Bioinspired Syntheses of Dimeric Hydroxycinnamic Acids (Lignans) and Hybrids, Using Phenol Oxidative Coupling as Key Reaction, and Medicinal Significance Thereof †
Abstract
:1. Introduction
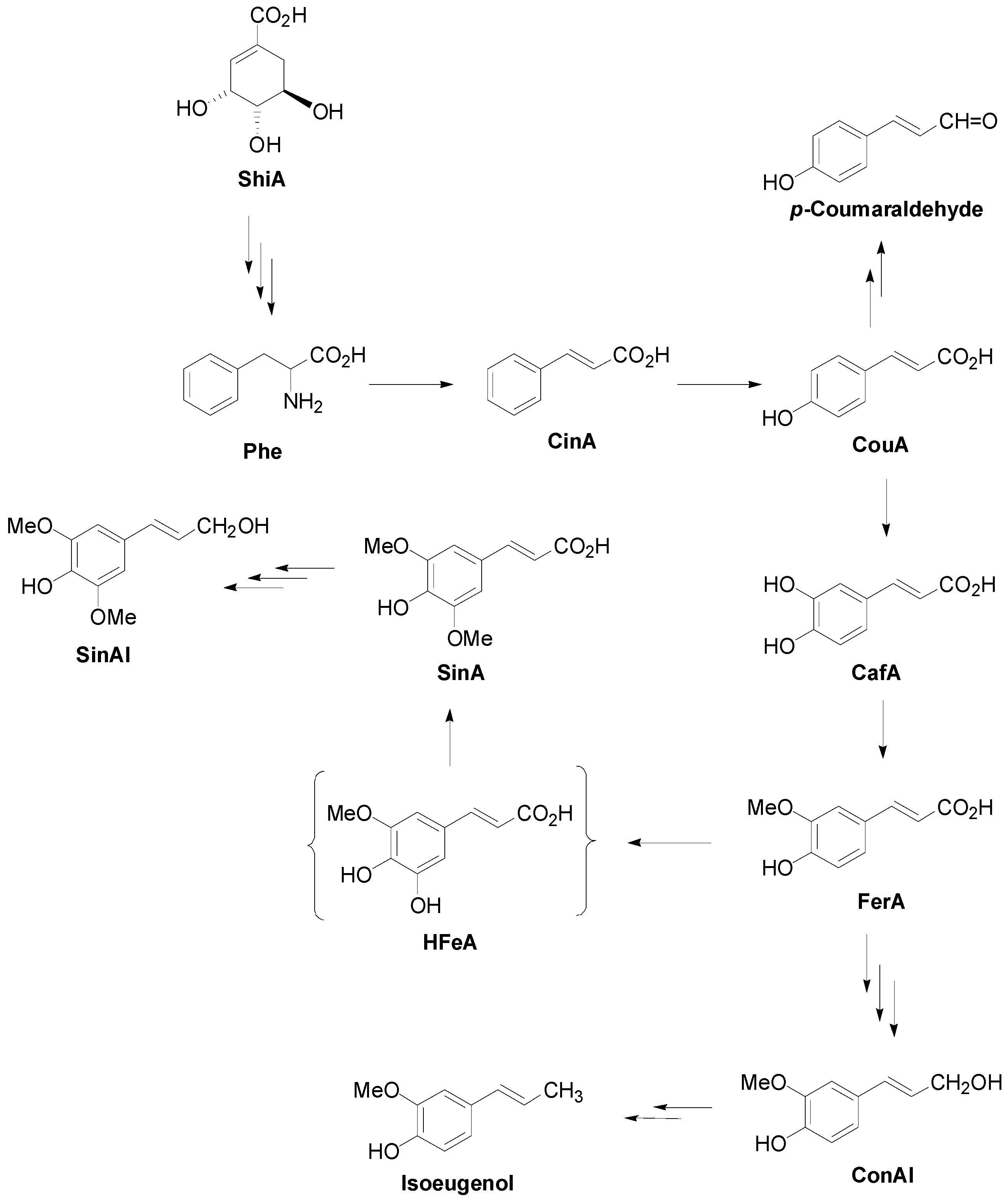
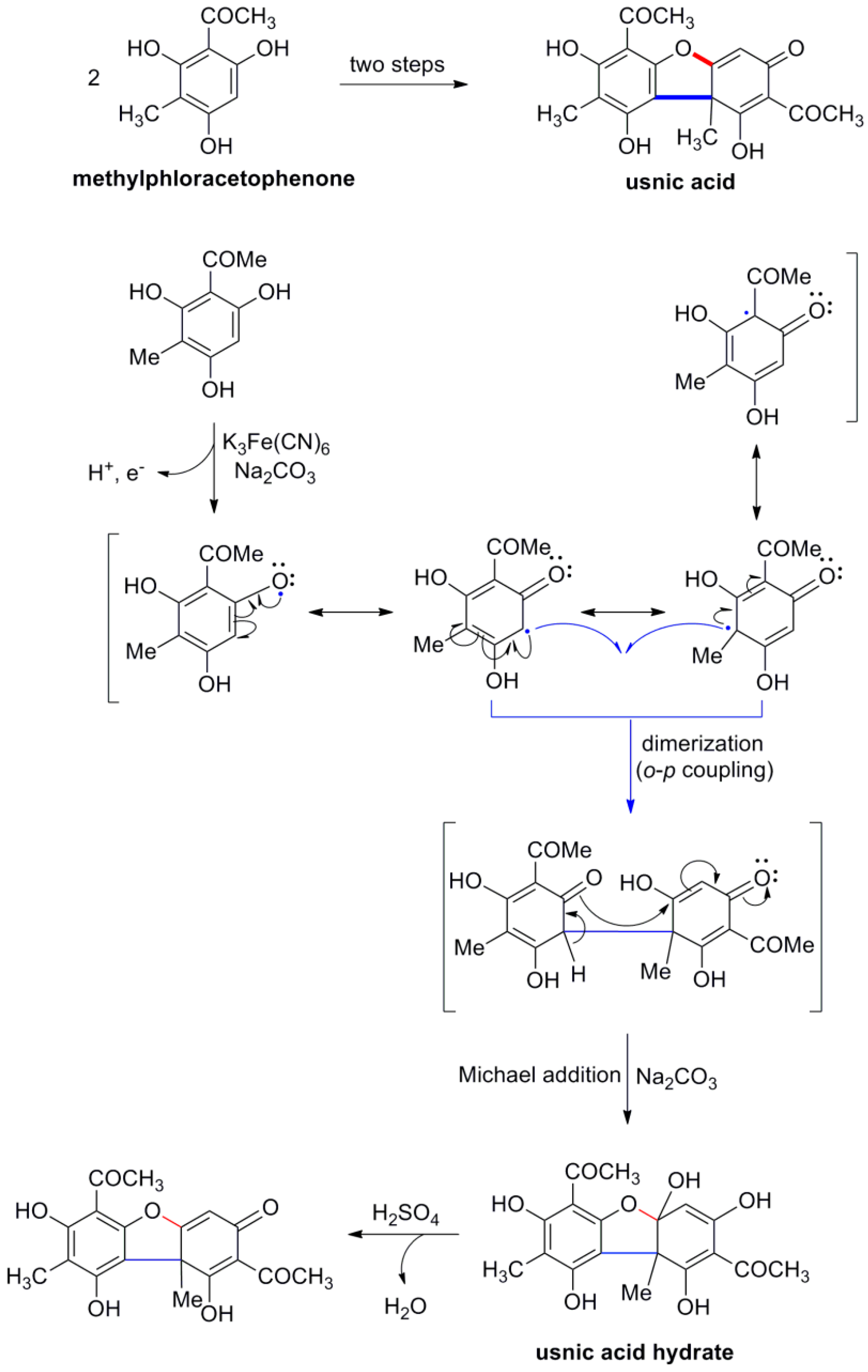
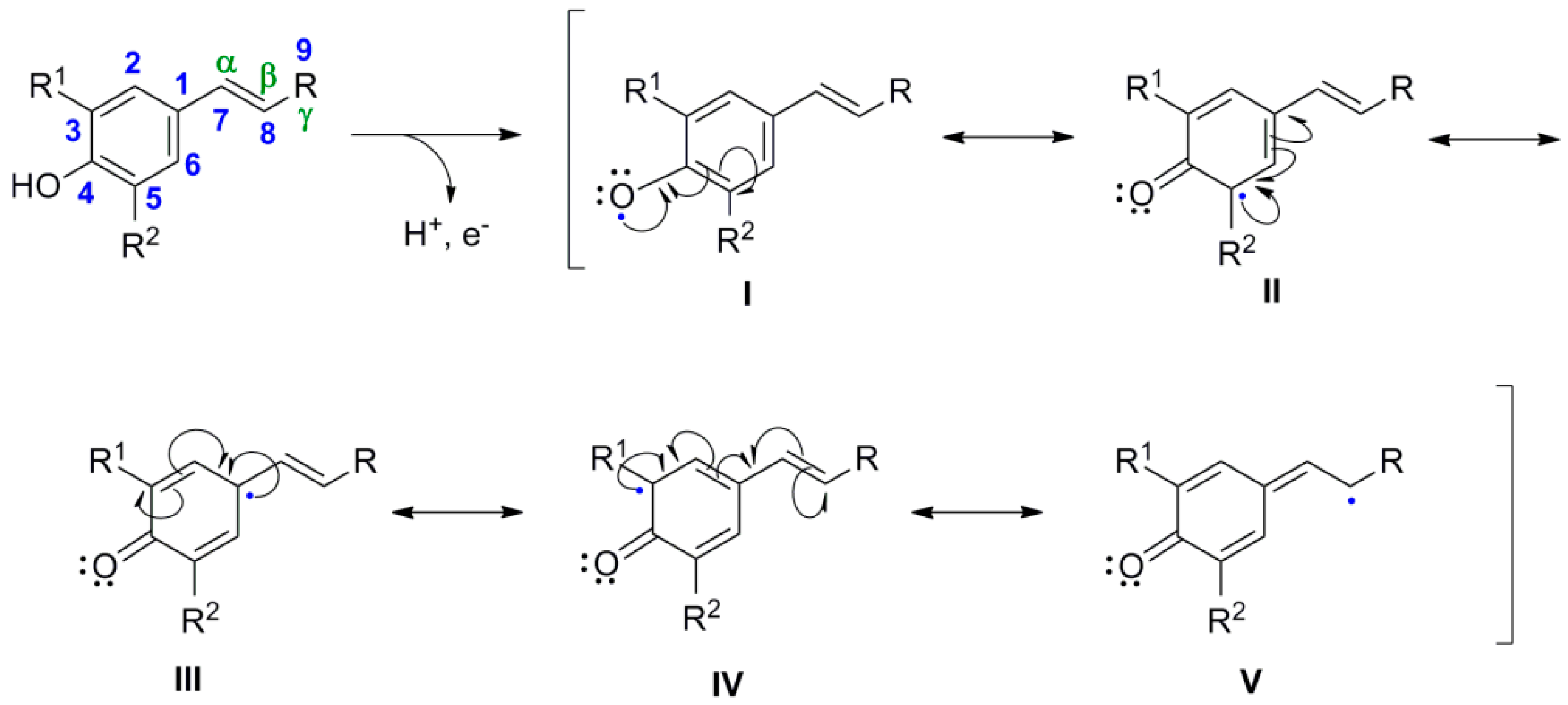

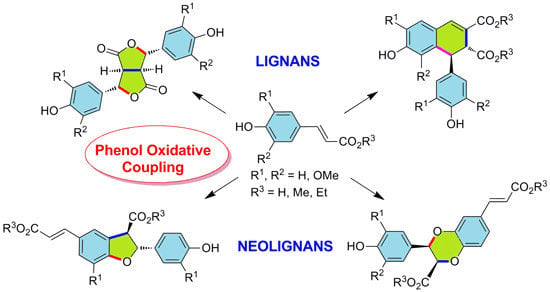
