HIV-1 and Its Resistance to Peptidic Carbohydrate-Binding Agents (CBAs): An Overview
Abstract
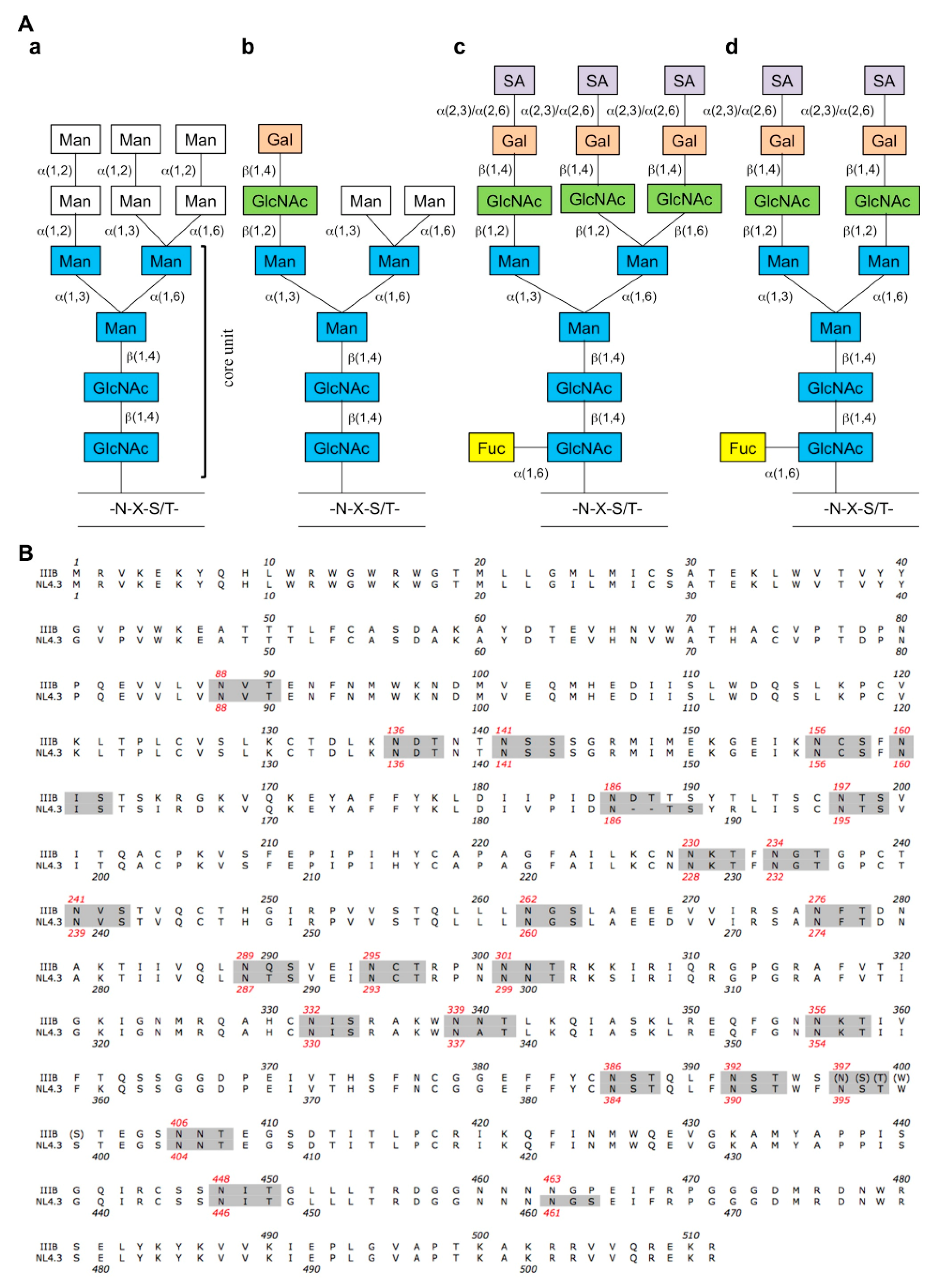
:1. Introduction

| Sequon a | N-glycan | NL4.32G12res. | IIIB2G12res. | IIIBHHAres. | IIIBGNAres. | IIIBAHres. | IIIBCV−Nres. | NL4.3CV−Nres. | NL4.3MVNres. | IIIBBanLecres. | IIIBGRFTres. | IIIBUDAres. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NVT (N88) | “potential” complex | x | x | x | ||||||||
| NDT (N136) | “potential” complex | x | x | |||||||||
| NSS (N141) | “potential” complex | (NG) d | ||||||||||
| NCS (N156) | “potential” complex | |||||||||||
| NIS (N160) | “potential” complex | x | x | |||||||||
| NDT (N186) | “potential” complex | x | x e | |||||||||
| NTS (N197) | “potential” complex | |||||||||||
| NKT (N230) | high-mannose | x | x e | x | x | x | x | |||||
| NGT (N234) | high-mannose | x | x | x | x | x | ||||||
| NVS (N241) | high-mannose | |||||||||||
| NGS (N262) | high-mannose | |||||||||||
| NFT (N276) | “potential” complex | x | ||||||||||
| NQS (N289) | high-mannose | x | x | x e | x | x | ||||||
| NCT (N295) | high-mannose | x | x | x | x | x | x | x | ||||
| NNT (N301) | “potential” complex | x | x | x | ||||||||
| NIS (N332) | high-mannose | x | x | x | ||||||||
| NNT (N339) | high-mannose | x | x | x | x | x | x | |||||
| NKT (N356) | “potential” complex | |||||||||||
| NST (N386) | high-mannose | x | x e | x | x | x | ||||||
| NST (N392) | high-mannose | x | x | x e | x | x | x | x | ||||
| NST (N397) b | “potential” complex | |||||||||||
| NNT (N406) | “potential” complex | x | x b | x | ||||||||
| NIT (N448) | high-mannose | x | x | x | x | |||||||
| NES (N463) c | “potential” complex | (NG) d | ||||||||||
| Total | 1 | 3 | 8 | 7 | 12 | 6 | 3 | 4 | 6 | 5 | 9 | |

2. Resistance and Cross-Resistance Pattern of CBAs with Potent Anti-HIV Activity
2.1. Mannose-Specific CBAs
2.1.1. Monoclonal Antibody 2G12
| CBAs | NL4.32G12res | IIIB2G12res | IIIBHHAres | IIIBGNAres | IIIBAHres | IIIBCV−Nres | NL4.3CV−Nres | NL4.3MVNres | IIIBBanLecres | IIIBGRFTres | IIIBUDAres |
|---|---|---|---|---|---|---|---|---|---|---|---|
| AH | N.D. a | N.D. | N.D. | N.D. | 19 b | N.D. | N.D. | N.D. | >66 | 118 | N.D. |
| MVN | 2 | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | >100 | N.D. | 4 | N.D. |
| 2G12 mAb | >50 | >36 | >15 | >15 | >25 | >45 | >45 | >38 | >25 | >35 | >29 |
| HHA | 12 | 2 | 919 | 490 | 1 | 17 | 4 | 3 | 3 | 5 | 117 |
| GNA | 6 | 1 | 926 | 581 | N.D. | 23 | 1 | 2 | N.D. | N.D. | 39 |
| BanLec | 1 | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | 5 | 24 | 13 | N.D. |
| GRFT | 10 | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | 14 | >106 | >1900 | N.D. |
| CV-N | 1 | 3 | 29 | 11 | N.D. | 20 | 7 | 4 | N.D. | N.D. | 83 |
| OAA | 3 | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | 2 | 2 | 2 | N.D. |
| UDA | 8 | N.D. | N.D. | N.D. | 1 | 1 | 3 | 3 | 1 | 3 | 24 |
| Increased sensitivity |
| 1 (none) |
| ≥2–9 (low) |
| ≥10–24 (moderate) |
| ≥25–49 (high) |
| ≥50 (very high) |
2.1.2. Hippeastrum Hybrid Agglutinin (HHA)
2.1.3. Galanthus Nivalis Agglutinin (GNA)
2.1.4. Actinohivin (AH)
2.1.5. Cyanovirin-N (CV-N)
2.1.6. Microvirin (MVN)
2.1.7. Banana Lectin (BanLec)
2.1.8. Griffithsin (GRFT)
2.1.9. Oscillatoria Agardhii Agglutinin (OAA)
2.2. N-Acetylglucosamine (GlcNAc)-Specific CBAs
2.2.1. Urtica Dioica Agglutinin (UDA)
2.2.2. Nicotiana Tabacum Agglutinin (NICTABA)
3. Inhibition of 2G12 mAb Binding to gp120 by CBAs
| CBA | Binding Inhibition | IC50 (nM) | References |
|---|---|---|---|
| HHA a | Yes | 28–39 | [22]/unpublished data |
| GNA a | Yes | 23–76 | [22] |
| AH | Yes | 93 | [29] |
| CV-N a | Yes | 16–28 | [22,26] |
| MVN | Yes | 259 | [26] |
| BanLec b | Yes | 4.5–14 | [59]/unpublished data |
| GRFT b | Yes | 0.57 | [61]/unpublished data |
| OAA | Yes | 21 | [27] |
| UDA | Yes | 1000 | [78] |
| NICTABA | No | >1000 | [78] |
4. CBAs: A Role in HIV Prevention or Treatment?
Acknowledgments
Conflicts of Interest
References
- Wilen, C.B.; Tilton, J.C.; Doms, R.W. HIV: Cell binding and entry. Cold Spring Harb. Perspect Med. 2012, 2. [Google Scholar] [CrossRef]
