Riesling wines. Anal. Chim. Acta 2006, 563, 319–324. [Google Scholar] [CrossRef]
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panighel, A.; Flamini, R. Applications of Solid-Phase Microextraction and Gas Chromatography/Mass Spectrometry (SPME-GC/MS) in the Study of Grape and Wine Volatile Compounds. Molecules 2014, 19, 21291-21309. https://doi.org/10.3390/molecules191221291
Panighel A, Flamini R. Applications of Solid-Phase Microextraction and Gas Chromatography/Mass Spectrometry (SPME-GC/MS) in the Study of Grape and Wine Volatile Compounds. Molecules. 2014; 19(12):21291-21309. https://doi.org/10.3390/molecules191221291
Chicago/Turabian StylePanighel, Annarita, and Riccardo Flamini. 2014. "Applications of Solid-Phase Microextraction and Gas Chromatography/Mass Spectrometry (SPME-GC/MS) in the Study of Grape and Wine Volatile Compounds" Molecules 19, no. 12: 21291-21309. https://doi.org/10.3390/molecules191221291
APA StylePanighel, A., & Flamini, R. (2014). Applications of Solid-Phase Microextraction and Gas Chromatography/Mass Spectrometry (SPME-GC/MS) in the Study of Grape and Wine Volatile Compounds. Molecules, 19(12), 21291-21309. https://doi.org/10.3390/molecules191221291