2. Lignan Skeleton Assembly with β-β Bond Formation as the Key Step
2.1. Syntheses of Lignans Based on the Key Intermediate 4-cis,8-cis-Diaryl-3,7-dioxabicyclo[3.3.0]-octane-2,6-dione



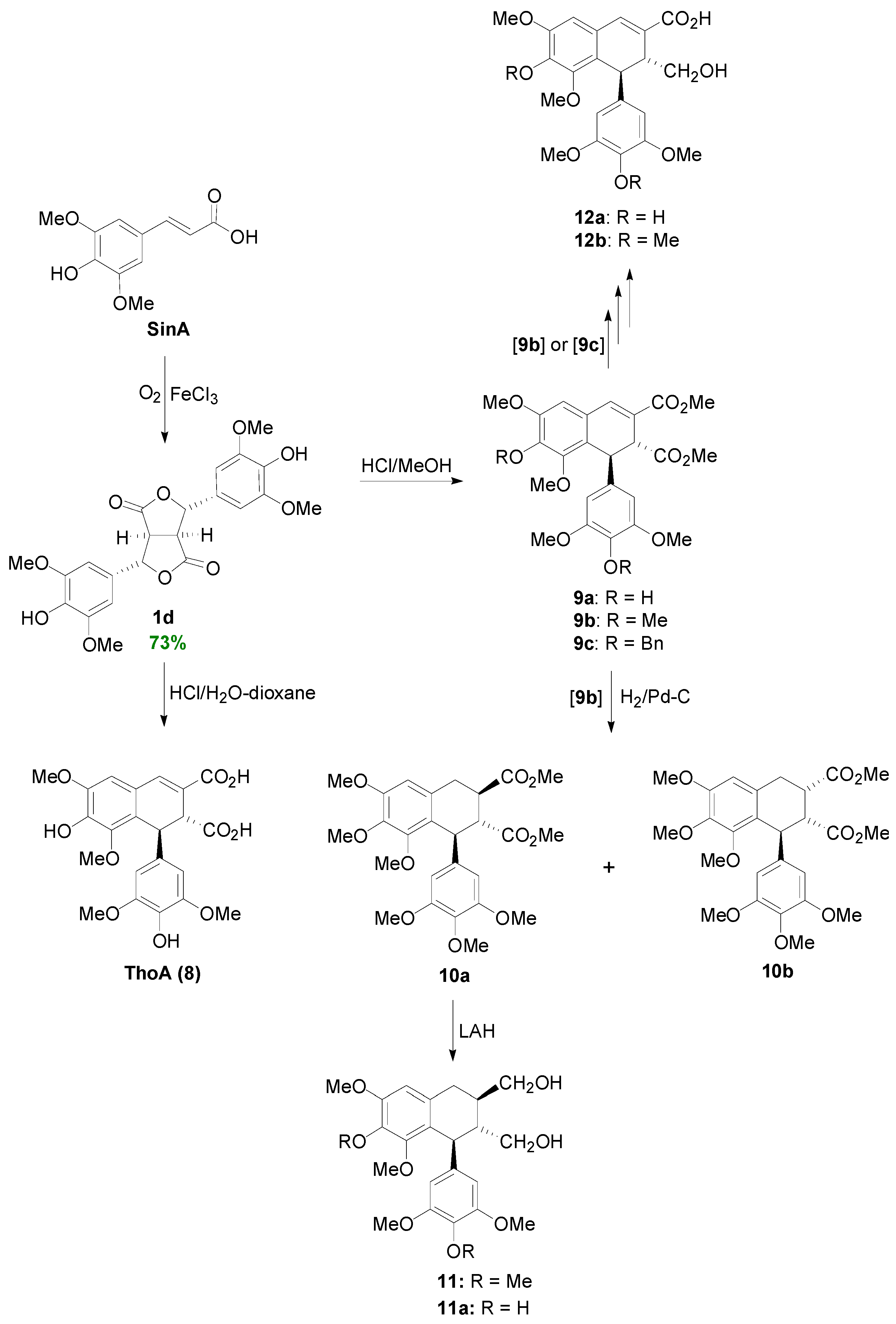
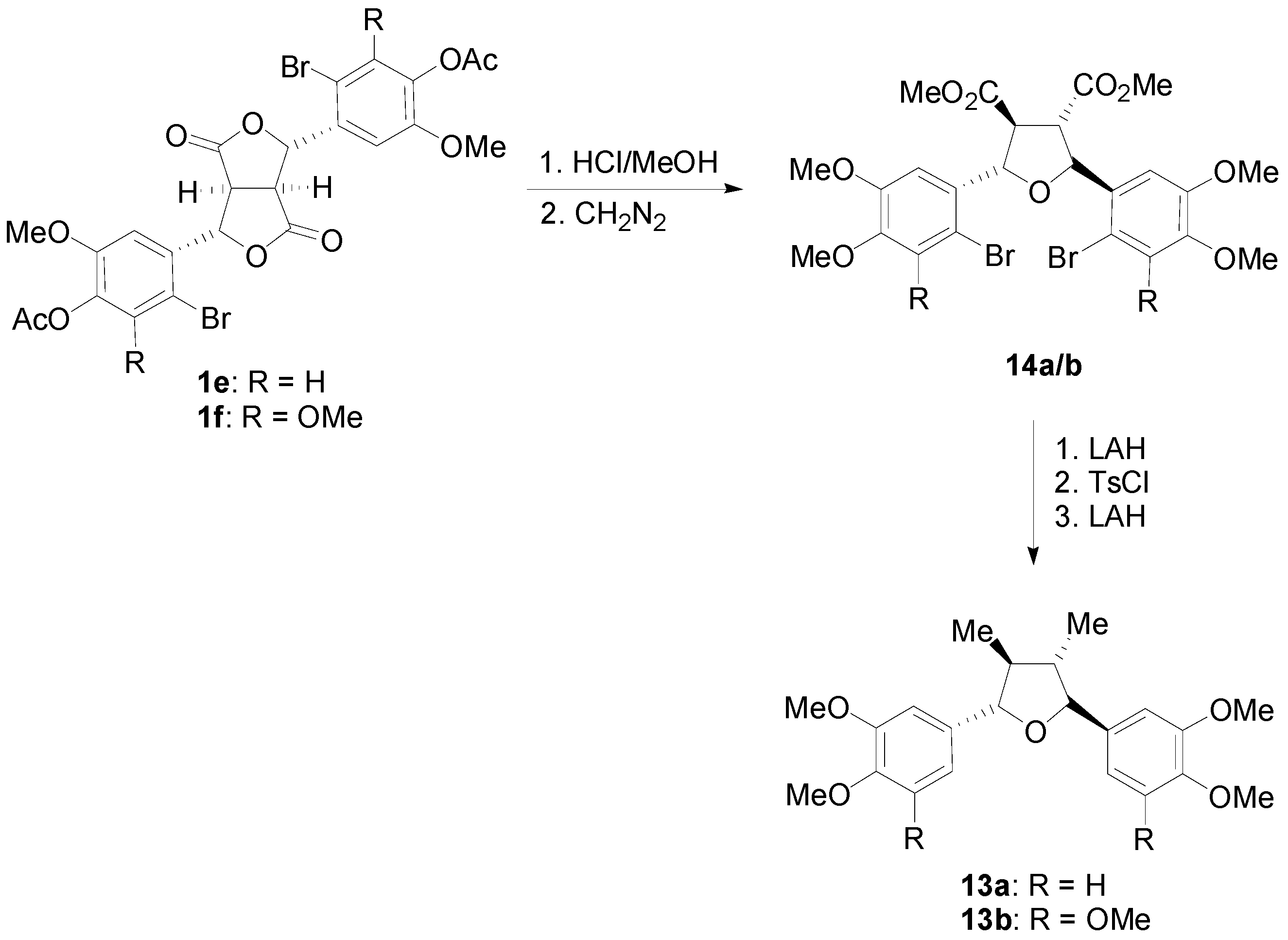

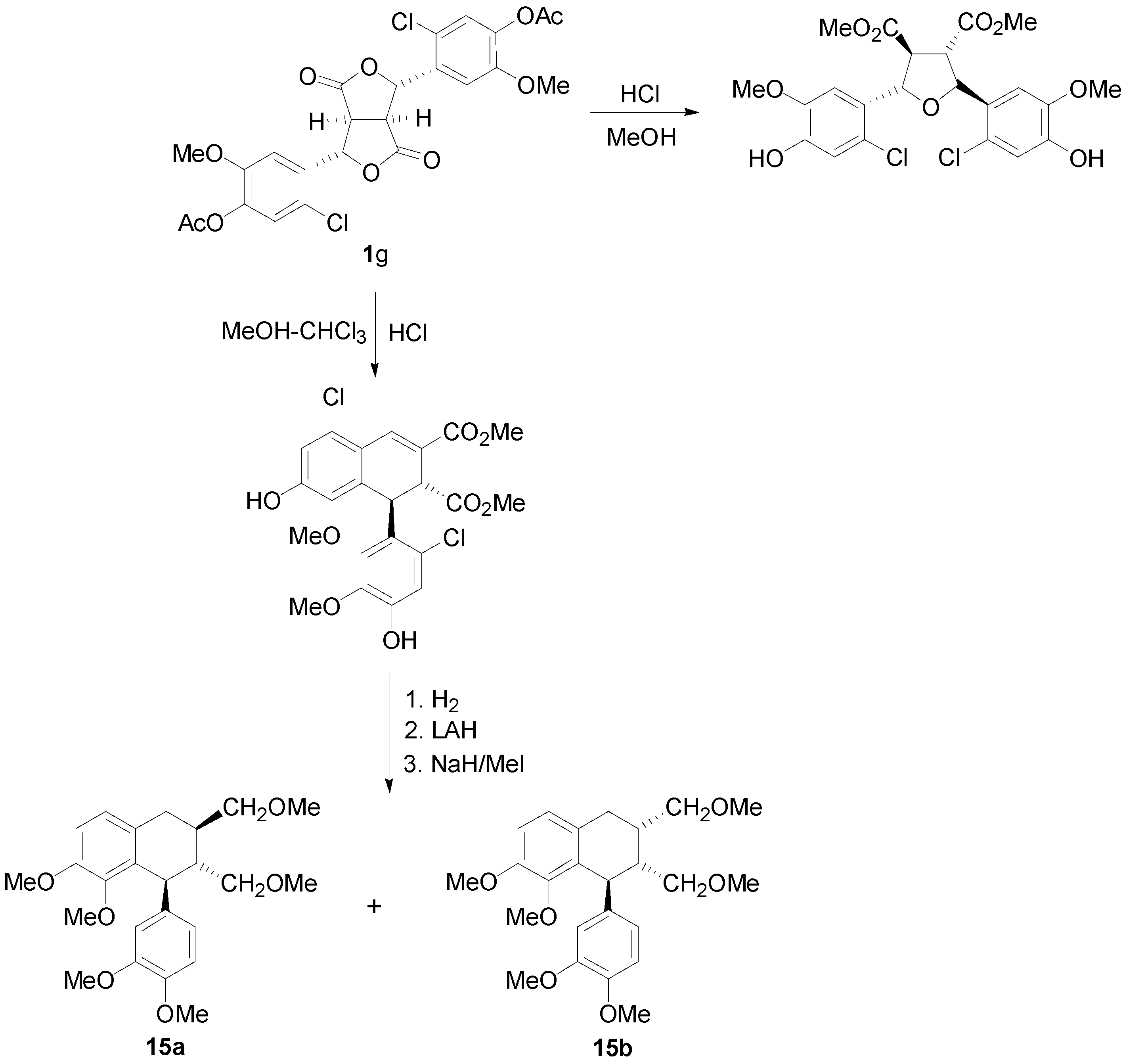