- Miyauchi, K.; Kim, Y.; Latinovic, O.; Morozov, V.; Melikyan, G.B. HIV enters cells via endocytosis and dynamin-dependent fusion with endosomes. Cell 2009, 137, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Leonard, C.K.; Spellman, M.W.; Riddle, L.; Harris, R.J.; Thomas, J.N.; Gregory, T.J. Assignment of intrachain disulfide bonds and characterization of potential glycosylation sites of the type 1 recombinant human immunodeficiency virus envelope glycoprotein (gp120) expressed in chinese hamster ovary cells. J. Biol. Chem. 1990, 265, 10373–10382. [Google Scholar] [PubMed]
- Scanlan, C.N.; Offer, J.; Zitzmann, N.; Dwek, R.A. Exploiting the defensive sugars of HIV-1 for drug and vaccine design. Nature 2007, 446, 1038–1045. [Google Scholar] [CrossRef] [PubMed]
- Helenius, A.; Aebi, M. Intracellular functions of n-linked glycans. Science 2001, 291, 2364–2369. [Google Scholar] [CrossRef] [PubMed]
- Mizuochi, T.; Spellman, M.W.; Larkin, M.; Solomon, J.; Basa, L.J.; Feizi, T. Structural characterization by chromatographic profiling of the oligosaccharides of human immunodeficiency virus (HIV) recombinant envelope glycoprotein gp120 produced in chinese hamster ovary cells. Biomed. Chromatogr. 1988, 2, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Mizuochi, T.; Spellman, M.W.; Larkin, M.; Solomon, J.; Basa, L.J.; Feizi, T. Carbohydrate structures of the human-immunodeficiency-virus (HIV) recombinant envelope glycoprotein gp120 produced in chinese-hamster ovary cells. Biochem. J. 1988, 254, 599–603. [Google Scholar] [PubMed]
- Doores, K.J.; Bonomelli, C.; Harvey, D.J.; Vasiljevic, S.; Dwek, R.A.; Burton, D.R.; Crispin, M.; Scanlan, C.N. Envelope glycans of immunodeficiency virions are almost entirely oligomannose antigens. Proc. Natl. Acad. Sci. USA 2010, 107, 13800–13805. [Google Scholar] [CrossRef] [PubMed]
- Bonomelli, C.; Doores, K.J.; Dunlop, D.C.; Thaney, V.; Dwek, R.A.; Burton, D.R.; Crispin, M.; Scanlan, C.N. The glycan shield of HIV is predominantly oligomannose independently of production system or viral clade. PLoS One 2011, 6, e23521. [Google Scholar] [CrossRef] [PubMed]
- Weis, W.I.; Taylor, M.E.; Drickamer, K. The c-type lectin superfamily in the immune system. Immunol. Rev. 1998, 163, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Kwong, P.D.; Wyatt, R.; Robinson, J.; Sweet, R.W.; Sodroski, J.; Hendrickson, W.A. Structure of an HIV gp120 envelope glycoprotein in complex with the cd4 receptor and a neutralizing human antibody. Nature 1998, 393, 648–659. [Google Scholar] [CrossRef] [PubMed]
- Balzarini, J. Targeting the glycans of glycoproteins: A novel paradigm for antiviral therapy. Nat. Rev. Microbiol. 2007, 5, 583–597. [Google Scholar] [CrossRef] [PubMed]
- Van Liempt, E.; Bank, C.M.; Mehta, P.; Garcia-Vallejo, J.J.; Kawar, Z.S.; Geyer, R.; Alvarez, R.A.; Cummings, R.D.; van Kooyk, Y.; van Die, I. Specificity of dc-sign for mannose- and fucose-containing glycans. FEBS Lett. 2006, 580, 6123–6131. [Google Scholar] [CrossRef] [PubMed]
- Van Kooyk, Y.; Geijtenbeek, T.B. DC-SIGN: Escape mechanism for pathogens. Nat. Rev. Immunol. 2003, 3, 697–709. [Google Scholar] [CrossRef] [PubMed]
- Cavrois, M.; Neidleman, J.; Greene, W.C. The achilles heel of the trojan horse model of HIV-1 trans-infection. PLoS Pathog. 2008, 4, e1000051. [Google Scholar] [CrossRef] [PubMed]
- Buchacher, A.; Predl, R.; Strutzenberger, K.; Steinfellner, W.; Trkola, A.; Purtscher, M.; Gruber, G.; Tauer, C.; Steindl, F.; Jungbauer, A.; et al. Generation of human monoclonal antibodies against HIV-1 proteins; electrofusion and epstein-barr virus transformation for peripheral blood lymphocyte immortalization. AIDS Res. Hum. Retrovir. 1994, 10, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Trkola, A.; Purtscher, M.; Muster, T.; Ballaun, C.; Buchacher, A.; Sullivan, N.; Srinivasan, K.; Sodroski, J.; Moore, J.P.; Katinger, H. Human monoclonal antibody 2g12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. J. Virol. 1996, 70, 1100–1108. [Google Scholar] [PubMed]
- Scanlan, C.N.; Pantophlet, R.; Wormald, M.R.; Ollmann Saphire, E.; Stanfield, R.; Wilson, I.A.; Katinger, H.; Dwek, R.A.; Rudd, P.M.; Burton, D.R. The broadly neutralizing anti-human immunodeficiency virus type 1 antibody 2g12 recognizes a cluster of α1→2 mannose residues on the outer face of gp120. J. Virol. 2002, 76, 7306–7321. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, R.; Kwong, P.D.; Desjardins, E.; Sweet, R.W.; Robinson, J.; Hendrickson, W.A.; Sodroski, J.G. The antigenic structure of the HIV gp120 envelope glycoprotein. Nature 1998, 393, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Calarese, D.A.; Scanlan, C.N.; Zwick, M.B.; Deechongkit, S.; Mimura, Y.; Kunert, R.; Zhu, P.; Wormald, M.R.; Stanfield, R.L.; Roux, K.H.; et al. Antibody domain exchange is an immunological solution to carbohydrate cluster recognition. Science 2003, 300, 2065–2071. [Google Scholar] [CrossRef] [PubMed]