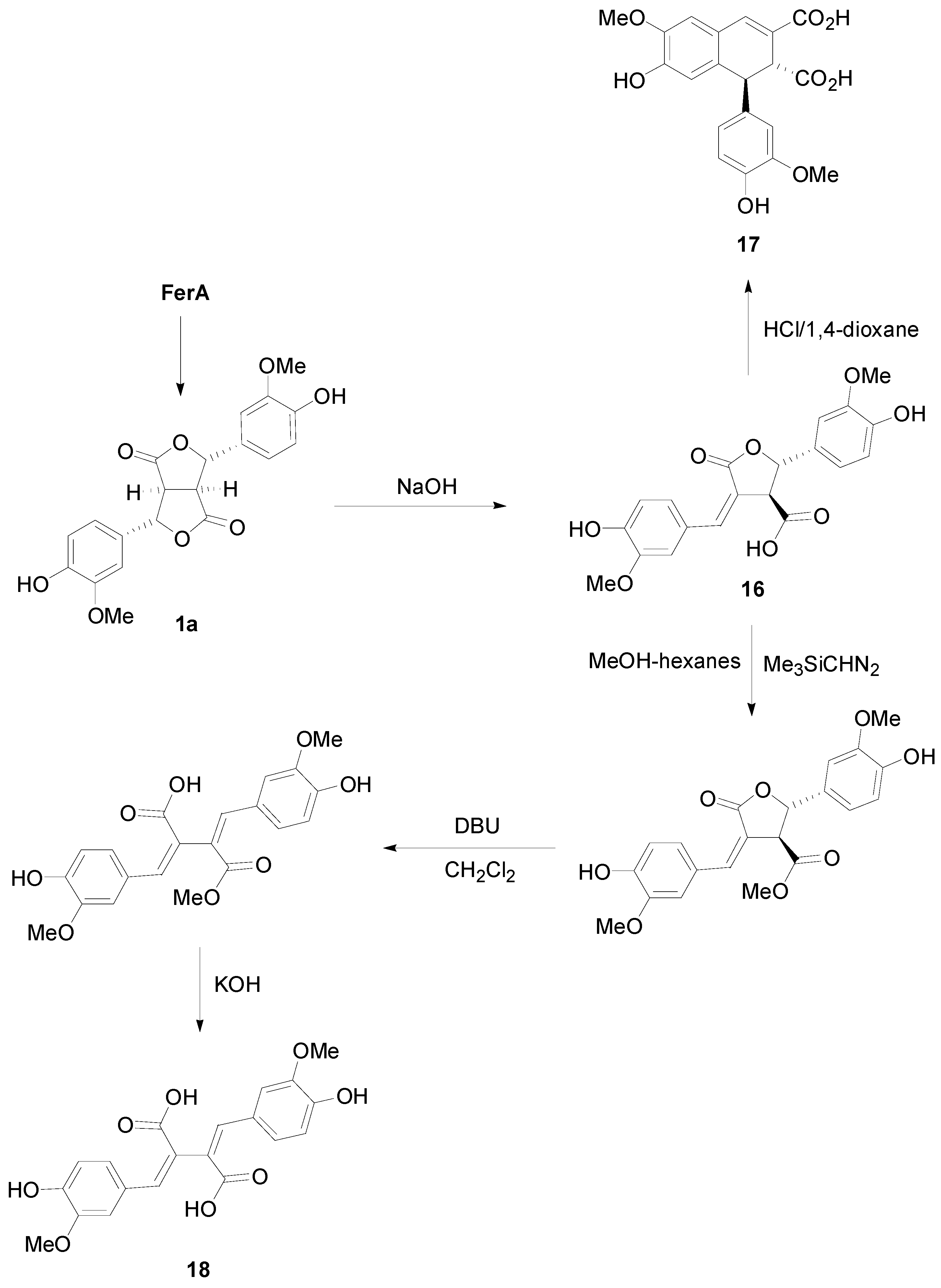
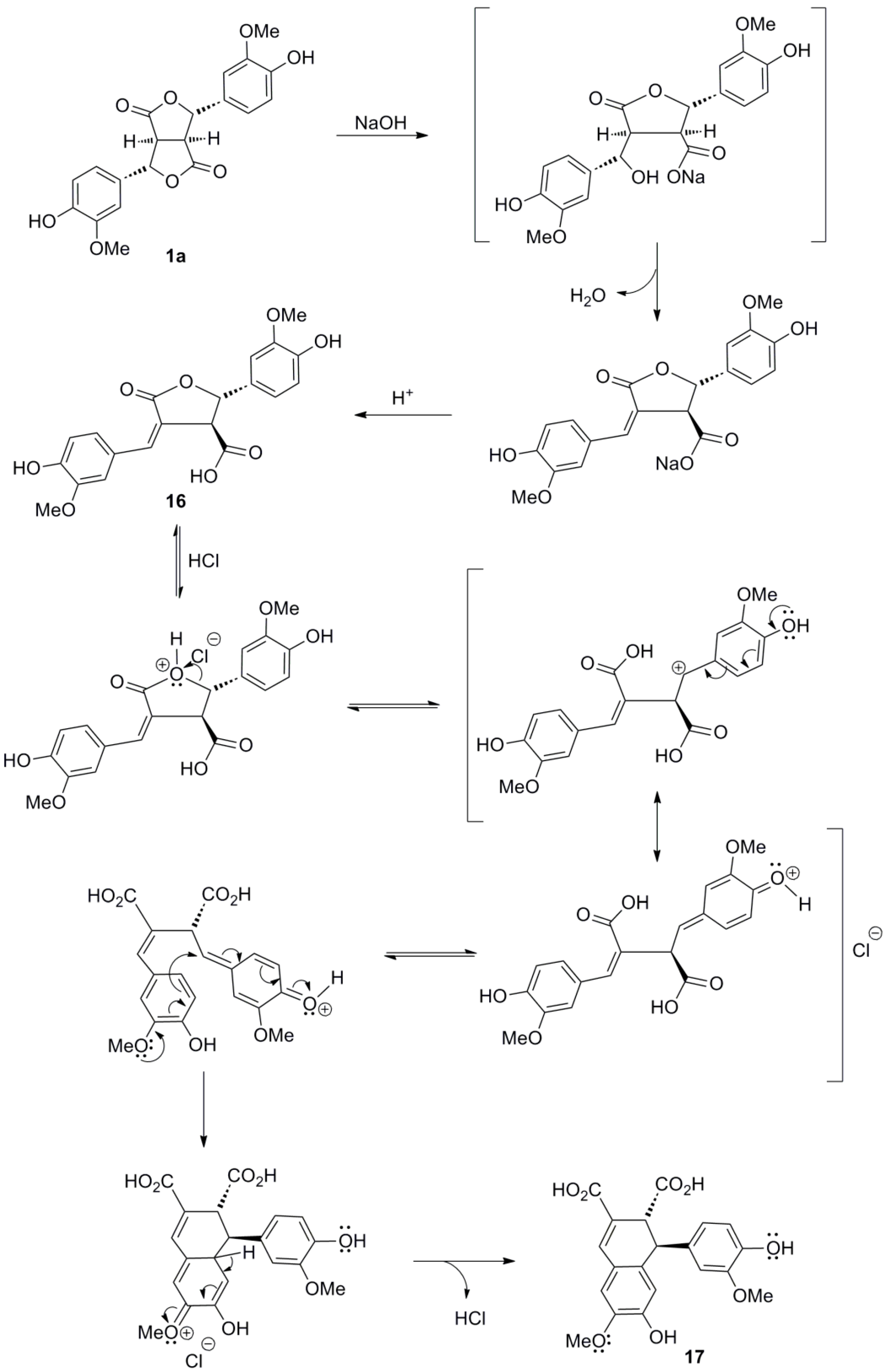
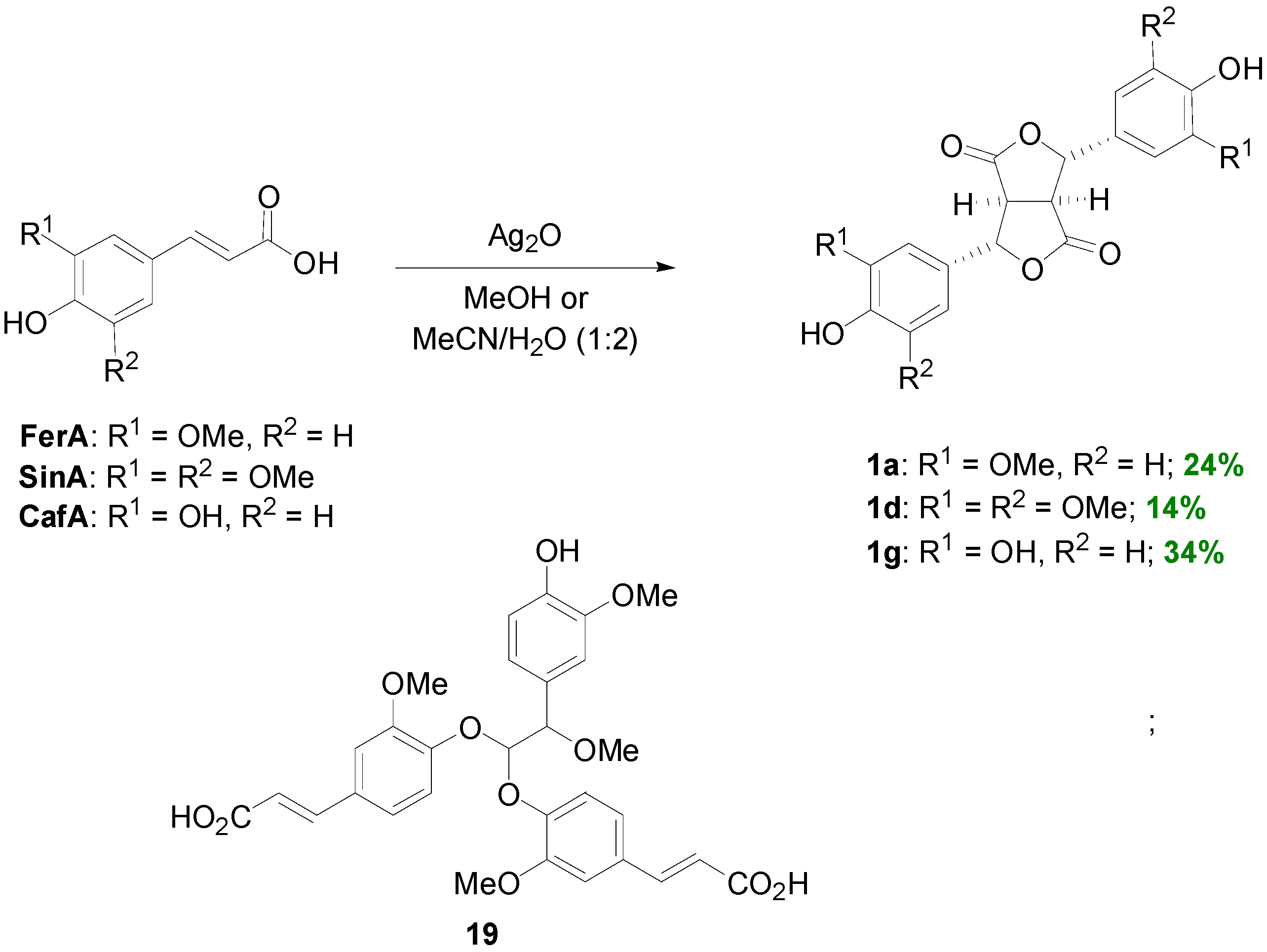
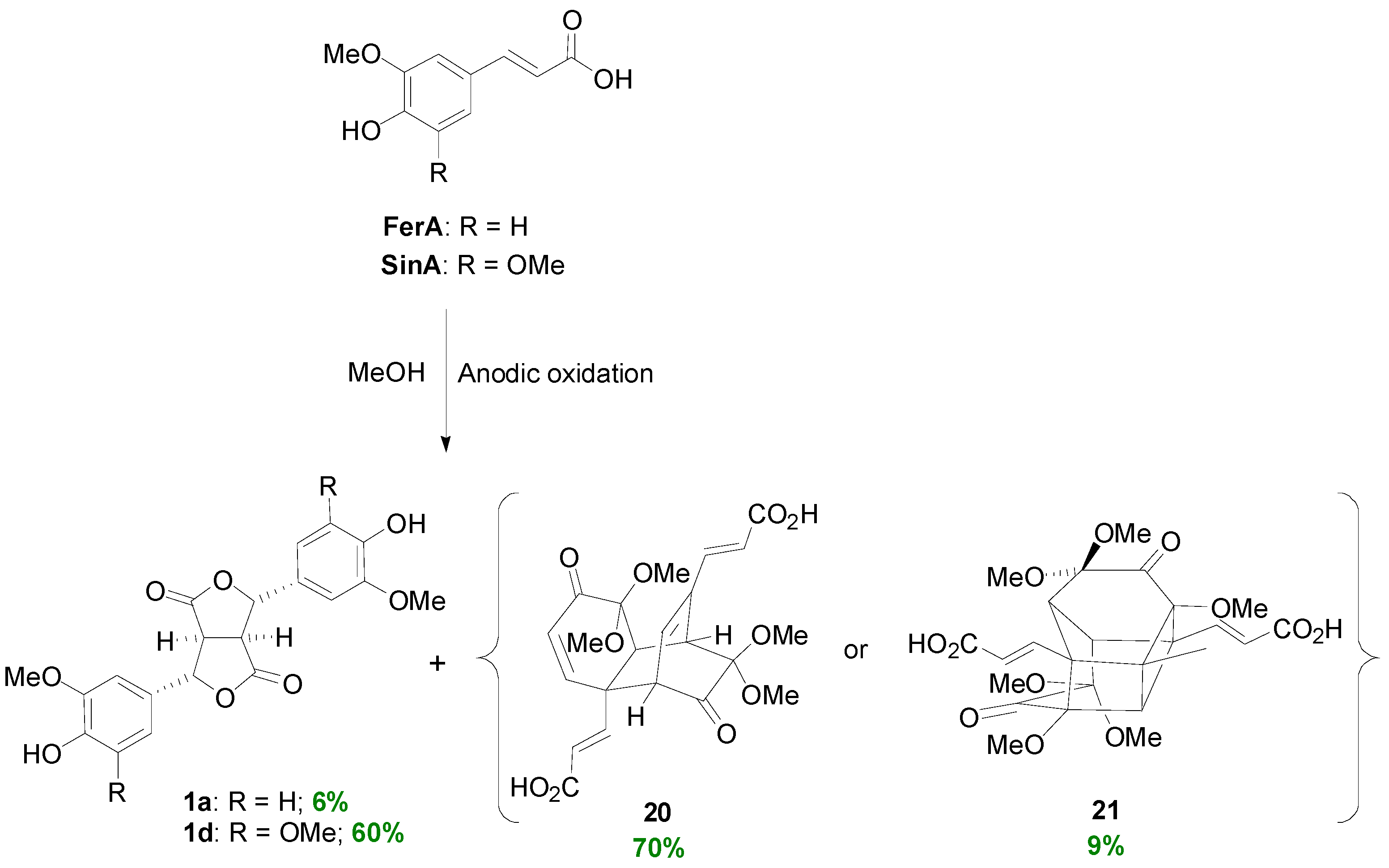
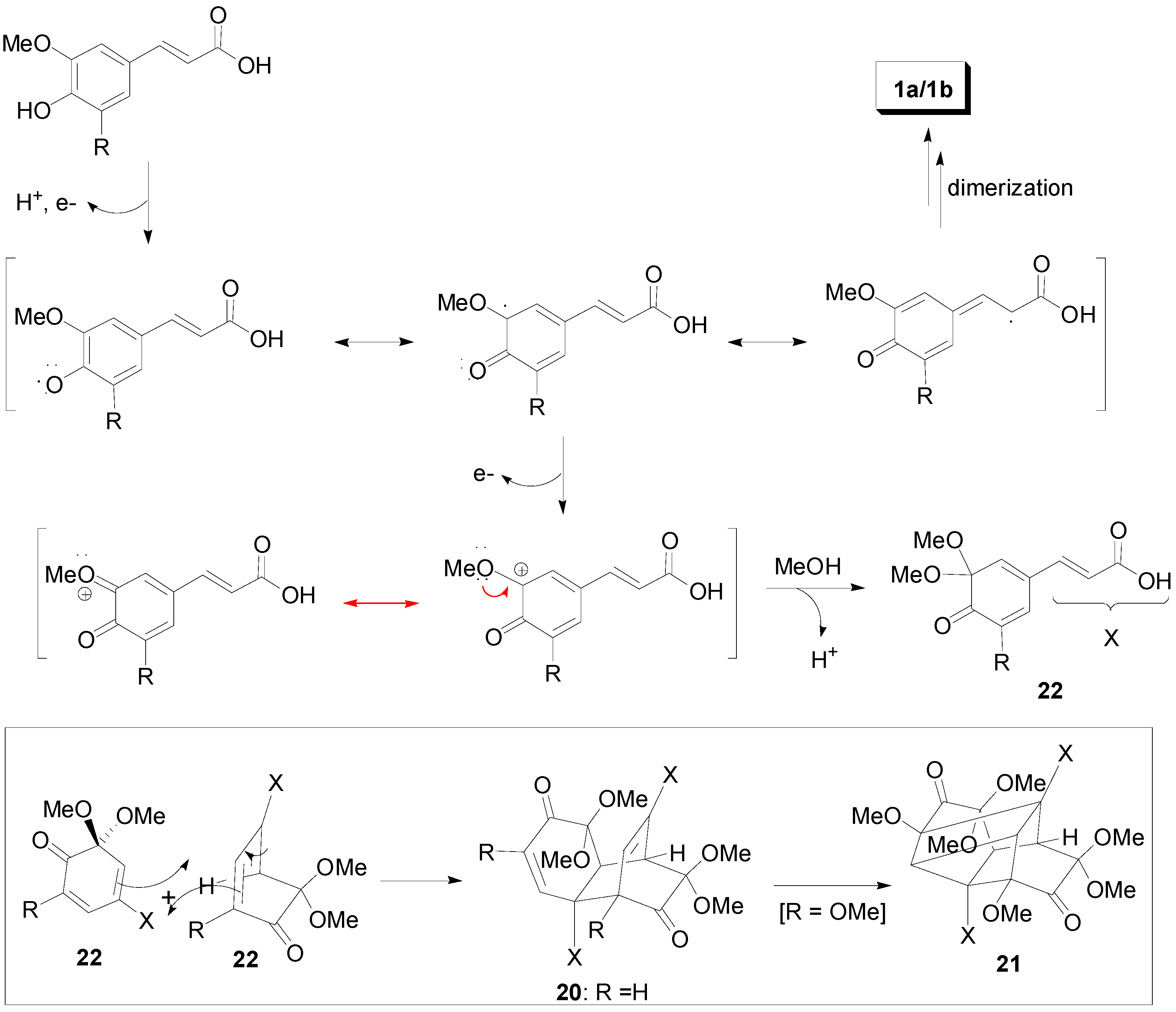
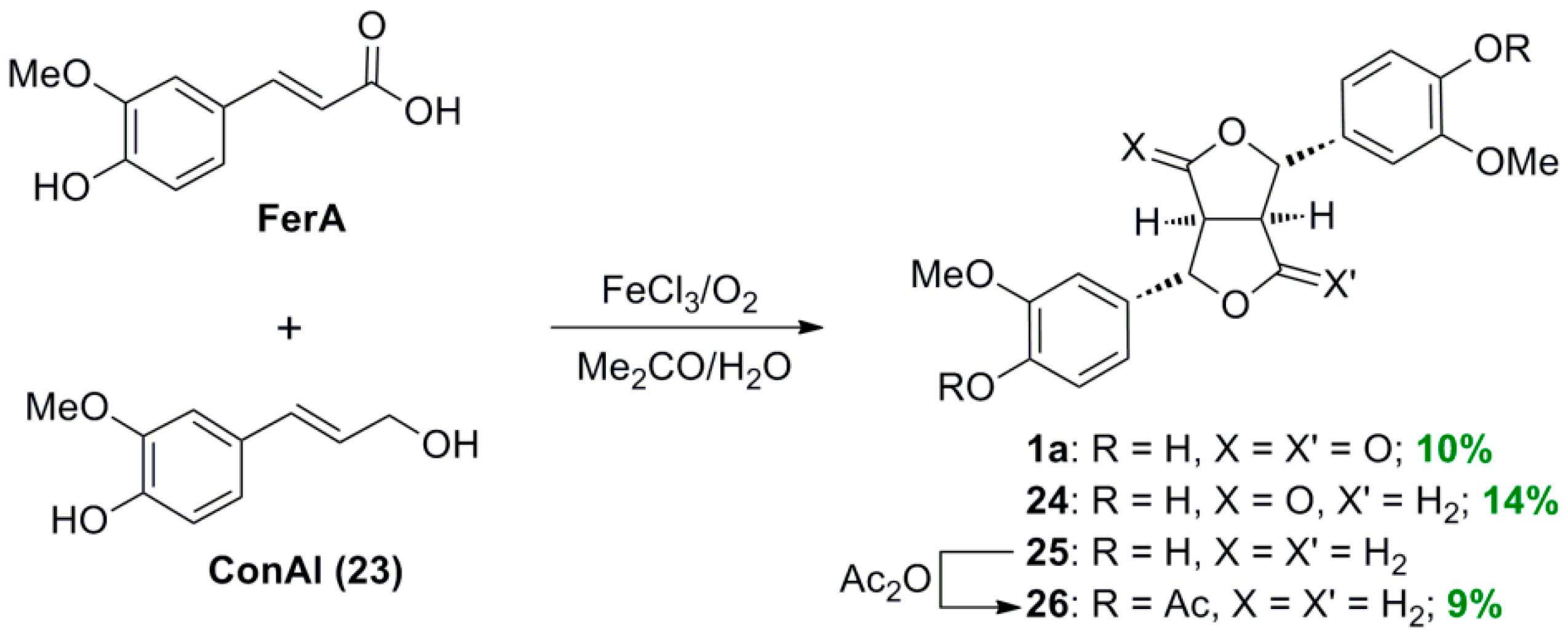
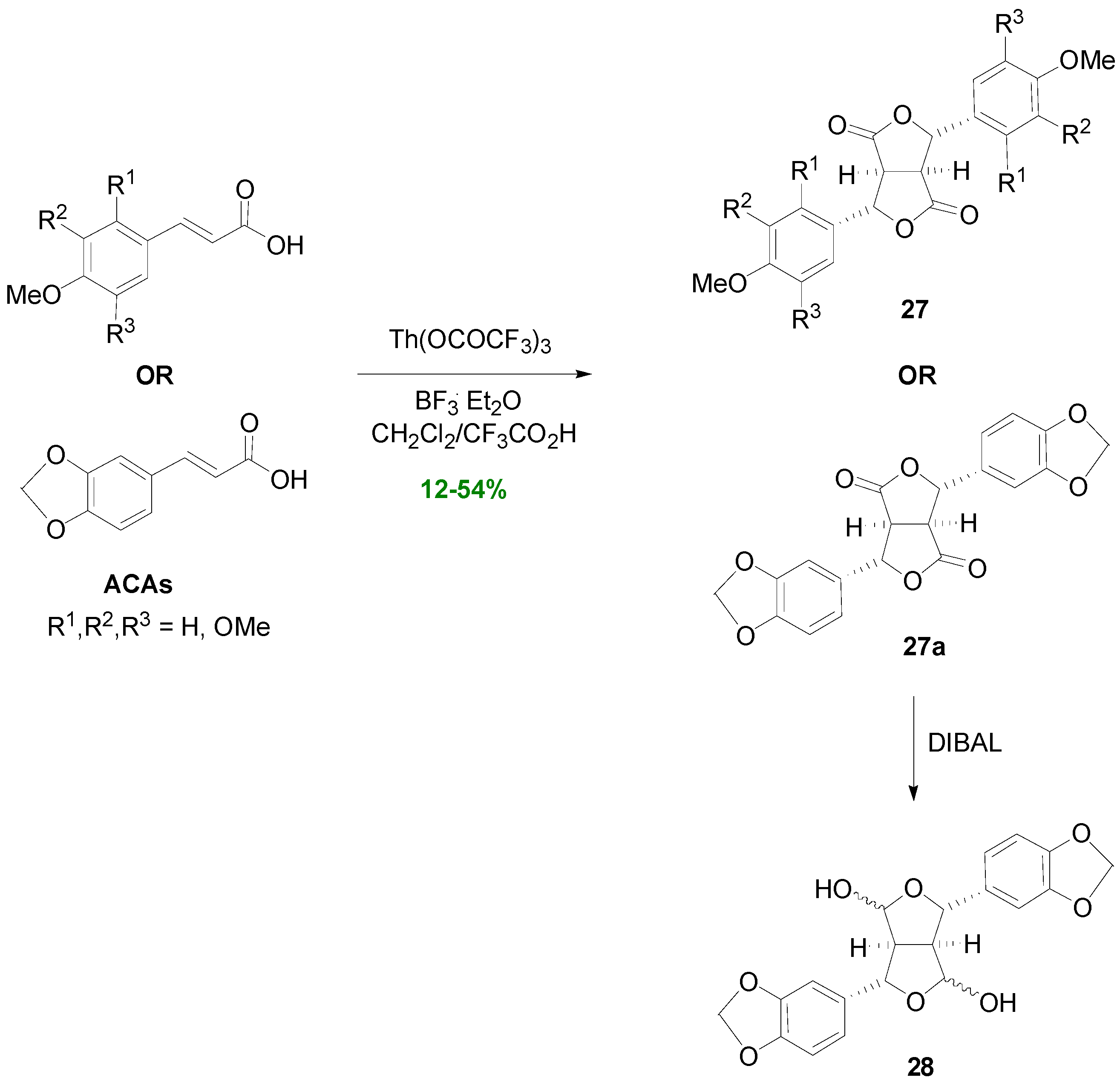

2.2. Syntheses of Lignans Based on the Key Intermediate Dialkyl 1,2-dihydro-1-arylnaphthalene-2,3-dicarboxylate
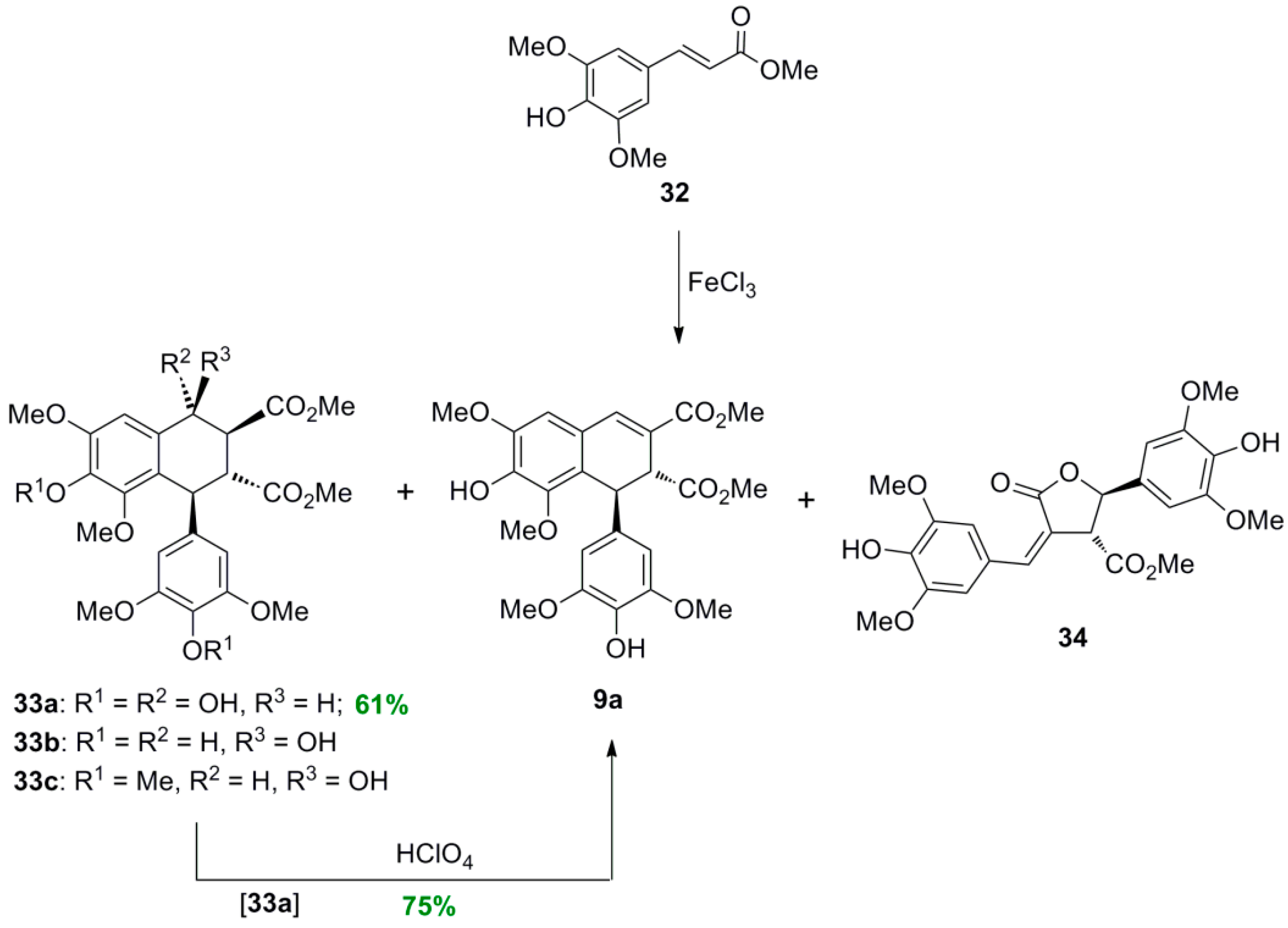
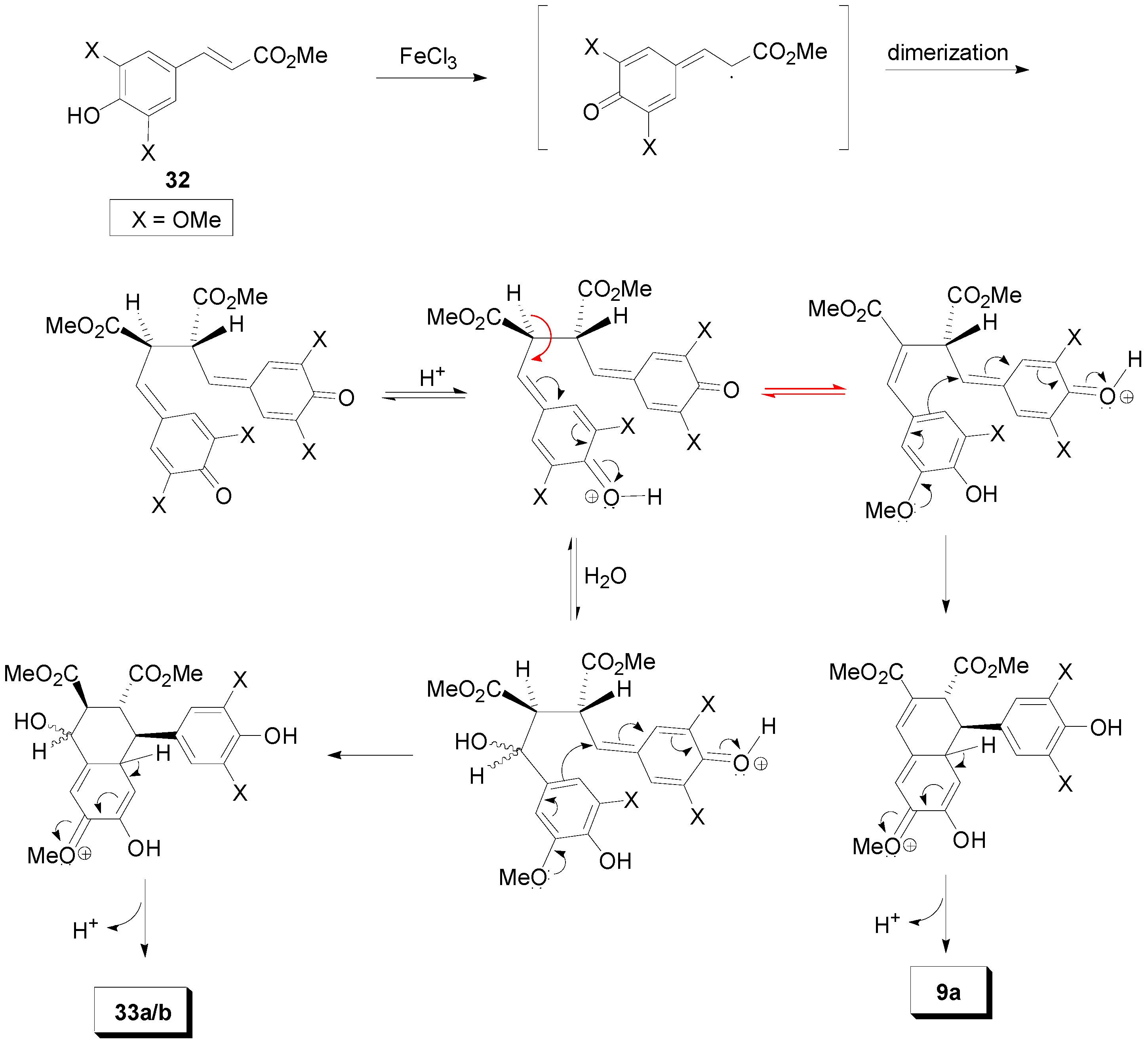
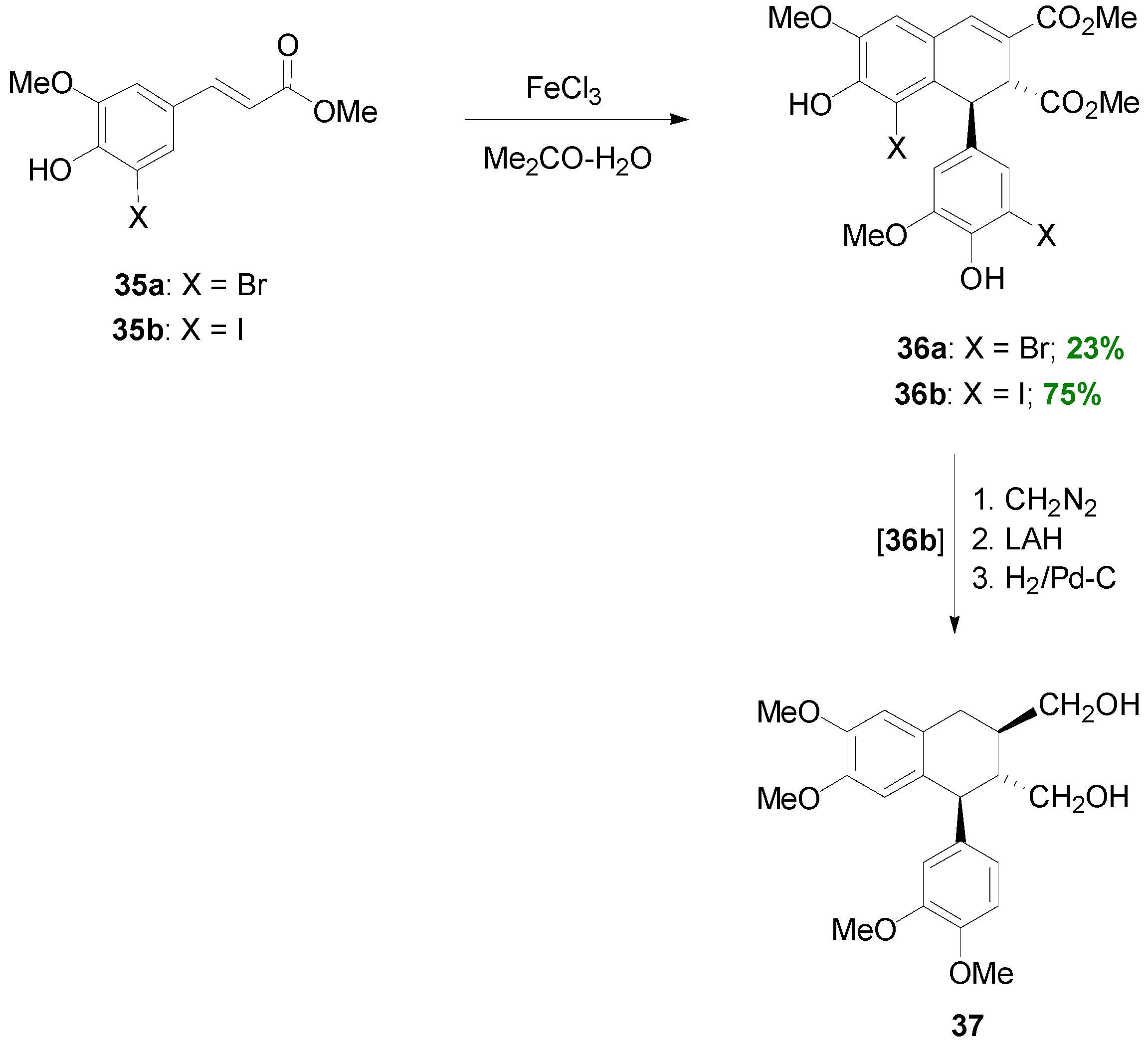

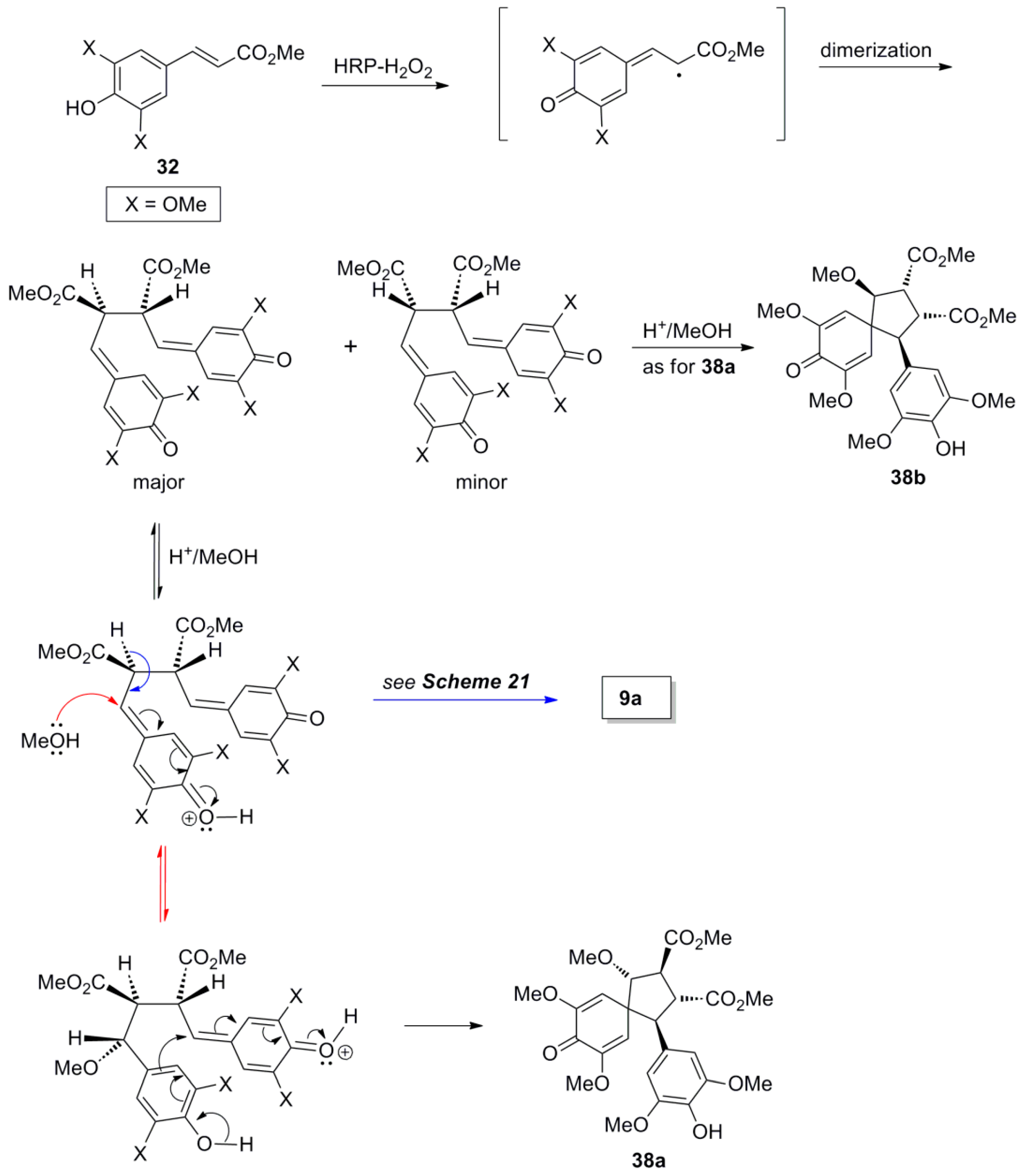

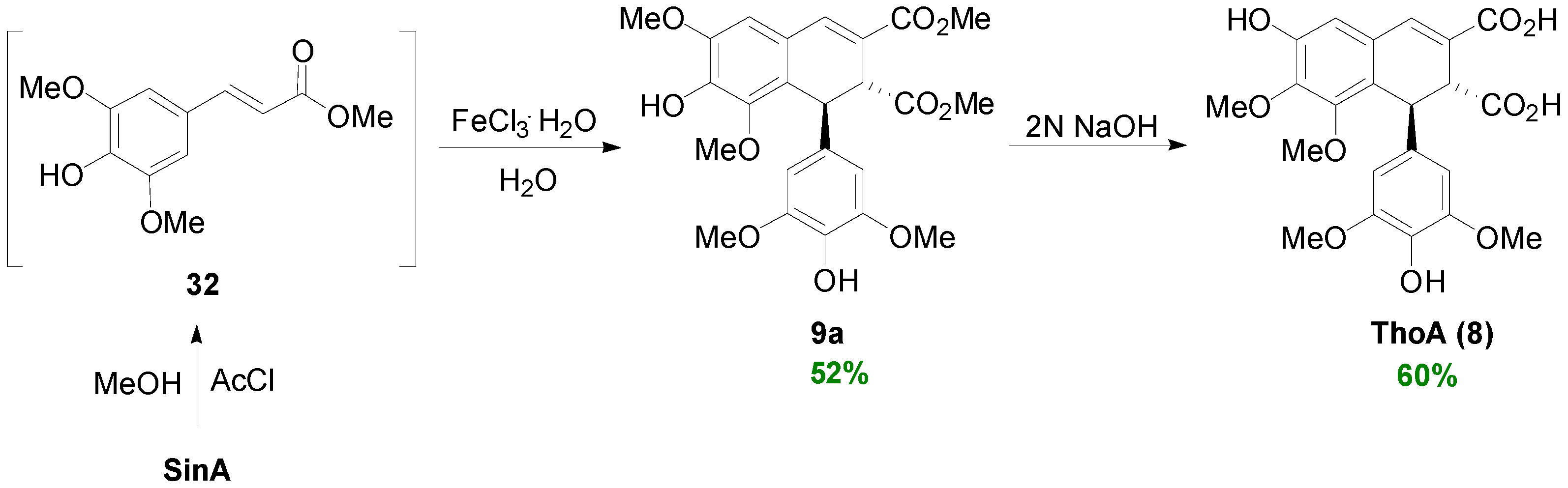


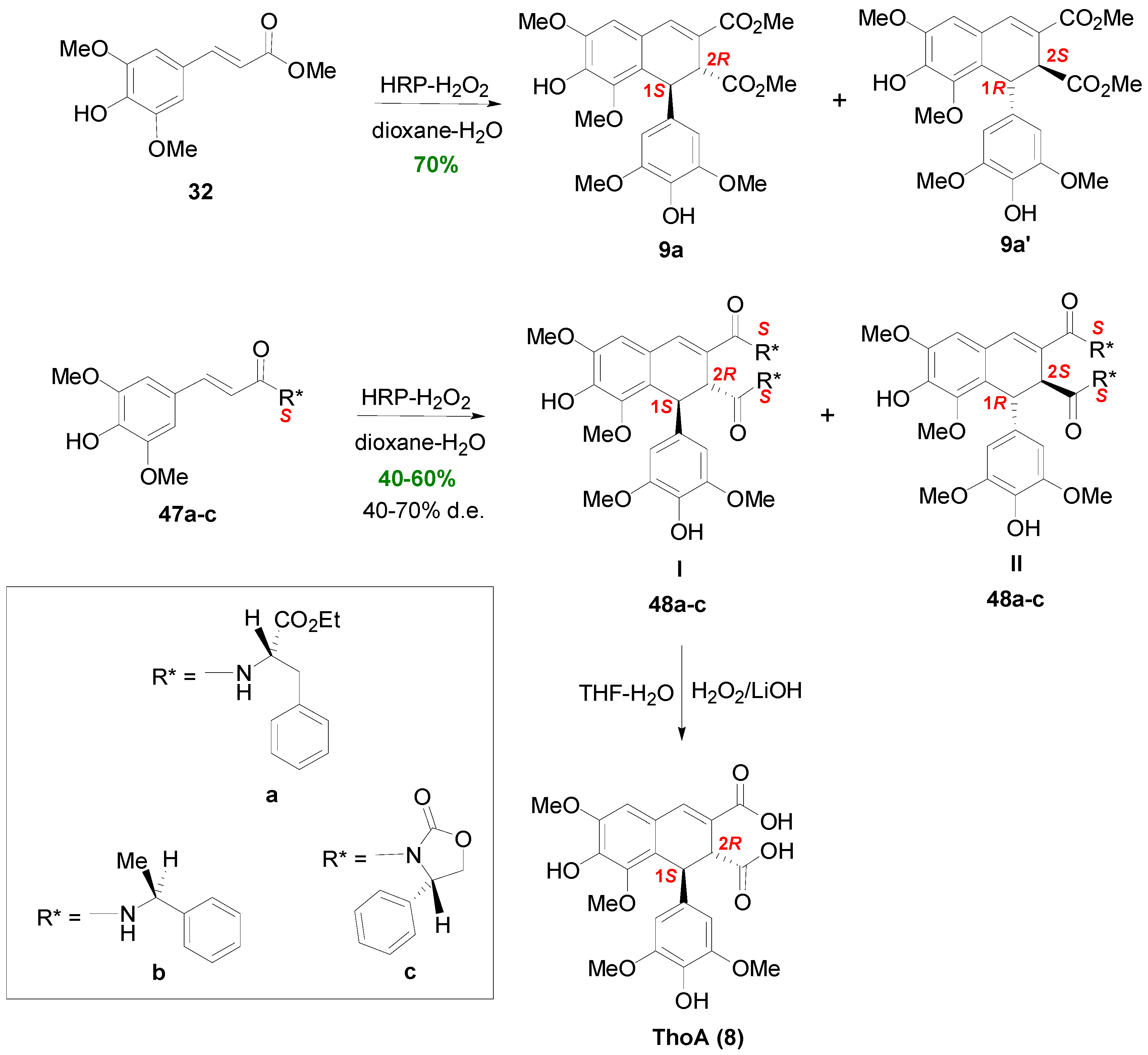
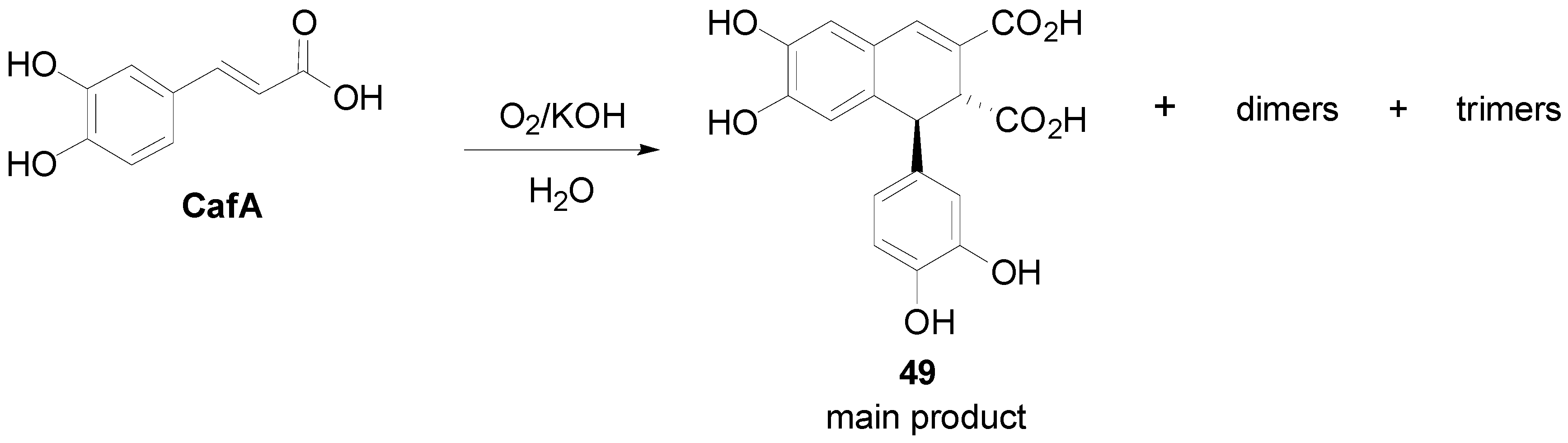