- Trkola, A.; Pomales, A.B.; Yuan, H.; Korber, B.; Maddon, P.J.; Allaway, G.P.; Katinger, H.; Barbas, C.F., 3rd; Burton, D.R.; Ho, D.D.; et al. Cross-clade neutralization of primary isolates of human immunodeficiency virus type 1 by human monoclonal antibodies and tetrameric cd4-igg. J. Virol. 1995, 69, 6609–6617. [Google Scholar] [PubMed]
- Huskens, D.; van Laethem, K.; Vermeire, K.; Balzarini, J.; Schols, D. Resistance of HIV-1 to the broadly HIV-1-neutralizing, anti-carbohydrate antibody 2g12. Virology 2007, 360, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Férir, G.; Huskens, D.; Palmer, K.E.; Boudreaux, D.M.; Swanson, M.D.; Markovitz, D.M.; Balzarini, J.; Schols, D. Combinations of griffithsin with other carbohydrate-binding agents demonstrate superior activity against HIV type 1, HIV type 2, and selected carbohydrate-binding agent-resistant HIV type 1 strains. AIDS Res. Hum. Retrovir. 2012, 28, 1513–1523. [Google Scholar] [CrossRef] [PubMed]
- Sanders, R.W.; Venturi, M.; Schiffner, L.; Kalyanaraman, R.; Katinger, H.; Lloyd, K.O.; Kwong, P.D.; Moore, J.P. The mannose-dependent epitope for neutralizing antibody 2g12 on human immunodeficiency virus type 1 glycoprotein gp120. J. Virol. 2002, 76, 7293–7305. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Xu, X.; Bishop, A.; Jones, I.M. Reintroduction of the 2g12 epitope in an HIV-1 clade c gp120. AIDS 2005, 19, 833–835. [Google Scholar] [CrossRef] [PubMed]
- Huskens, D.; Férir, G.; Vermeire, K.; Kehr, J.C.; Balzarini, J.; Dittmann, E.; Schols, D. Microvirin, a novel {alpha}(1,2)-mannose-specific lectin isolated from microcystis aeruginosa, has comparable anti-HIV-1 activity as cyanovirin-n, but a much higher safety profile. J. Biol. Chem. 2010, 285, 24845–24854. [Google Scholar] [CrossRef] [PubMed]
- Férir, G.; Huskens, D.; Noppen, S.; Koharudin, L.M.; Gronenborn, A.M.; Schols, D. Broad anti-HIV activity of the oscillatoria agardhii agglutinin homologue lectin family. J. Antimicrob. Chemother. 2014, 69, 2746–2758. [Google Scholar] [CrossRef] [PubMed]
- Kaku, H.; van Damme, E.J.; Peumans, W.J.; Goldstein, I.J. Carbohydrate-binding specificity of the daffodil (Narcissus pseudonarcissus) and amaryllis (Hippeastrum hybr.) bulb lectins. Arch. Biochem. Biophys. 1990, 279, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Hoorelbeke, B.; Huskens, D.; Férir, G.; François, K.; Takahashi, A.; van Laethem, K.; Schols, D.; Tanaka, H.; Balzarini, J. Actinohivin, a broadly neutralizing prokaryotic lectin, inhibits HIV-1 infection by specically targeting high-mannose-type glycans on the gp120 envelope. Antimicrob. Agents Chemother. 2010, 54, 3287–3301. [Google Scholar] [CrossRef] [PubMed]
- Férir, G.; Vermeire, K.; Huskens, D.; Balzarini, J.; van Damme, E.J.; Kehr, J.C.; Dittmann, E.; Swanson, M.D.; Markovitz, D.M.; Schols, D. Synergistic in vitro anti-HIV type 1 activity of tenofovir with carbohydrate-binding agents (cbas). Antivir. Res. 2011, 90, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Pollicita, M.; Schols, D.; Aquaro, S.; Peumans, W.J.; van Damme, E.J.; Perno, C.F.; Balzarini, J. Carbohydrate-binding agents (cbas) inhibit HIV-1 infection in human primary monocyte-derived macrophages (mdms) and efficiently prevent mdm-directed viral capture and subsequent transmission to cd4+ t lymphocytes. Virology 2008, 370, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Balzarini, J.; Hatse, S.; Vermeire, K.; Princen, K.; Aquaro, S.; Perno, C.F.; de Clercq, E.; Egberink, H.; Vanden Mooter, G.; Peumans, W.; et al. Mannose-specific plant lectins from the amaryllidaceae family qualify as efficient microbicides for prevention of human immunodeficiency virus infection. Antimicrob. Agents Chemother. 2004, 48, 3858–3870. [Google Scholar] [CrossRef] [PubMed]
- Balzarini, J.; Schols, D.; Neyts, J.; van Damme, E.; Peumans, W.; de Clercq, E. Alpha-(1–3)- and alpha-(1–6)-d-mannose-specific plant lectins are markedly inhibitory to human immunodeficiency virus and cytomegalovirus infections in vitro. Antimicrob. Agents Chemother. 1991, 35, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Balzarini, J.; van Laethem, K.; Hatse, S.; Froeyen, M.; Peumans, W.; van Damme, E.; Schols, D. Carbohydrate-binding agents cause deletions of highly conserved glycosylation sites in HIV gp120: A new therapeutic concept to hit the achilles heel of HIV. J. Biol. Chem. 2005, 280, 41005–41014. [Google Scholar] [CrossRef] [PubMed]
- Balzarini, J.; van Laethem, K.; Hatse, S.; Froeyen, M.; van Damme, E.; Bolmstedt, A.; Peumans, W.; de Clercq, E.; Schols, D. Marked depletion of glycosylation sites in HIV-1 gp120 under selection pressure by the mannose-specific plant lectins of hippeastrum hybrid and galanthus nivalis. Mol. Pharmacol. 2005, 67, 1556–1565. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, N.; Goldstein, I.J.; van Damme, E.J.; Peumans, W.J. Binding properties of a mannose-specific lectin from the snowdrop (galanthus nivalis) bulb. J. Biol. Chem. 1988, 263, 728–734. [Google Scholar] [PubMed]
- Chiba, H.; Inokoshi, J.; Okamoto, M.; Asanuma, S.; Matsuzaki, K.; Iwama, M.; Mizumoto, K.; Tanaka, H.; Oheda, M.; Fujita, K.; et al. Actinohivin, a novel anti-HIV protein from an actinomycete that inhibits syncytium formation: Isolation, characterization, and biological activities. Biochem. Biophys. Res. Commun. 2001, 282, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Chiba, H.; Inokoshi, J.; Kuno, A.; Sugai, T.; Takahashi, A.; Ito, Y.; Tsunoda, M.; Suzuki, K.; Takenaka, A.; et al. Mechanism by which the lectin actinohivin blocks HIV infection of target cells. Proc. Natl. Acad. Sci. USA 2009, 106, 15633–15638. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Inokoshi, J.; Hachiya, A.; Oka, S.; Omura, S.; Tanaka, H. The high mannose-type glycan binding lectin actinohivin: Dimerization greatly improves anti-HIV activity. J. Antibiot. (Tokyo) 2011, 64, 551–557. [Google Scholar] [CrossRef]
- Takahashi, A.; Inokoshi, J.; Tsunoda, M.; Suzuki, K.; Takenaka, A.; Sekiguchi, T.; Omura, S.; Tanaka, H. Actinohivin: Specific amino acid residues essential for anti-HIV activity. J. Antibiot. (Tokyo) 2010, 63, 661–665. [Google Scholar] [CrossRef]
- Chiba, H.; Inokoshi, J.; Nakashima, H.; Omura, S.; Tanaka, H. Actinohivin, a novel anti-human immunodeficiency virus protein from an actinomycete, inhibits viral entry to cells by binding high-mannose type sugar chains of gp120. Biochem. Biophys. Res. Commun. 2004, 316, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Boyd, M.R.; Gustafson, K.R.; McMahon, J.B.; Shoemaker, R.H.; O’Keefe, B.R.; Mori, T.; Gulakowski, R.J.; Wu, L.; Rivera, M.I.; Laurencot, C.M.; et al. Discovery of cyanovirin-n, a novel human immunodeficiency virus-inactivating protein that binds viral surface envelope glycoprotein gp120: Potential applications to microbicide development. Antimicrob. Agents Chemother. 1997, 41, 1521–1530. [Google Scholar] [PubMed]
- Bewley, C.A.; Gustafson, K.R.; Boyd, M.R.; Covell, D.G.; Bax, A.; Clore, G.M.; Gronenborn, A.M. Solution structure of cyanovirin-N, a potent HIV-inactivating protein. Nat. Struct. Biol. 1998, 5, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Bewley, C.A.; Louis, J.M.; Gustafson, K.R.; Boyd, M.R.; Gronenborn, A.M.; Clore, G.M.; Wlodawer, A. Crystal structure of cyanovirin-N, a potent HIV-inactivating protein, shows unexpected domain swapping. J. Mol. Biol. 1999, 288, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, L.G.; Louis, J.M.; Botos, I.; Mori, T.; Han, Z.; O’Keefe, B.R.; Boyd, M.R.; Wlodawer, A.; Gronenborn, A.M. The domain-swapped dimer of cyanovirin-N is in a metastable folded state: Reconciliation of X-ray and nmr structures. Structure 2002, 10, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Bewley, C.A.; Otero-Quintero, S. The potent anti-HIV protein cyanovirin-n contains two novel carbohydrate binding sites that selectively bind to man(8) d1d3 and man(9) with nanomolar affinity: Implications for binding to the HIV envelope protein gp120. J. Am. Chem. Soc. 2001, 123, 3892–3902. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, S.R.; O’Keefe, B.R.; Bolmstedt, A.J.; Cartner, L.K.; Boyd, M.R. Selective interactions of the human immunodeficiency virus-inactivating protein cyanovirin-n with high-mannose oligosaccharides on gp120 and other glycoproteins. J. Pharmacol. Exp. Ther. 2001, 297, 704–710. [Google Scholar] [PubMed]