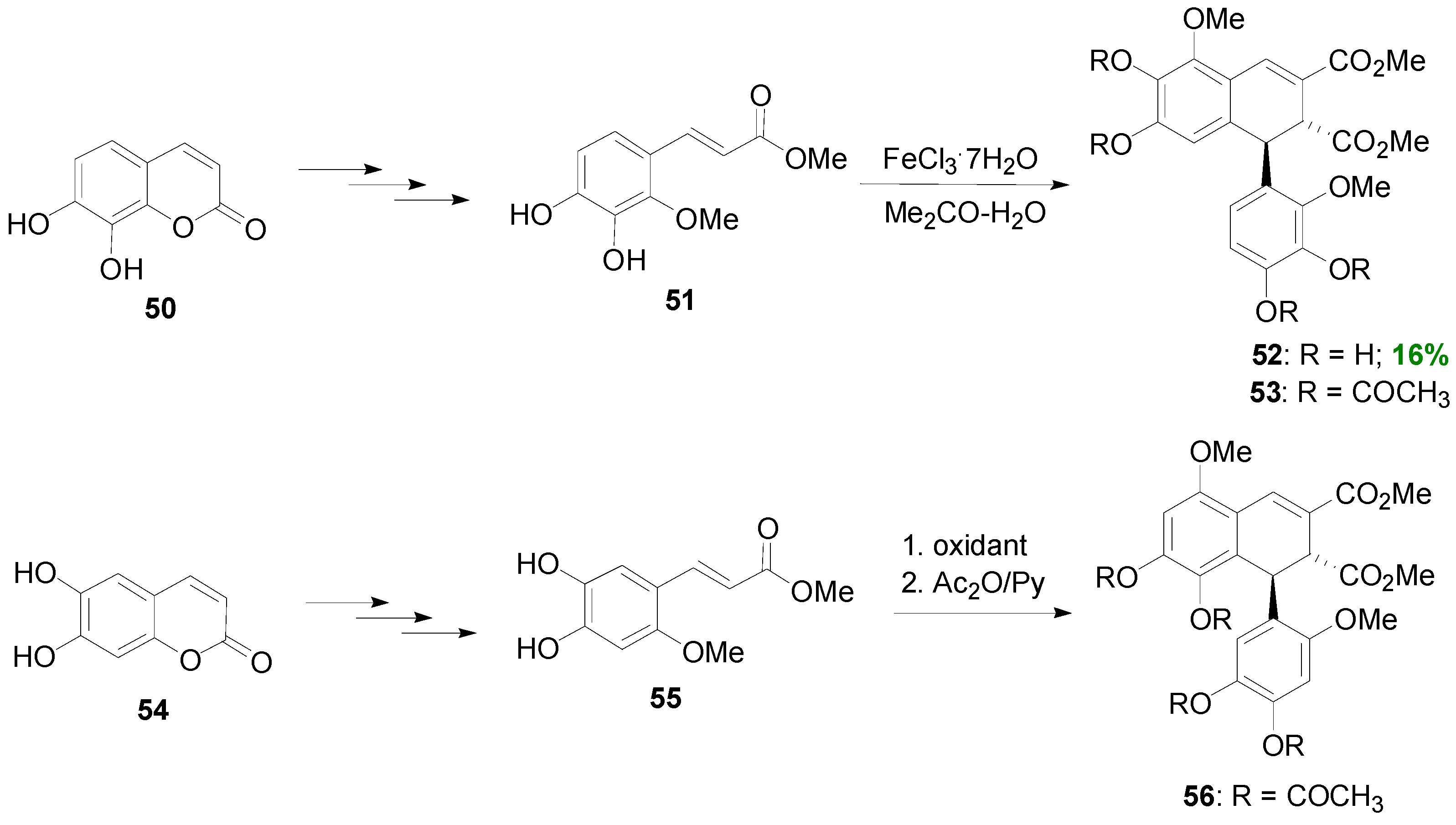
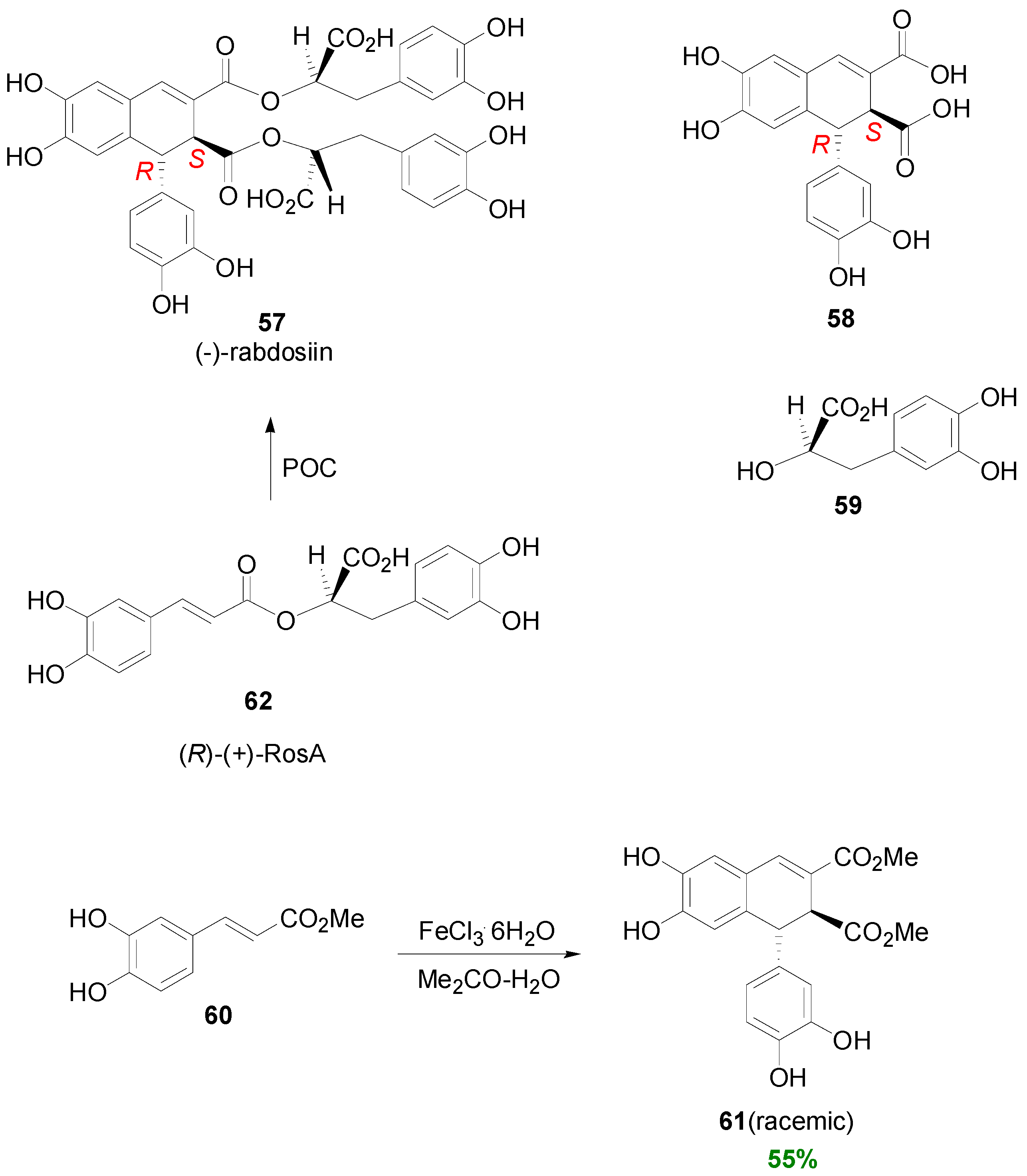

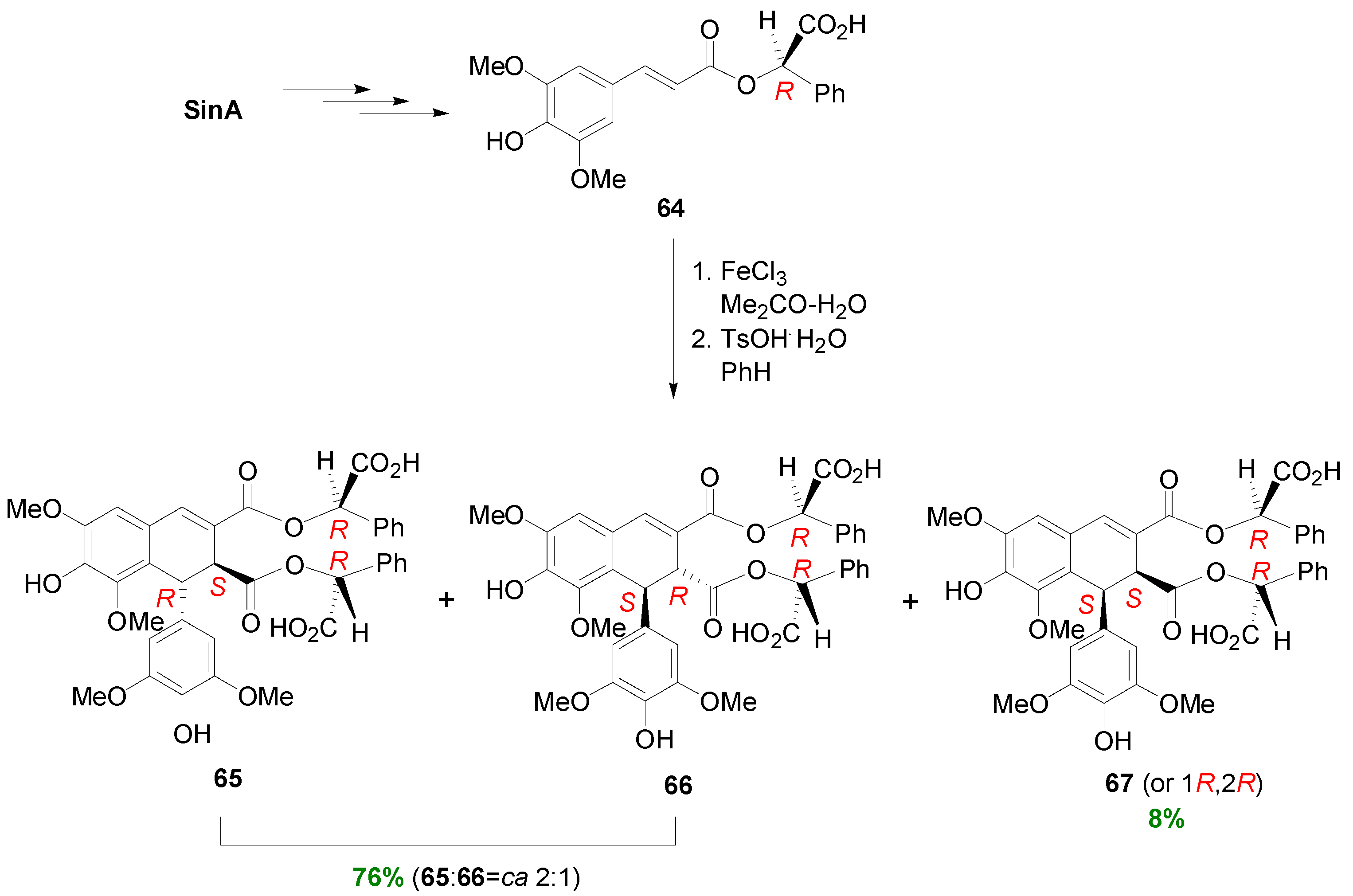


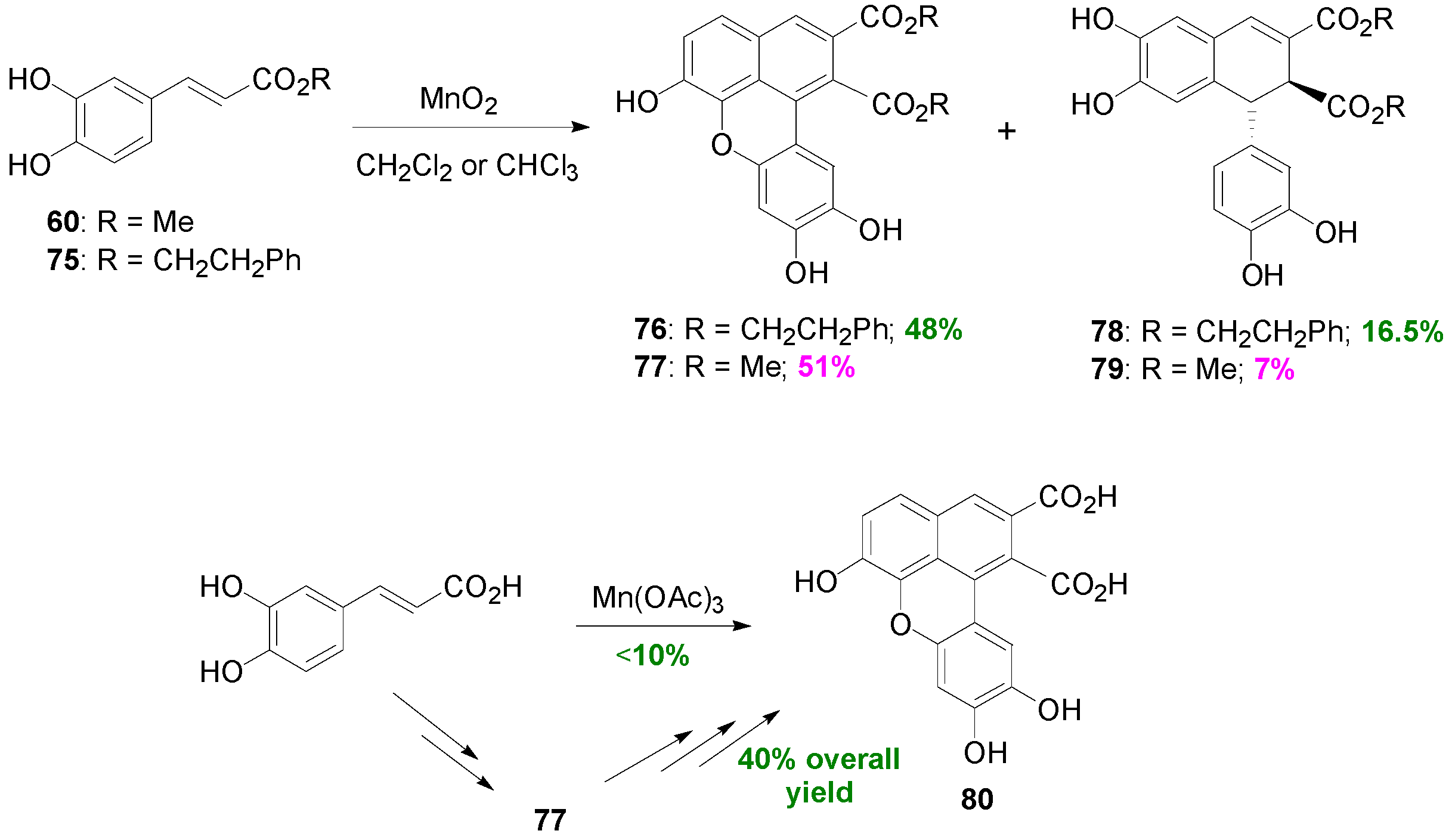
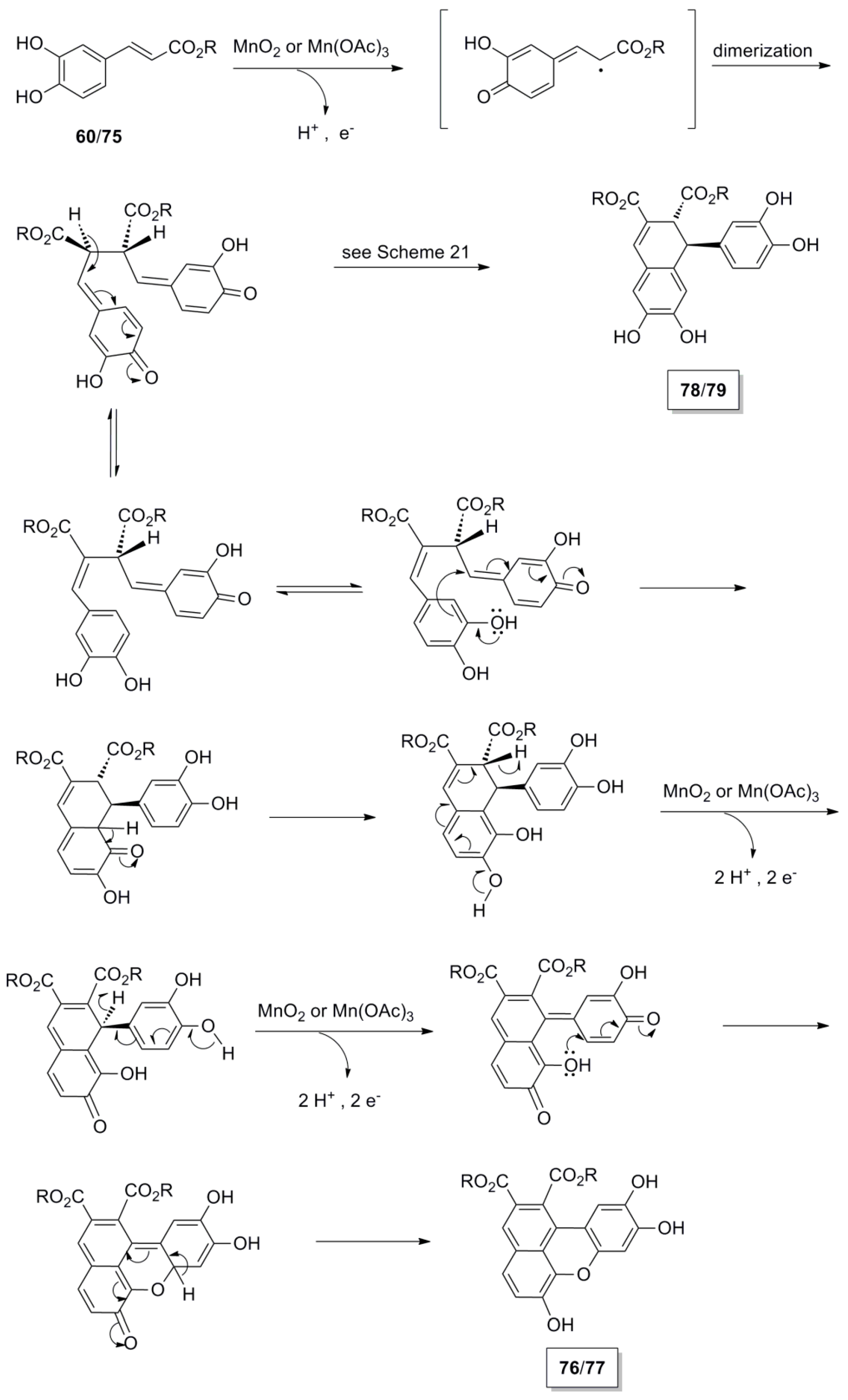
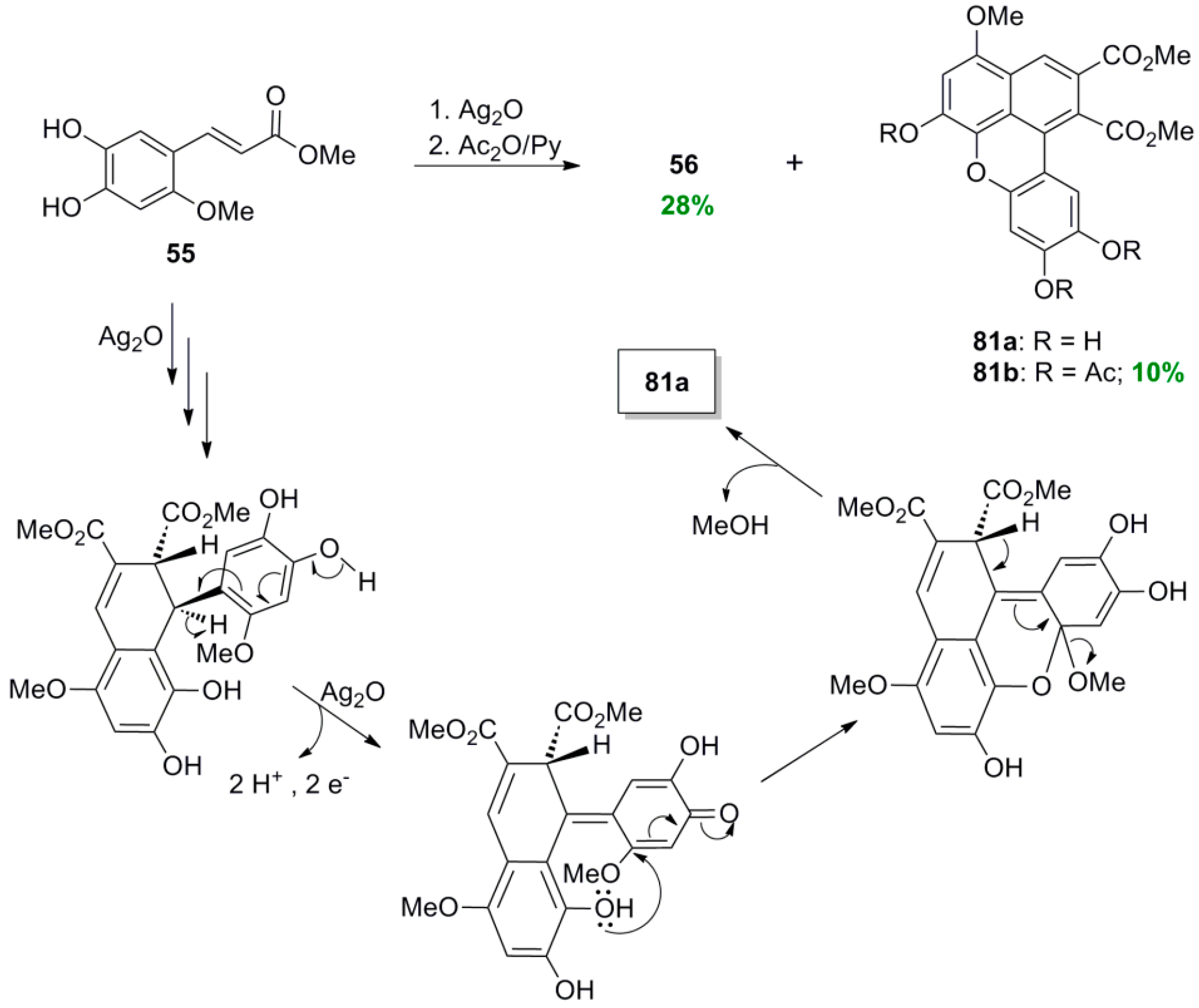


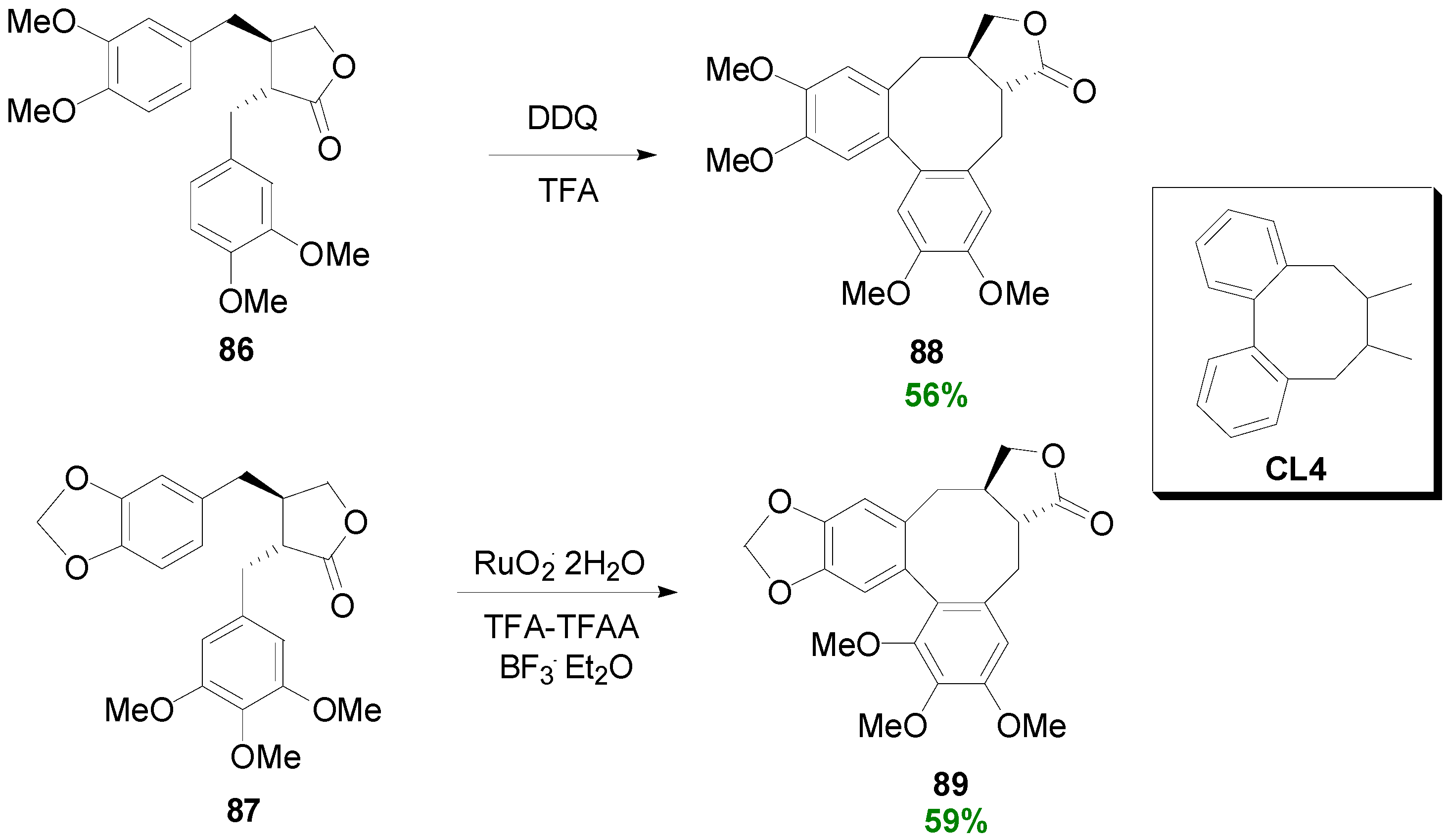
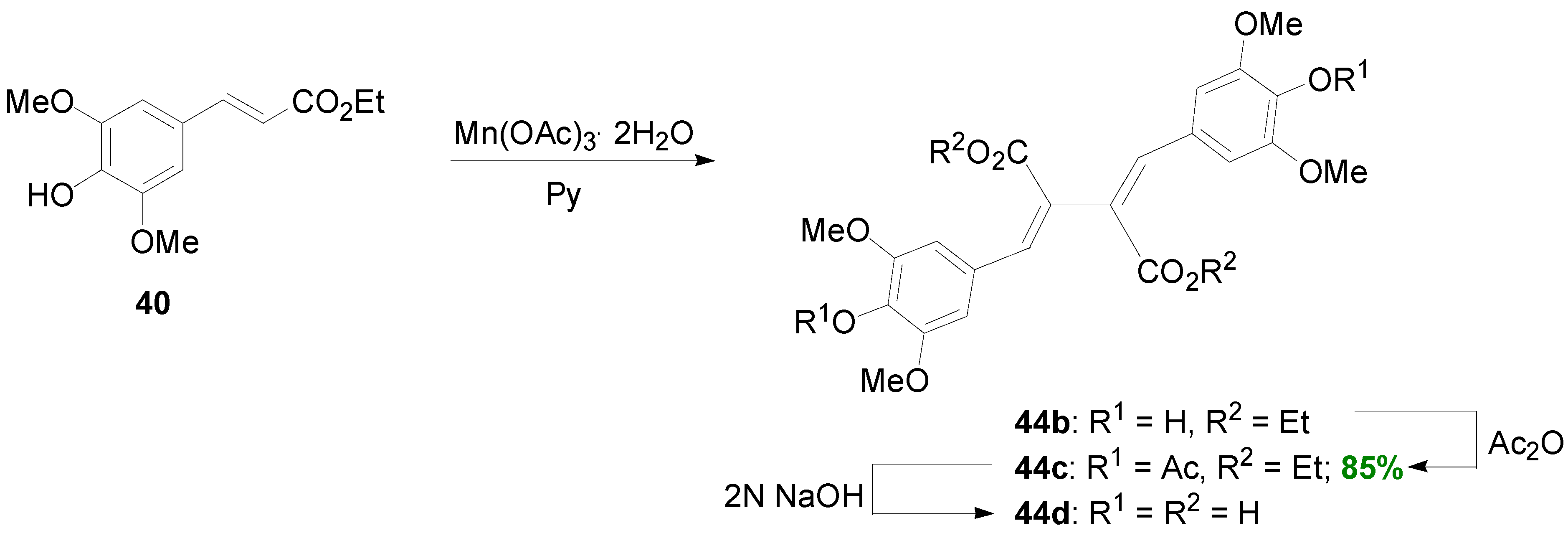
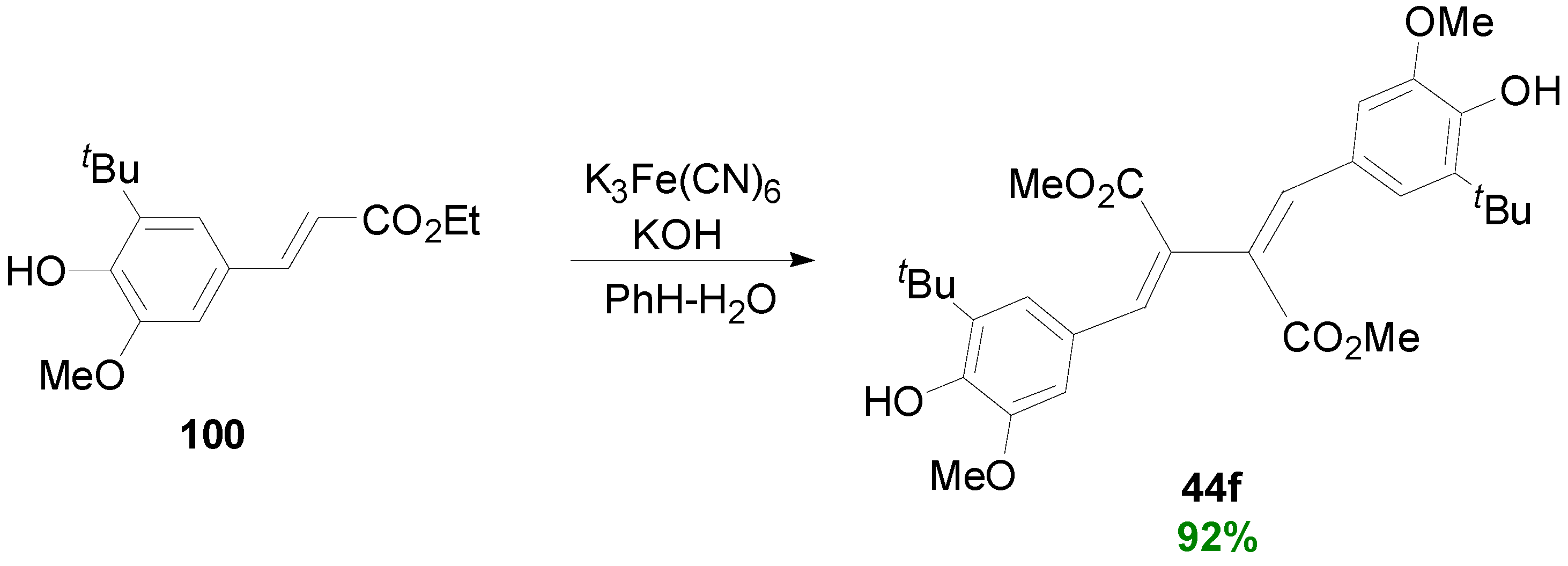
2.3. Syntheses of Lignans Based on the Key Intermediate Dialkyl Bis(arylmethyle-ne)succinate
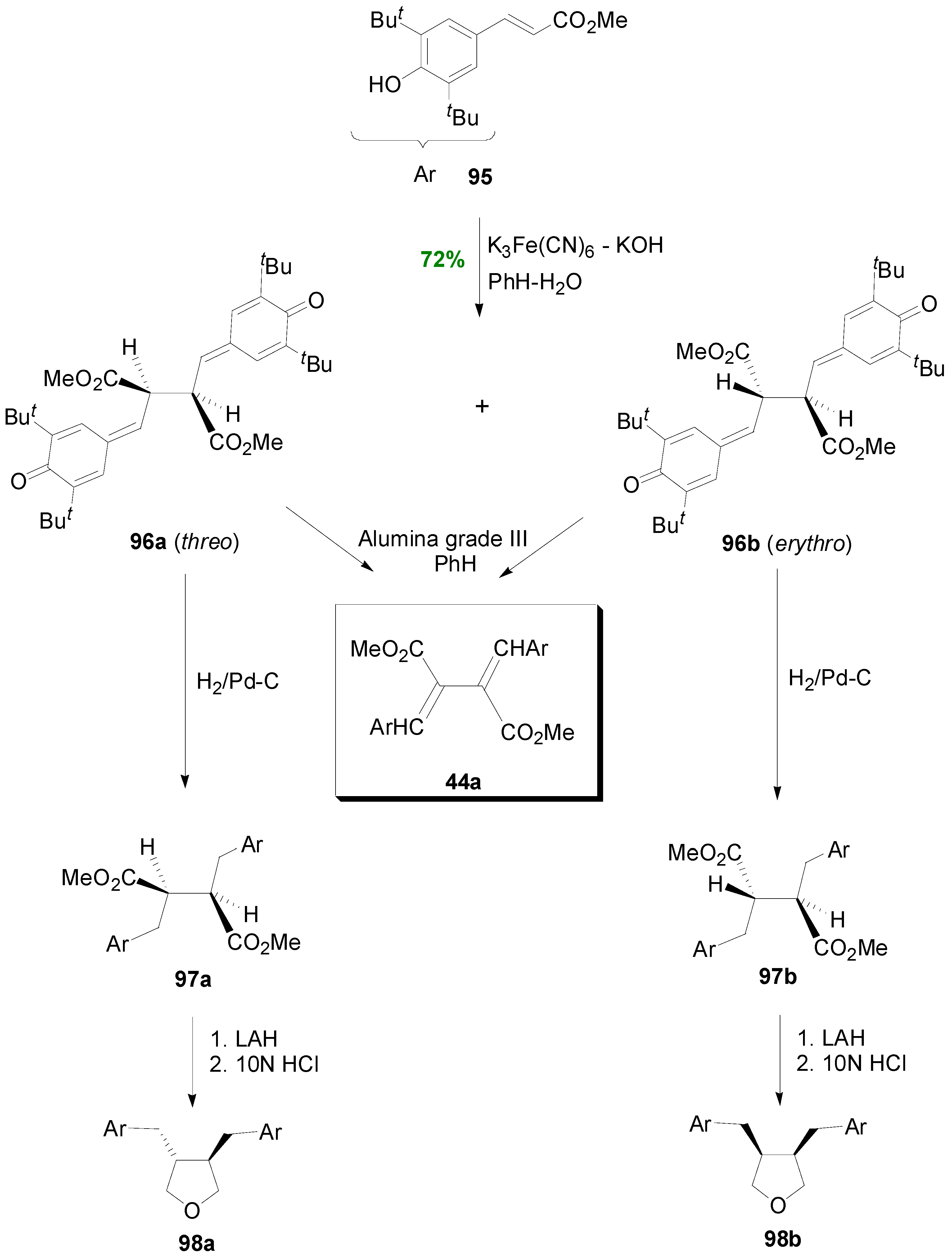
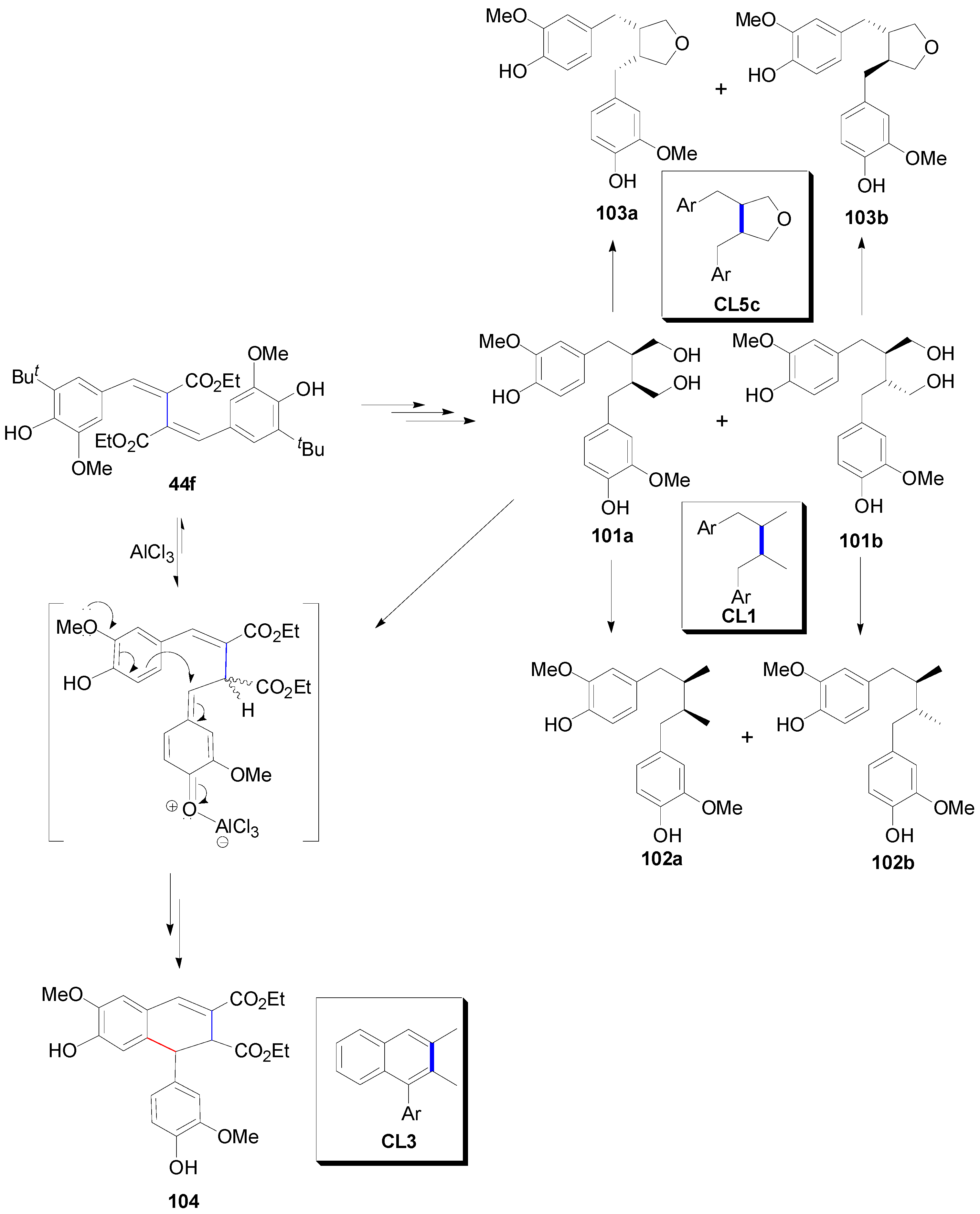

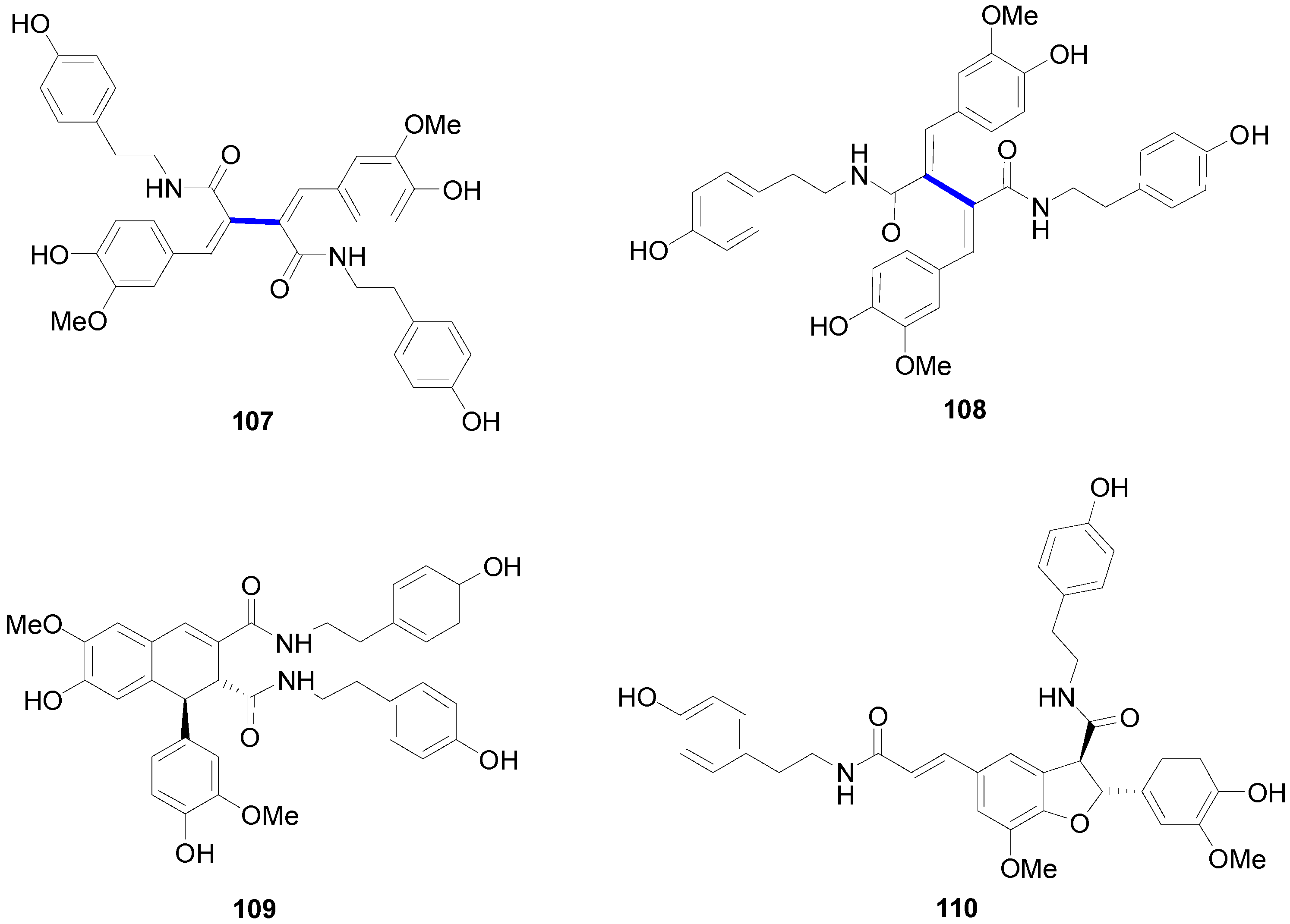
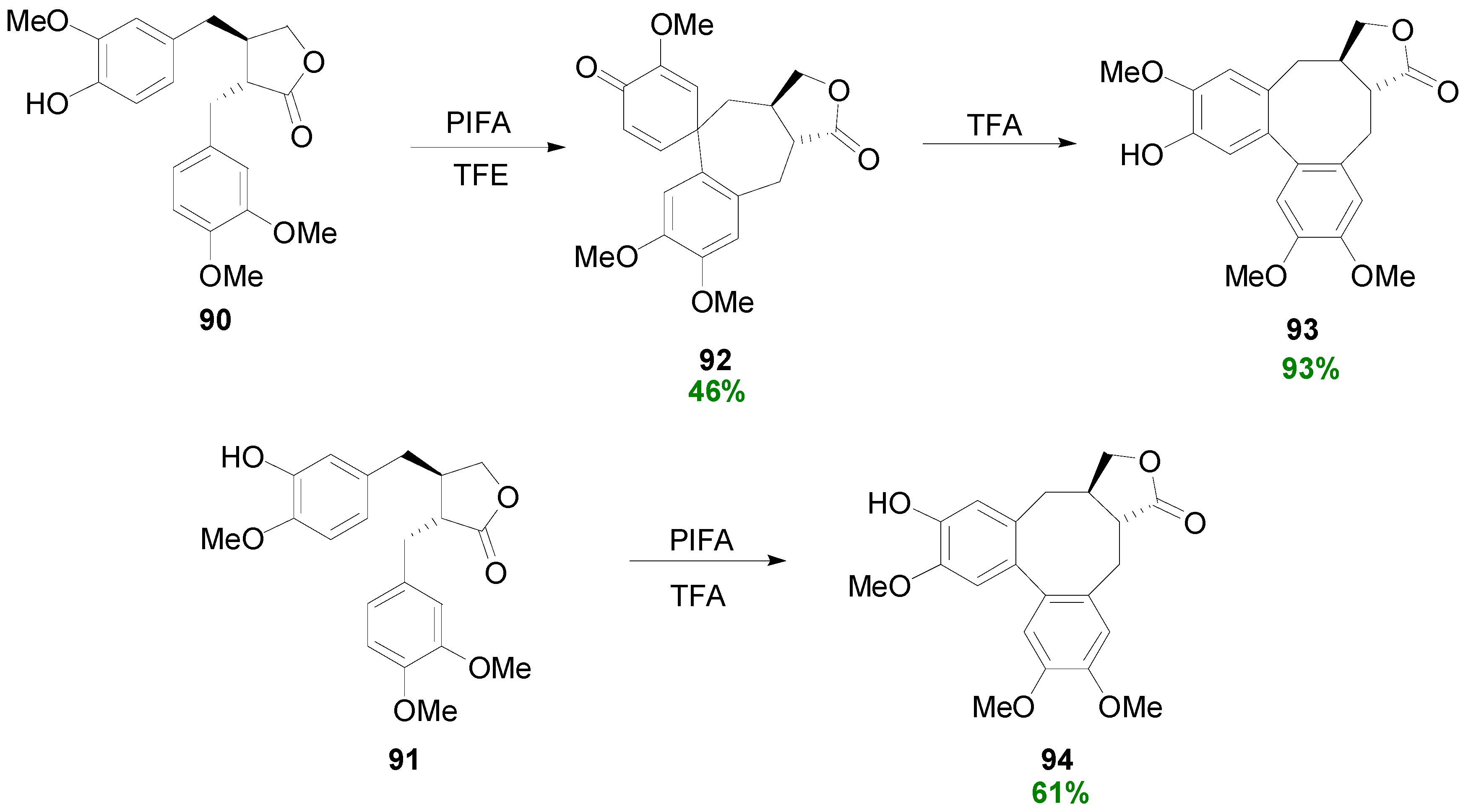
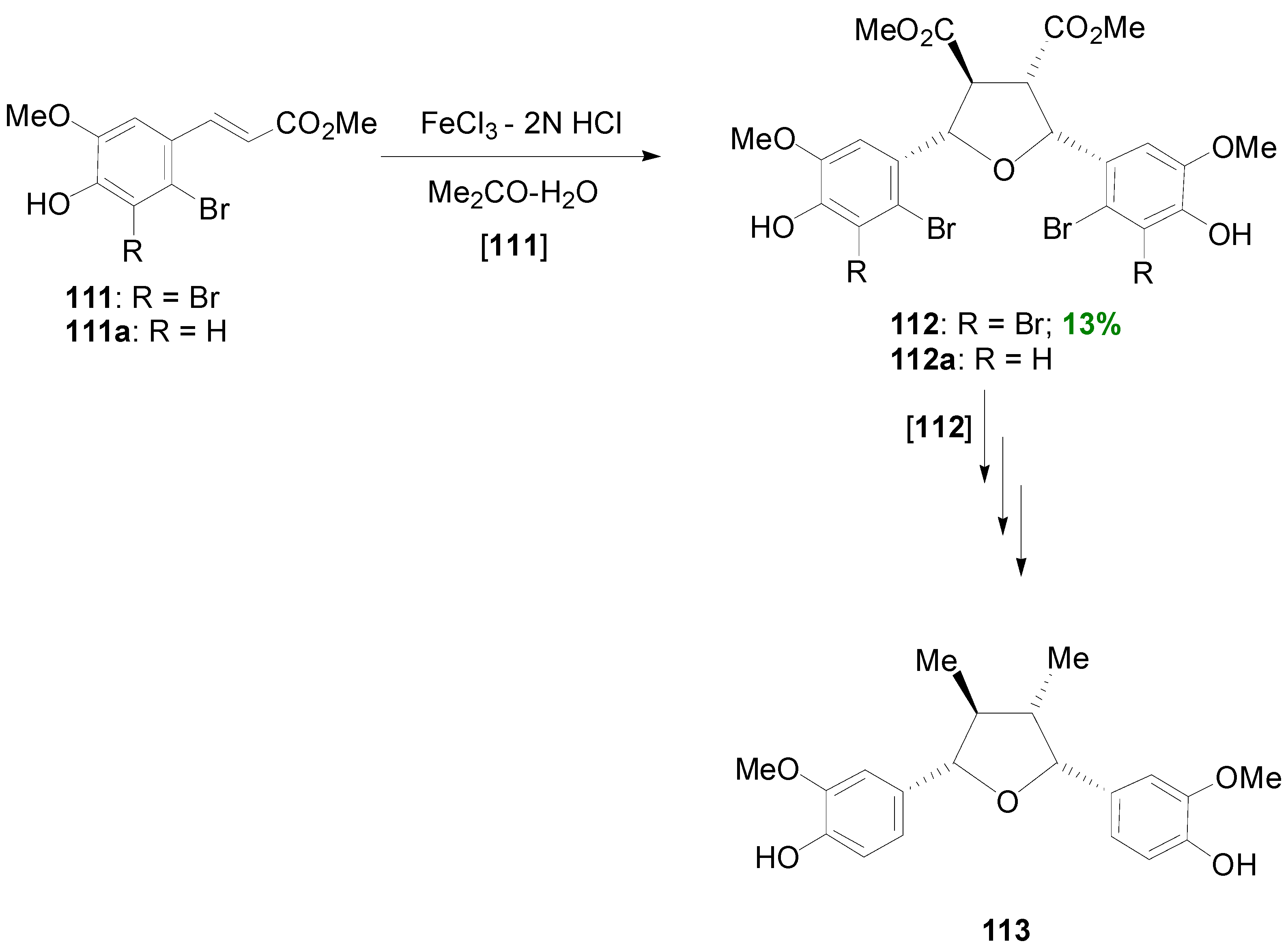