- Botos, I.; O’Keefe, B.R.; Shenoy, S.R.; Cartner, L.K.; Ratner, D.M.; Seeberger, P.H.; Boyd, M.R.; Wlodawer, A. Structures of the complexes of a potent anti-HIV protein cyanovirin-N and high mannose oligosaccharides. J. Biol. Chem. 2002, 277, 34336–34342. [Google Scholar] [CrossRef] [PubMed]
- Balzarini, J.; van Laethem, K.; Peumans, W.J.; van Damme, E.J.; Bolmstedt, A.; Gago, F.; Schols, D. Mutational pathways, resistance profile, and side effects of cyanovirin relative to human immunodeficiency virus type 1 strains with N-glycan deletions in their gp120 envelopes. J. Virol. 2006, 80, 8411–8421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buffa, V.; Stieh, D.; Mamhood, N.; Hu, Q.; Fletcher, P.; Shattock, R.J. Cyanovirin-N potently inhibits human immunodeficiency virus type 1 infection in cellular and cervical explant models. J. Gen. Virol. 2009, 90, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Mahmood, N.; Shattock, R.J. High-mannose-specific deglycosylation of HIV-1 gp120 induced by resistance to cyanovirin-N and the impact on antibody neutralization. Virology 2007, 368, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Kehr, J.C.; Zilliges, Y.; Springer, A.; Disney, M.D.; Ratner, D.D.; Bouchier, C.; Seeberger, P.H.; de Marsac, N.T.; Dittmann, E. A mannan binding lectin is involved in cell-cell attachment in a toxic strain of microcystis aeruginosa. Mol. Microbiol. 2006, 59, 893–906. [Google Scholar] [CrossRef] [PubMed]
- Shahzad-ul-Hussan, S.; Gustchina, E.; Ghirlando, R.; Clore, G.M.; Bewley, C.A. Solution structure of the monovalent lectin microvirin in complex with man(alpha)(1–2)man provides a basis for anti-HIV activity with low toxicity. J. Biol. Chem. 2011, 286, 20788–20796. [Google Scholar] [CrossRef] [PubMed]
- Koshte, V.L.; van Dijk, W.; van der Stelt, M.E.; Aalberse, R.C. Isolation and characterization of banlec-I, a mannoside-binding lectin from musa paradisiac (banana). Biochem. J. 1990, 272, 721–726. [Google Scholar] [PubMed]
- Peumans, W.J.; Zhang, W.; Barre, A.; Houles Astoul, C.; Balint-Kurti, P.J.; Rovira, P.; Rouge, P.; May, G.D.; van Leuven, F.; Truffa-Bachi, P.; et al. Fruit-specific lectins from banana and plantain. Planta 2000, 211, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Meagher, J.L.; Winter, H.C.; Ezell, P.; Goldstein, I.J.; Stuckey, J.A. Crystal structure of banana lectin reveals a novel second sugar binding site. Glycobiology 2005, 15, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Mo, H.; Winter, H.C.; van Damme, E.J.; Peumans, W.J.; Misaki, A.; Goldstein, I.J. Carbohydrate binding properties of banana (musa acuminata) lectin I. Novel recognition of internal alpha1,3-linked glucosyl residues. Eur. J. Biochem. 2001, 268, 2609–2615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldstein, I.J.; Winter, H.C.; Mo, H.; Misaki, A.; van Damme, E.J.; Peumans, W.J. Carbohydrate binding properties of banana (musa acuminata) lectin II. Binding of laminaribiose oligosaccharides and beta-glucans containing beta1,6-glucosyl end groups. Eur. J. Biochem. 2001, 268, 2616–2619. [Google Scholar] [CrossRef] [PubMed]
- Swanson, M.D.; Winter, H.C.; Goldstein, I.J.; Markovitz, D.M. A lectin isolated from bananas is a potent inhibitor of HIV replication. J. Biol. Chem. 2010, 285, 8646–8655. [Google Scholar] [CrossRef] [PubMed]
- Férir, G.; Palmer, K.E.; Schols, D. Griffithsin, alone and combined with all classes of antiretroviral drugs, potently inhibits HIV cell-cell transmission and destruction of cd4+ t cells. J. Antivir. Antiretrovir. 2012, 4, 103–112. [Google Scholar] [CrossRef]
- Mori, T.; O’Keefe, B.R.; Sowder, R.C., 2nd; Bringans, S.; Gardella, R.; Berg, S.; Cochran, P.; Turpin, J.A.; Buckheit, R.W., Jr.; McMahon, J.B.; et al. Isolation and characterization of griffithsin, a novel HIV-inactivating protein, from the red alga Griffithsia sp. J. Biol. Chem. 2005, 280, 9345–9353. [Google Scholar] [CrossRef] [PubMed]
- Ziółkowska, N.E.; O’Keefe, B.R.; Mori, T.; Zhu, C.; Giomarelli, B.; Vojdani, F.; Palmer, K.E.; McMahon, J.B.; Wlodawer, A. Domain-swapped structure of the potent antiviral protein griffithsin and its mode of carbohydrate binding. Structure 2006, 14, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Ziółkowska, N.E.; Shenoy, S.R.; O’Keefe, B.R.; Wlodawer, A. Crystallographic studies of the complexes of antiviral protein griffithsin with glucose and N-acetylglucosamine. Protein Sci. 2007, 16, 1485–1489. [Google Scholar] [CrossRef] [PubMed]
- Ziółkowska, N.E.; Shenoy, S.R.; O’Keefe, B.R.; McMahon, J.B.; Palmer, K.E.; Dwek, R.A.; Wormald, M.R.; Wlodawer, A. Crystallographic, thermodynamic, and molecular modeling studies of the mode of binding of oligosaccharides to the potent antiviral protein griffithsin. Proteins 2007, 67, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Moulaei, T.; Shenoy, S.R.; Giomarelli, B.; Thomas, C.; McMahon, J.B.; Dauter, Z.; O’Keefe, B.R.; Wlodawer, A. Monomerization of viral entry inhibitor griffithsin elucidates the relationship between multivalent binding to carbohydrates and anti-HIV activity. Structure 2010, 18, 1104–1115. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Hoorelbeke, B.; Kagiampakis, I.; Demeler, B.; Balzarini, J.; Liwang, P.J. The griffithsin dimer is required for high-potency inhibition of HIV-1: Evidence for manipulation of the structure of gp120 as part of the griffithsin dimer mechanism. Antimicrob. Agents Chemother. 2013, 57, 3976–3989. [Google Scholar] [CrossRef] [PubMed]
- Emau, P.; Tian, B.; O’Keefe B, R.; Mori, T.; McMahon, J.B.; Palmer, K.E.; Jiang, Y.; Bekele, G.; Tsai, C.C. Griffithsin, a potent hiv entry inhibitor, is an excellent candidate for anti-HIV microbicide. J. Med. Primatol. 2007, 36, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Jin, W.; Griffin, G.E.; Shattock, R.J.; Hu, Q. Removal of two high-mannose N-linked glycans on gp120 renders human immunodeficiency virus 1 largely resistant to the carbohydrate-binding agent griffithsin. J. Gen. Virol. 2011, 92, 2367–2373. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, K.B.; Gray, E.S.; Lambson, B.E.; Moore, P.L.; Choge, I.A.; Mlisana, K.; Karim, S.S.; McMahon, J.; O’Keefe, B.; Chikwamba, R.; et al. Mannose-rich glycosylation patterns on HIV-1 subtype c gp120 and sensitivity to the lectins, griffithsin, cyanovirin-N and scytovirin. Virology 2010, 402, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Férir, G.; Palmer, K.E.; Schols, D. Synergistic activity profile of griffithsin in combination with tenofovir, maraviroc and enfuvirtide against HIV-1 clade c. Virology 2011, 417, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Hoorelbeke, B.; Xue, J.; LiWang, P.J.; Balzarini, J. Role of the carbohydrate-binding sites of griffithsin in the prevention of dc-sign-mediated capture and transmission of HIV-1. PLoS One 2013, 8, e64132. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Murakami, M.; Miyazawa, K.; Hori, K. Purification and characterization of a novel lectin from a freshwater cyanobacterium, oscillatoria agardhii. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2000, 125, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Okuyama, S.; Hori, K. Primary structure and carbohydrate binding specificity of a potent anti-HIV lectin isolated from the filamentous cyanobacterium oscillatoria agardhii. J. Biol. Chem. 2007, 282, 11021–11029. [Google Scholar] [CrossRef] [PubMed]
- Koharudin, L.M.; Gronenborn, A.M. Structural basis of the anti-HIV activity of the cyanobacterial oscillatoria agardhii agglutinin. Structure 2011, 19, 1170–1181. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, N.; Goldstein, I.J.; Shafer, J.A.; Peumans, W.J.; Broekaert, W.F. Carbohydrate binding properties of the stinging nettle (urtica dioica) rhizome lectin. Arch. Biochem. Biophys. 1986, 249, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Peumans, W.; de Ley, M.; Broekaert, W.F. An unusual lectin from stinging nettle (urtica dioica) rhizomes. FEBS Lett. 1984, 177, 99–103. [Google Scholar] [CrossRef]
- Balzarini, J.; Neyts, J.; Schols, D.; Hosoya, M.; van Damme, E.; Peumans, W.; de Clercq, E. The mannose-specific plant lectins from cymbidium hybrid and epipactis helleborine and the (N-acetylglucosamine)n-specific plant lectin from urtica dioica are potent and selective inhibitors of human immunodeficiency virus and cytomegalovirus replication in vitro. Antiviral Res. 1992, 18, 191–207. [Google Scholar] [CrossRef] [PubMed]
- Gordts, S.C.; Renders, M.; Férir, G.; Huskens, D.; van Damme, E.; Peumans, W.; Balzarini, J.; Schols, D. Nictaba and uda, two glcnac-binding lectins with unique anti-viral activity profile. J. Antimicrob. Chemother. 2014. submitted. [Google Scholar]
- Chen, Y.; Peumans, W.J.; Hause, B.; Bras, J.; Kumar, M.; Proost, P.; Barre, A.; Rouge, P.; van Damme, E.J. Jasmonic acid methyl ester induces the synthesis of a cytoplasmic/nuclear chito-oligosaccharide binding lectin in tobacco leaves. FASEB J. 2002, 16, 905–907. [Google Scholar] [PubMed]