2.4. Syntheses of Lignans Based on the Key Intermediate 3,4-Disubstituted 2,5-Di-aryltetrahydrofuran
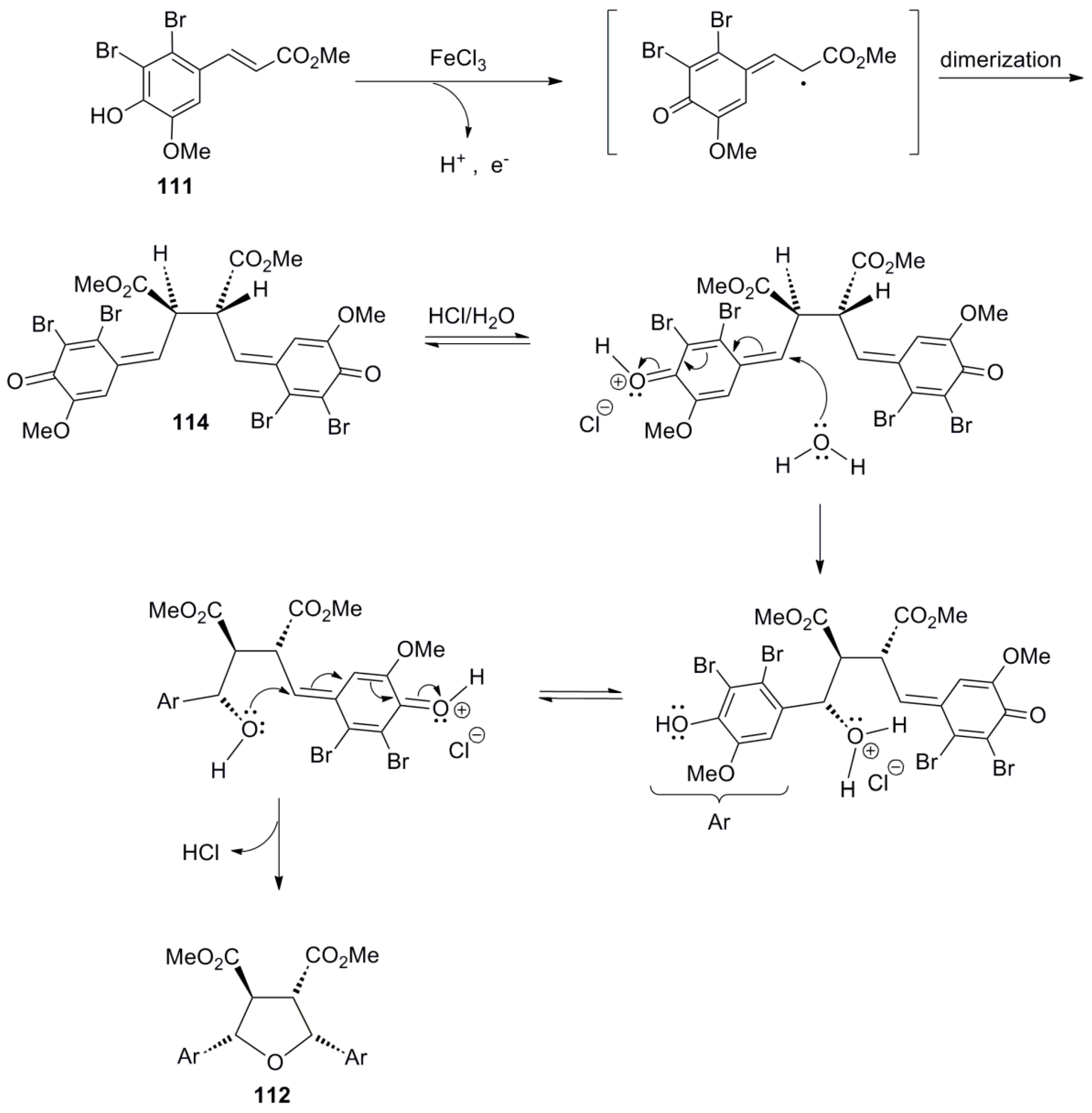
3. Neolignan Skeleton Assembly
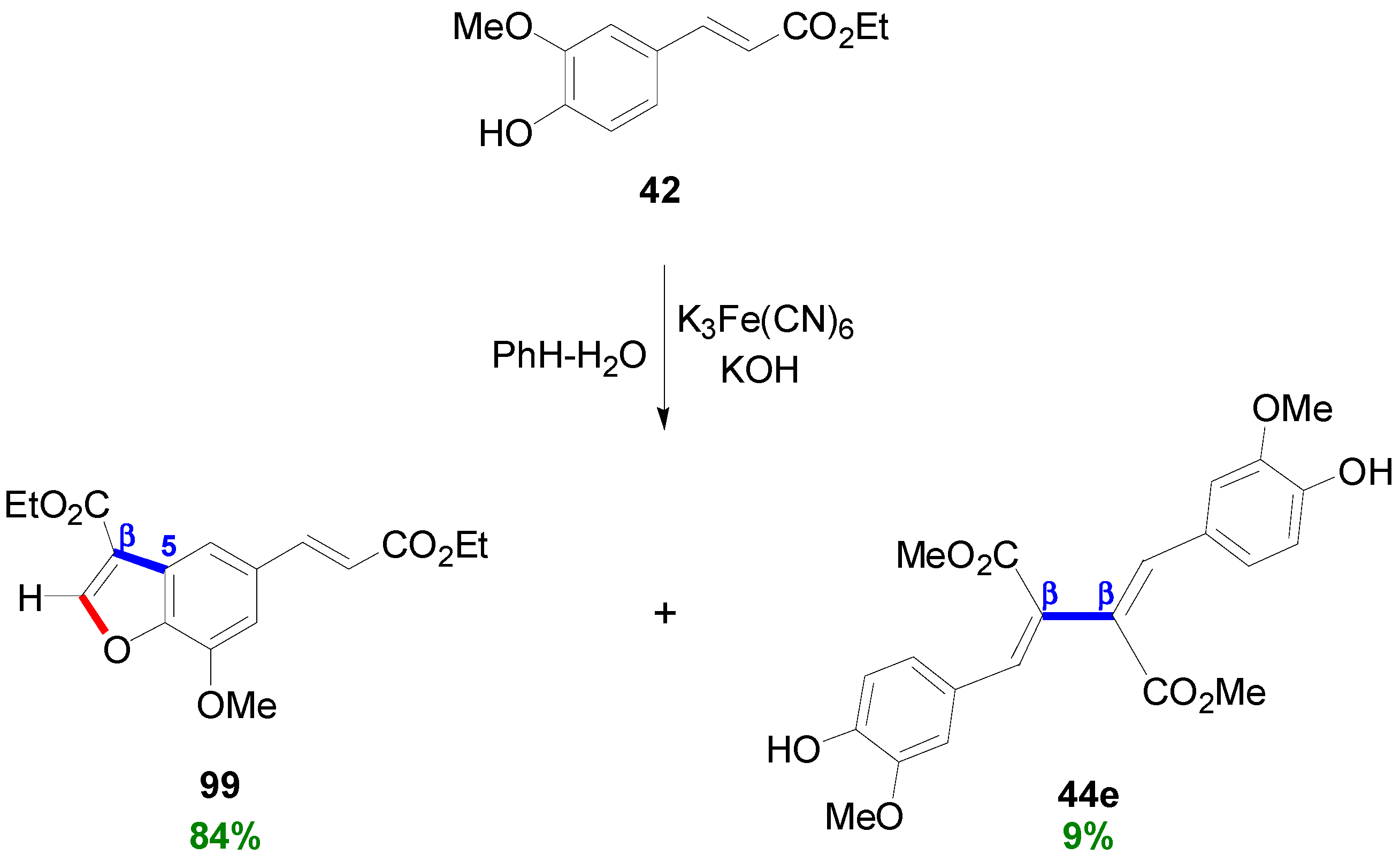
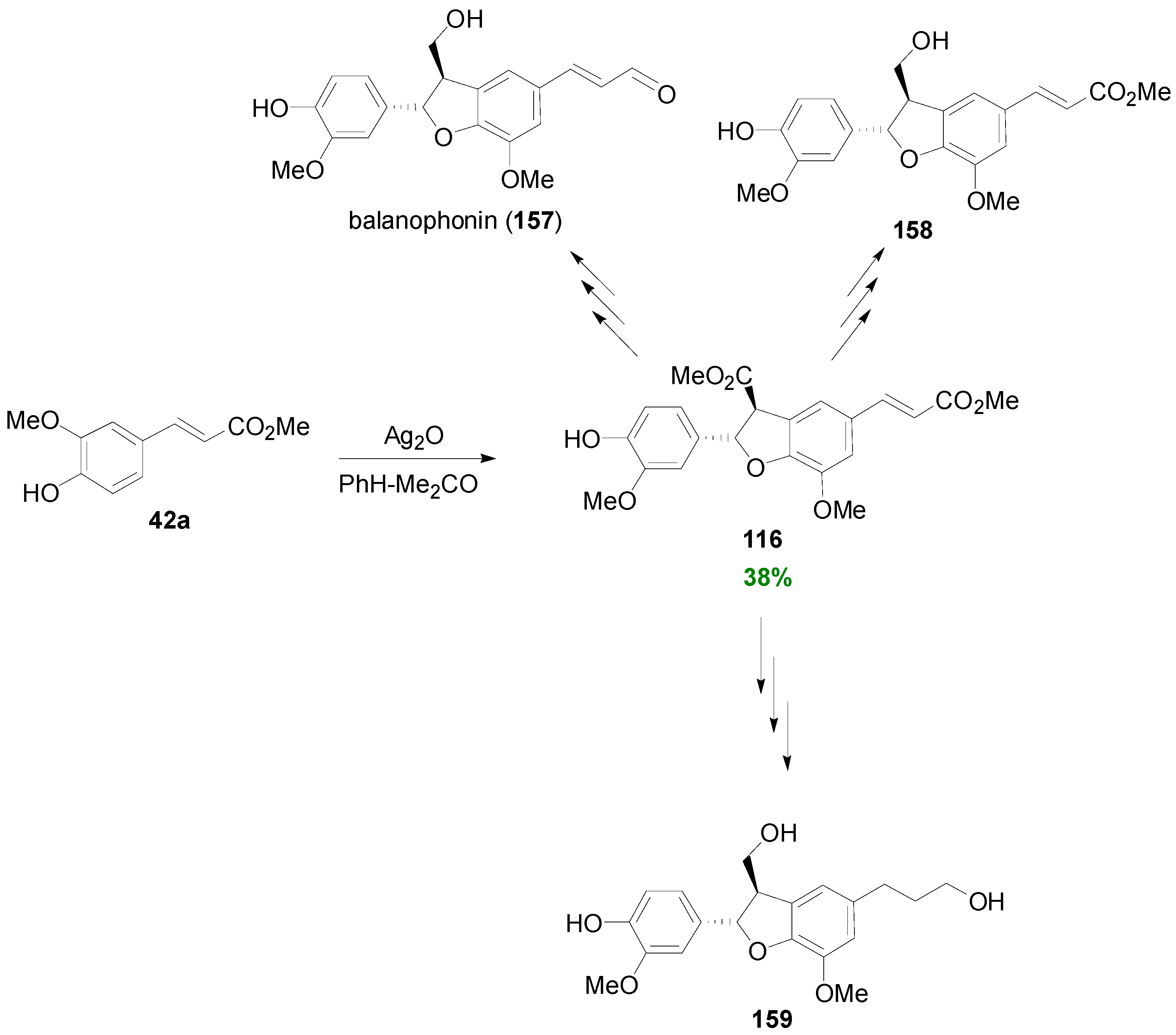
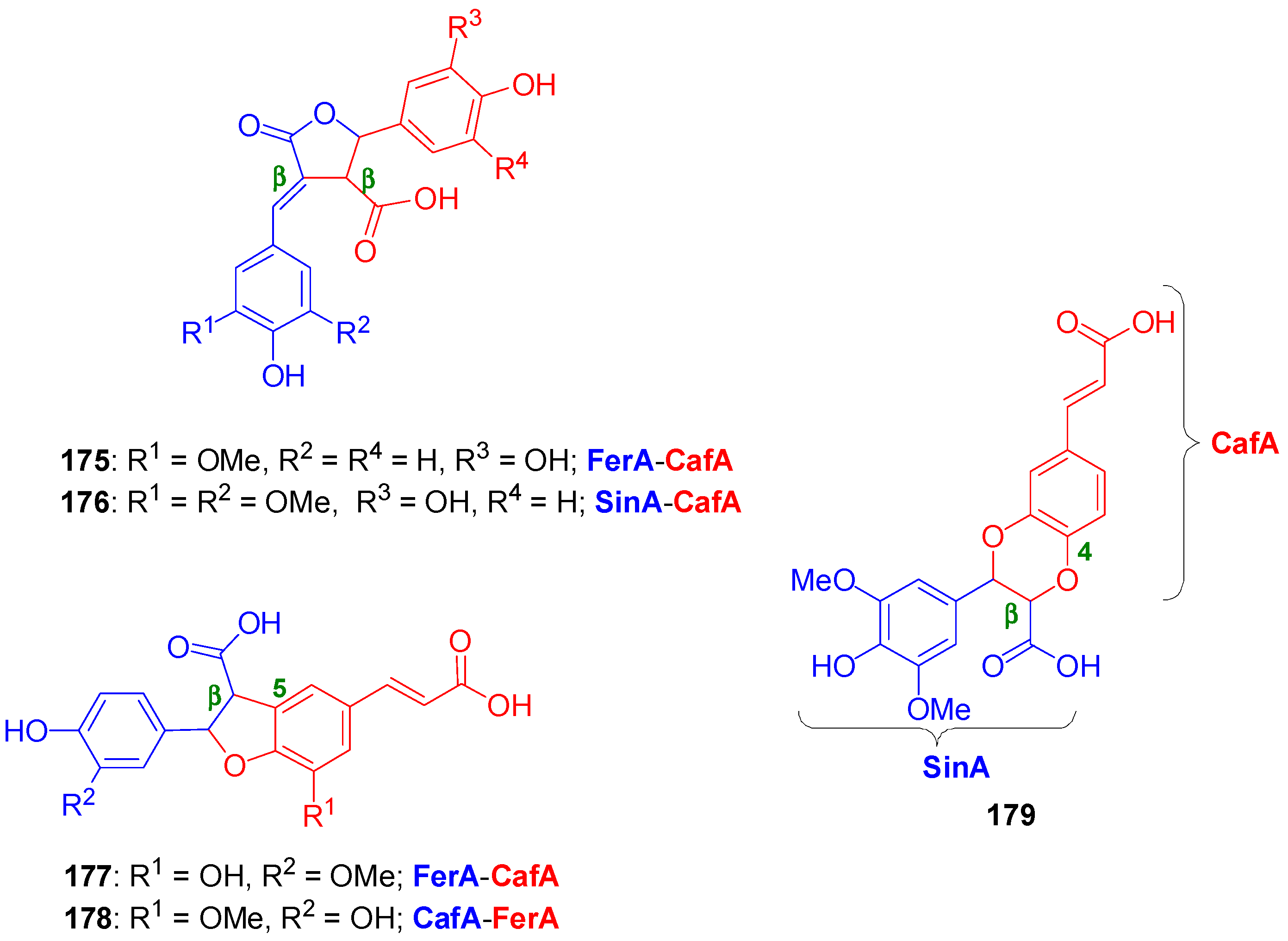
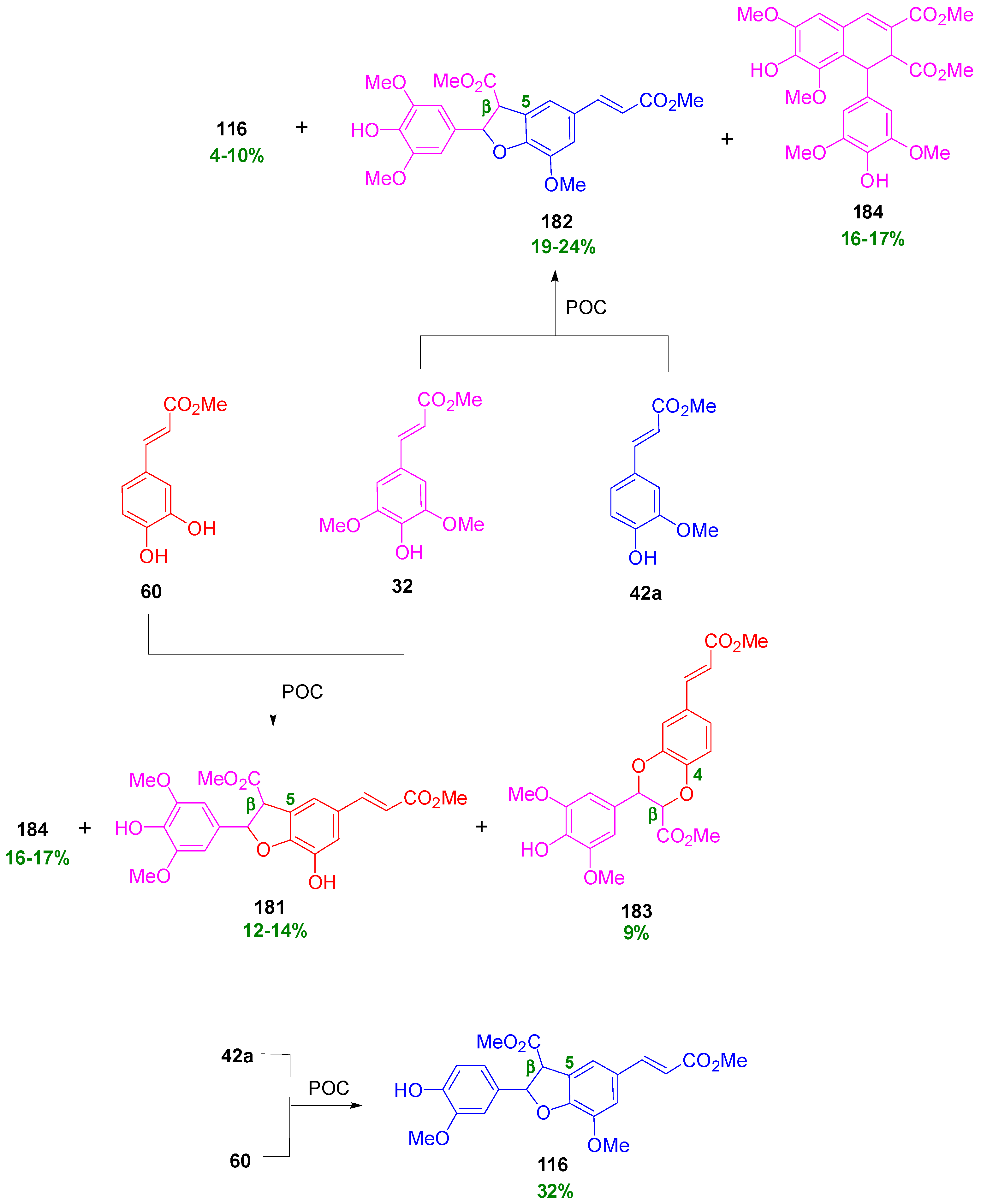