- Lannoo, N.; van Damme, E.J. Expression analysis of jasmonate-responsive lectins in plants. Methods Mol. Biol. 2013, 1011, 251–263. [Google Scholar] [PubMed]
- Schouppe, D.; Ghesquiere, B.; Menschaert, G.; de Vos, W.H.; Bourque, S.; Trooskens, G.; Proost, P.; Gevaert, K.; van Damme, E.J. Interaction of the tobacco lectin with histone proteins. Plant Physiol. 2011, 155, 1091–1102. [Google Scholar] [CrossRef] [PubMed]
- Lannoo, N.; Peumans, W.J.; Pamel, E.V.; Alvarez, R.; Xiong, T.C.; Hause, G.; Mazars, C.; van Damme, E.J. Localization and in vitro binding studies suggest that the cytoplasmic/nuclear tobacco lectin can interact in situ with high-mannose and complex N-glycans. FEBS Lett. 2006, 580, 6329–6337. [Google Scholar] [CrossRef] [PubMed]
- Abdool Karim, Q.; Abdool Karim, S.S.; Frohlich, J.A.; Grobler, A.C.; Baxter, C.; Mansoor, L.E.; Kharsany, A.B.; Sibeko, S.; Mlisana, K.P.; Omar, Z.; et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science 2010, 329, 1168–1174. [Google Scholar] [CrossRef] [PubMed]
- Reitter, J.N.; Means, R.E.; Desrosiers, R.C. A role for carbohydrates in immune evasion in aids. Nat. Med. 1998, 4, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Mathys, L.; Balzarini, J. Exposure of HIV-1 to a combination of two carbohydrate-binding agents markedly delays drug resistance development and selects for virus strains with compromised fitness. J. Antimicrob. Chemother. 2014, 69, 582–593. [Google Scholar] [CrossRef] [PubMed]
- Auwerx, J.; François, K.O.; Covens, K.; van Laethem, K.; Balzarini, J. Glycan deletions in the HIV-1 gp120 v1/v2 domain compromise viral infectivity, sensitize the mutant virus strains to carbohydrate-binding agents and represent a specific target for therapeutic intervention. Virology 2008, 382, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.N.; McLellan, J.S.; Huang, W.; Orwenyo, J.; Burton, D.R.; Koff, W.C.; Kwong, P.D.; Wang, L.X. Synthetic glycopeptides reveal the glycan specificity of HIV-neutralizing antibodies. Nat. Chem. Biol. 2013, 9, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Julien, J.P.; Sok, D.; Khayat, R.; Lee, J.H.; Doores, K.J.; Walker, L.M.; Ramos, A.; Diwanji, D.C.; Pejchal, R.; Cupo, A.; et al. Broadly neutralizing antibody pgt121 allosterically modulates cd4 binding via recognition of the HIV-1 gp120 v3 base and multiple surrounding glycans. PLoS Pathog. 2013, 9, e1003342. [Google Scholar] [CrossRef] [PubMed]
- Mouquet, H.; Scharf, L.; Euler, Z.; Liu, Y.; Eden, C.; Scheid, J.F.; Halper-Stromberg, A.; Gnanapragasam, P.N.P.; Spencer, D.I.R.; Seaman, M.S.; et al. Complex-type N-glycan recognition by potent broadly neutralizing HIV antibodies. Proc. Natl. Acad. Sci. USA 2012, 109, E3268–E3277. [Google Scholar] [CrossRef] [PubMed]
- François, K.O.; Balzarini, J. The highly conserved glycan at asparagine 260 of HIV-1 gp120 is indispensable for viral entry. J. Biol. Chem. 2011, 286, 42900–42910. [Google Scholar] [CrossRef] [PubMed]
- Willey, R.L.; Smith, D.H.; Lasky, L.A.; Theodore, T.S.; Earl, P.L.; Moss, B.; Capon, D.J.; Martin, M.A. In vitro mutagenesis identifies a region within the envelope gene of the human immunodeficiency virus that is critical for infectivity. J. Virol. 1988, 62, 139–147. [Google Scholar] [PubMed]
- Mathys, L.; François, K.O.; Quandte, M.; Braakman, I.; Balzarini, J. Deletion of the highly conserved n-glycan at asn260 of HIV-1 gp120 affects folding and lysosomal degradation of gp120, and results in loss of viral infectivity. PLoS One 2014, 9, e101181. [Google Scholar] [CrossRef] [PubMed]
- Petrova, M.I.; van den Broek, M.; Balzarini, J.; Vanderleyden, J.; Lebeer, S. Vaginal microbiota and its role in HIV transmission and infection. FEMS Microbiol. Rev. 2013, 37, 762–792. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.C.; Emau, P.; Jiang, Y.; Agy, M.B.; Shattock, R.J.; Schmidt, A.; Morton, W.R.; Gustafson, K.R.; Boyd, M.R. Cyanovirin-N inhibits aids virus infections in vaginal transmission models. AIDS Res. Hum. Retrovir. 2004, 20, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.C.; Emau, P.; Jiang, Y.; Tian, B.; Morton, W.R.; Gustafson, K.R.; Boyd, M.R. Cyanovirin-N gel as a topical microbicide prevents rectal transmission of shiv89.6p in macaques. AIDS Res. Hum. Retrovir. 2003, 19, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Lagenaur, L.A.; Sanders-Beer, B.E.; Brichacek, B.; Pal, R.; Liu, X.; Liu, Y.; Yu, R.; Venzon, D.; Lee, P.P.; Hamer, D.H. Prevention of vaginal shiv transmission in macaques by a live recombinant lactobacillus. Mucosal Immunol. 2011, 4, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Brichacek, B.; Lagenaur, L.A.; Lee, P.P.; Venzon, D.; Hamer, D.H. In vivo evaluation of safety and toxicity of a lactobacillus jensenii producing modified cyanovirin-n in a rhesus macaque vaginal challenge model. PLoS One 2013, 8, e78817. [Google Scholar] [CrossRef] [PubMed]
- Smee, D.F.; Bailey, K.W.; Wong, M.H.; O’Keefe, B.R.; Gustafson, K.R.; Mishin, V.P.; Gubareva, L.V. Treatment of influenza a (h1n1) virus infections in mice and ferrets with cyanovirin-N. Antivir. Res. 2008, 80, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Global Alert and Response (GAR). Available online: http://www.who.int/csr/disease/ebola/en/ (accessed on 23 September 2014).
- Barrientos, L.G.; O’Keefe, B.R.; Bray, M.; Sanchez, A.; Gronenborn, A.M.; Boyd, M.R. Cyanovirin-N binds to the viral surface glycoprotein, gp1,2 and inhibits infectivity of ebola virus. Antivir. Res. 2003, 58, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Michelow, I.C.; Lear, C.; Scully, C.; Prugar, L.I.; Longley, C.B.; Yantosca, L.M.; Ji, X.; Karpel, M.; Brudner, M.; Takahashi, K.; et al. High-dose mannose-binding lectin therapy for ebola virus infection. J. Infect. Dis. 2011, 203, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Alen, M.M.; Kaptein, S.J.; de Burghgraeve, T.; Balzarini, J.; Neyts, J.; Schols, D. Antiviral activity of carbohydrate-binding agents and the role of dc-sign in dengue virus infection. Virology 2009, 387, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Bertaux, C.; Daelemans, D.; Meertens, L.; Cormier, E.G.; Reinus, J.F.; Peumans, W.J.; van Damme, E.J.; Igarashi, Y.; Oki, T.; Schols, D.; et al. Entry of hepatitis c virus and human immunodeficiency virus is selectively inhibited by carbohydrate-binding agents but not by polyanions. Virology 2007, 366, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, V.; Shukla, S.Y.; Shukla, D. A sugar binding protein cyanovirin-N blocks herpes simplex virus type-1 entry and cell fusion. Antivir. Res. 2009, 84, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Huskens, D.; Vermeire, K.; Vandemeulebroucke, E.; Balzarini, J.; Schols, D. Safety concerns for the potential use of cyanovirin-n as a microbicidal anti-HIV agent. Int. J. Biochem. Cell Biol. 2008, 40, 2802–2814. [Google Scholar] [CrossRef] [PubMed]
- Kouokam, J.C.; Huskens, D.; Schols, D.; Johannemann, A.; Riedell, S.K.; Walter, W.; Walker, J.M.; Matoba, N.; O’Keefe, B.R.; Palmer, K.E. Investigation of griffithsin’s interactions with human cells confirms its outstanding safety and efficacy profile as a microbicide candidate. PLoS One 2011, 6, e22635. [Google Scholar] [CrossRef] [PubMed]
- Barton, C.; Kouokam, J.C.; Lasnik, A.B.; Foreman, O.; Cambon, A.; Brock, G.; Montefiori, D.C.; Vojdani, F.; McCormick, A.A.; O’Keefe, B.R.; et al. Activity of and effect of subcutaneous treatment with the broad-spectrum antiviral lectin griffithsin in two laboratory rodent models. Antimicrob. Agents Chemother. 2014, 58, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Parker, A.S.; Choi, Y.; Griswold, K.E.; Bailey-Kellogg, C. Structure-guided deimmunization of therapeutic proteins. J. Comput. Biol. 2013, 20, 152–165. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Griswold, K.E.; Bailey-Kellogg, C. Structure-based redesign of proteins for minimal T-cell epitope content. J. Comput. Chem. 2013, 34, 879–891. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Férir, G.; Gordts, S.C.; Schols, D. HIV-1 and Its Resistance to Peptidic Carbohydrate-Binding Agents (CBAs): An Overview. Molecules 2014, 19, 21085-21112. https://doi.org/10.3390/molecules191221085
Férir G, Gordts SC, Schols D. HIV-1 and Its Resistance to Peptidic Carbohydrate-Binding Agents (CBAs): An Overview. Molecules. 2014; 19(12):21085-21112. https://doi.org/10.3390/molecules191221085
Chicago/Turabian StyleFérir, Geoffrey, Stephanie C. Gordts, and Dominique Schols. 2014. "HIV-1 and Its Resistance to Peptidic Carbohydrate-Binding Agents (CBAs): An Overview" Molecules 19, no. 12: 21085-21112. https://doi.org/10.3390/molecules191221085