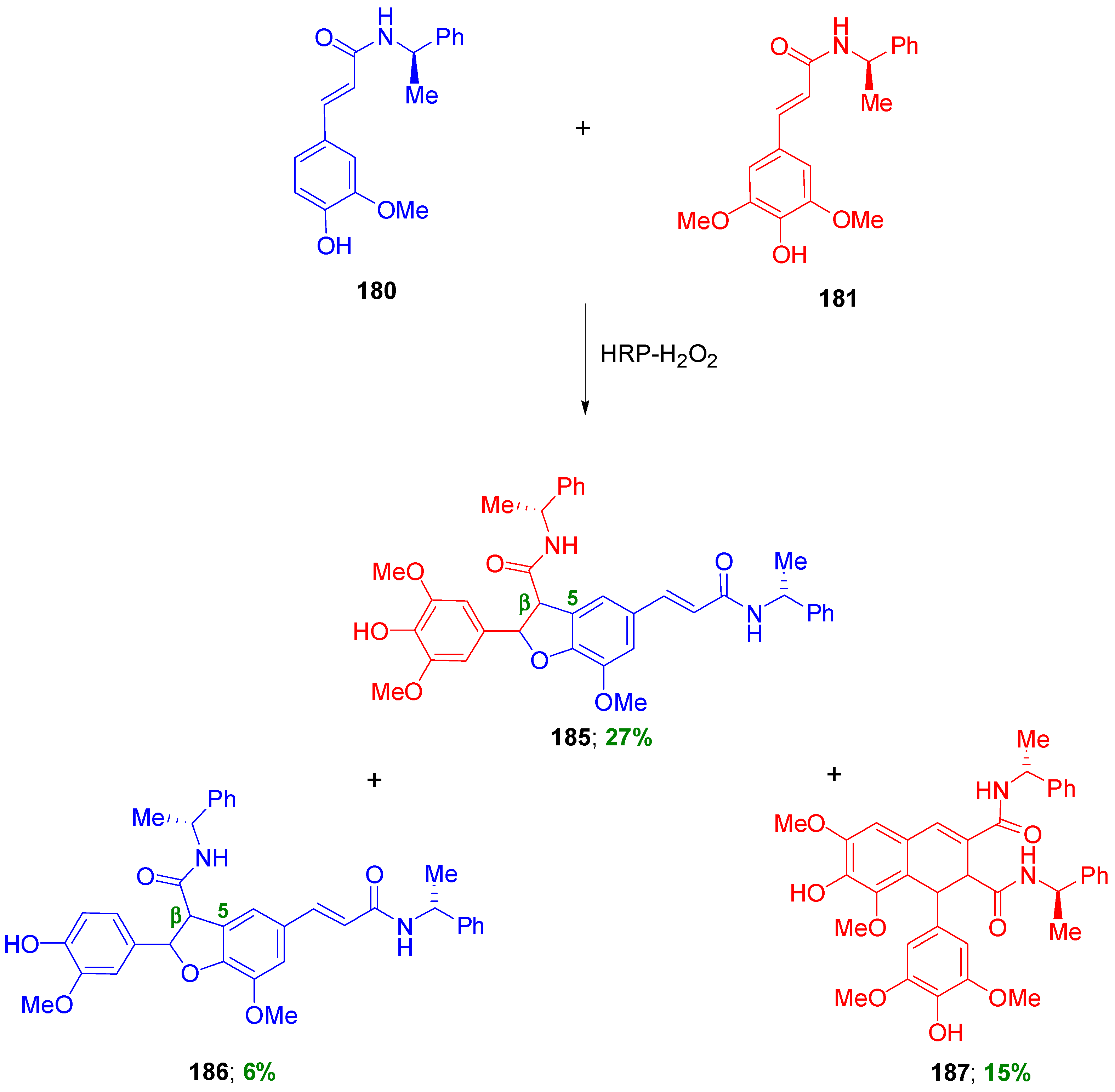
3.1. Synthesis of NLs of the Benzofuran Type—Formation of β-5 Bond as the Key Step

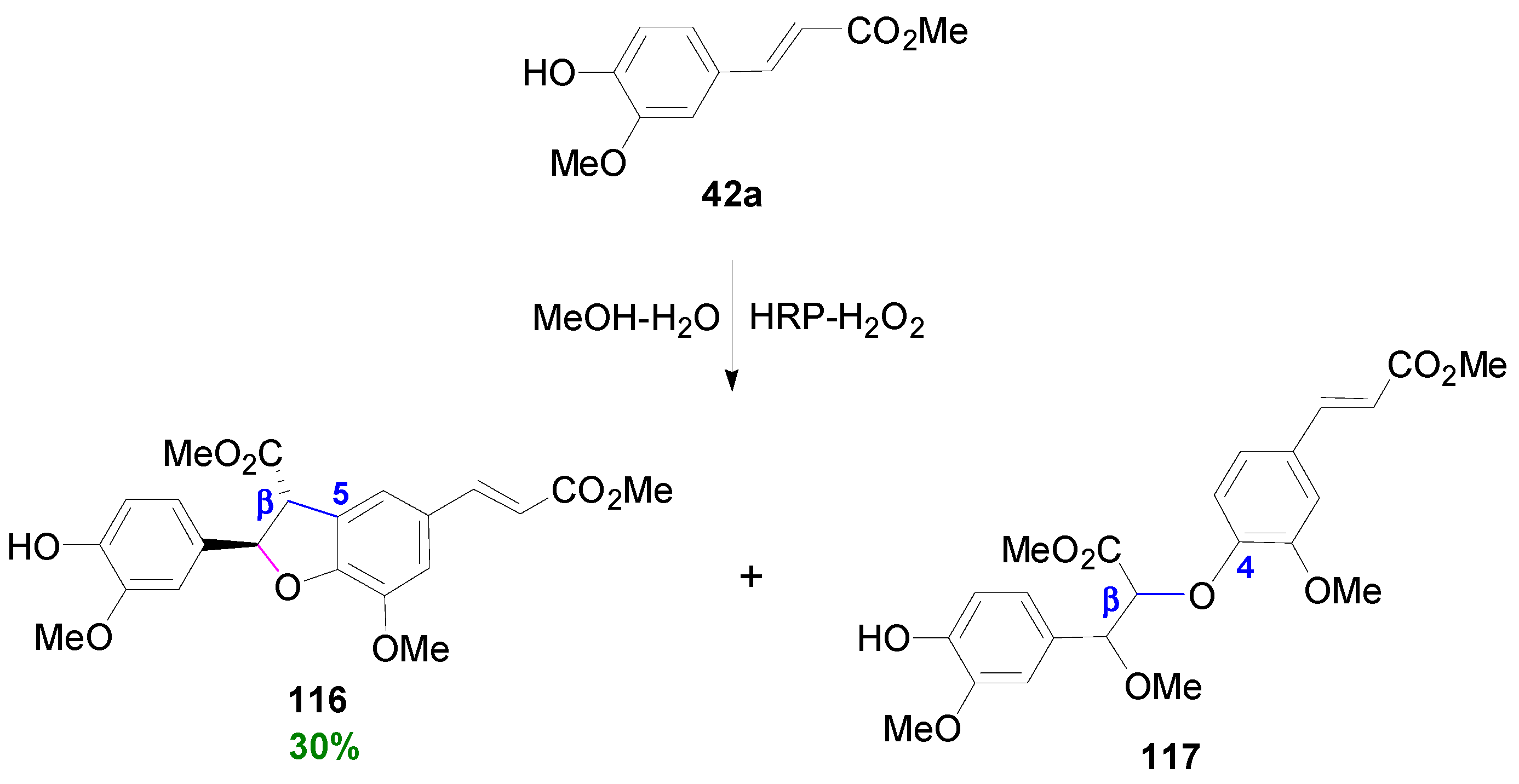
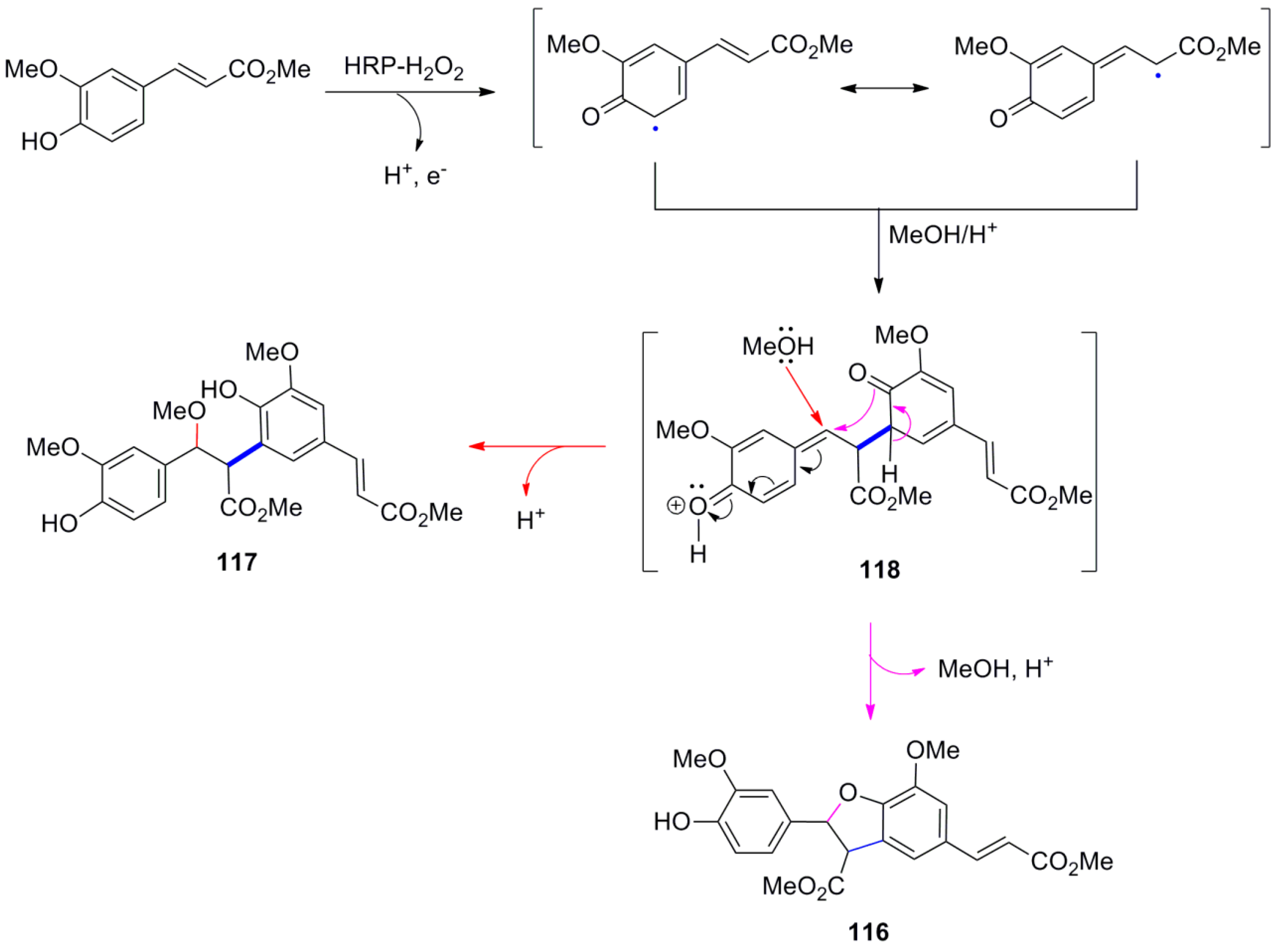
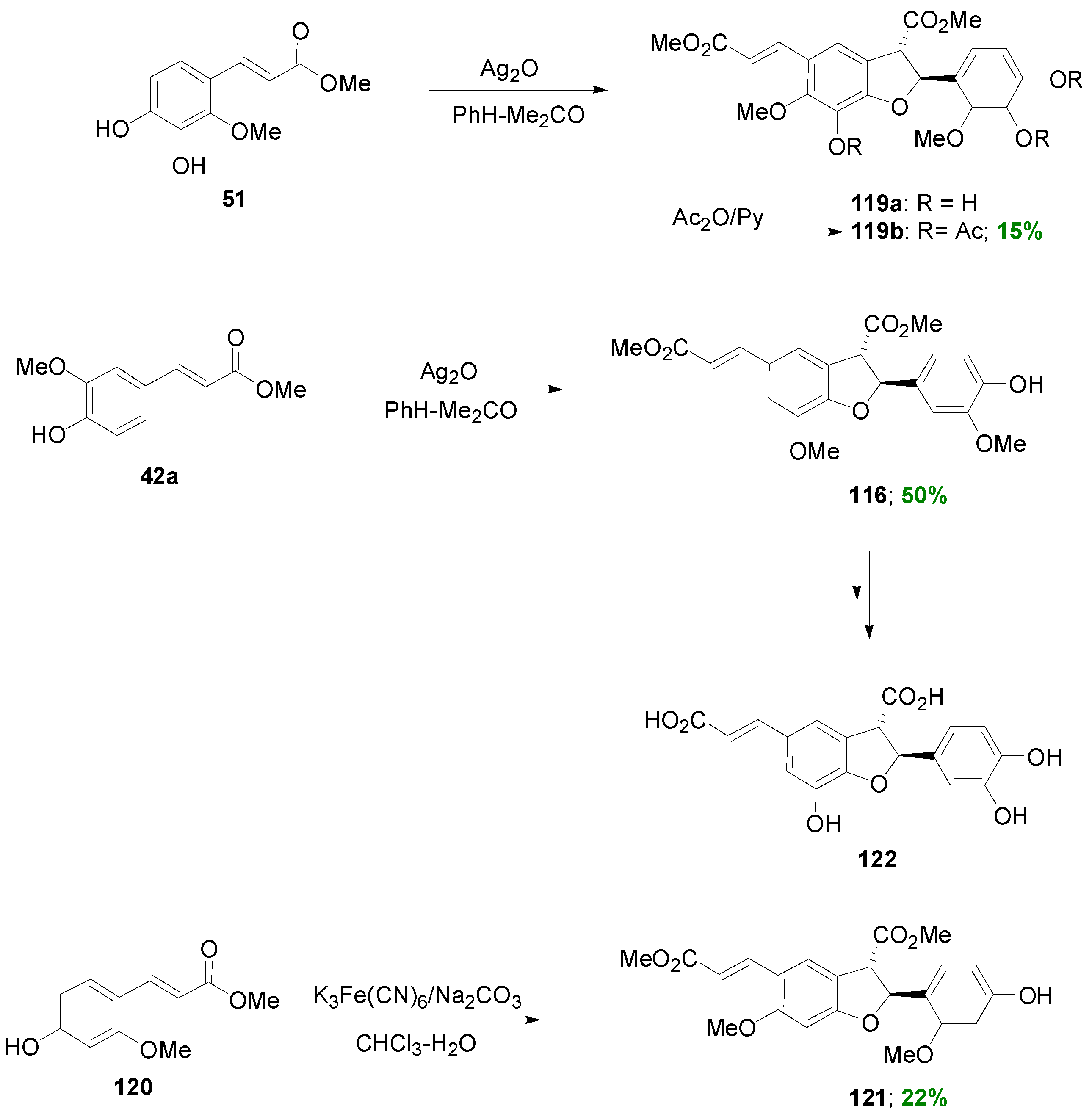


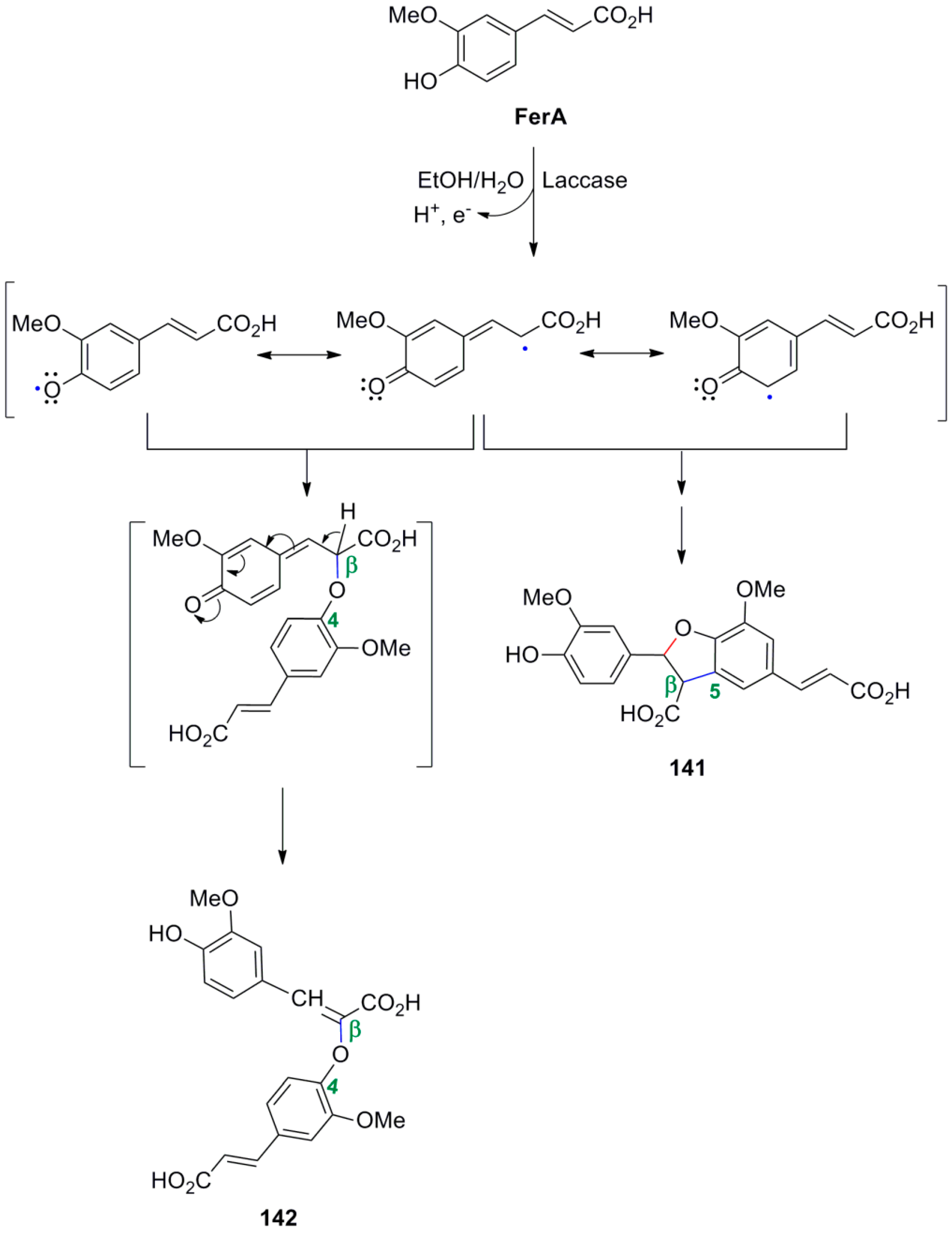

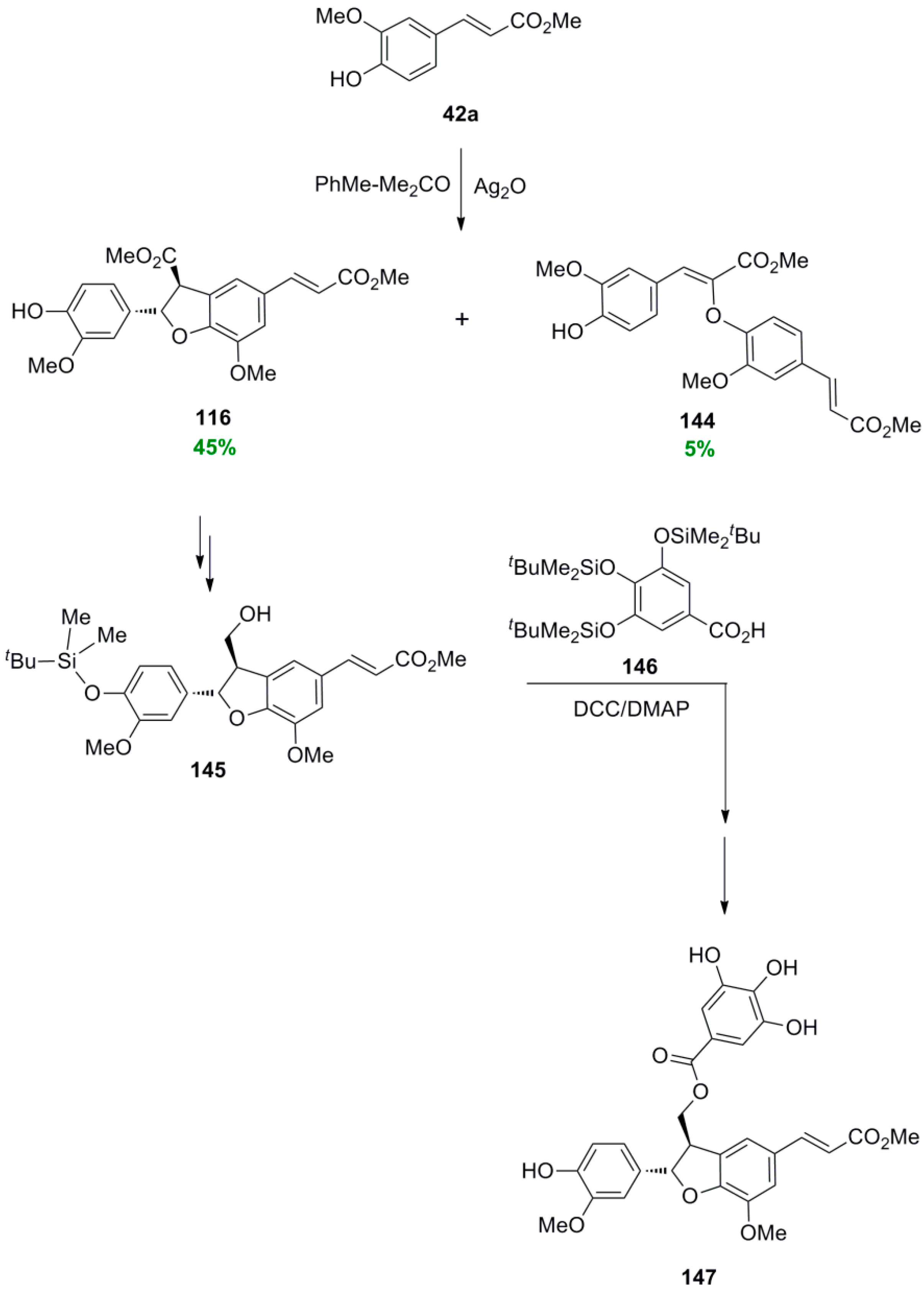

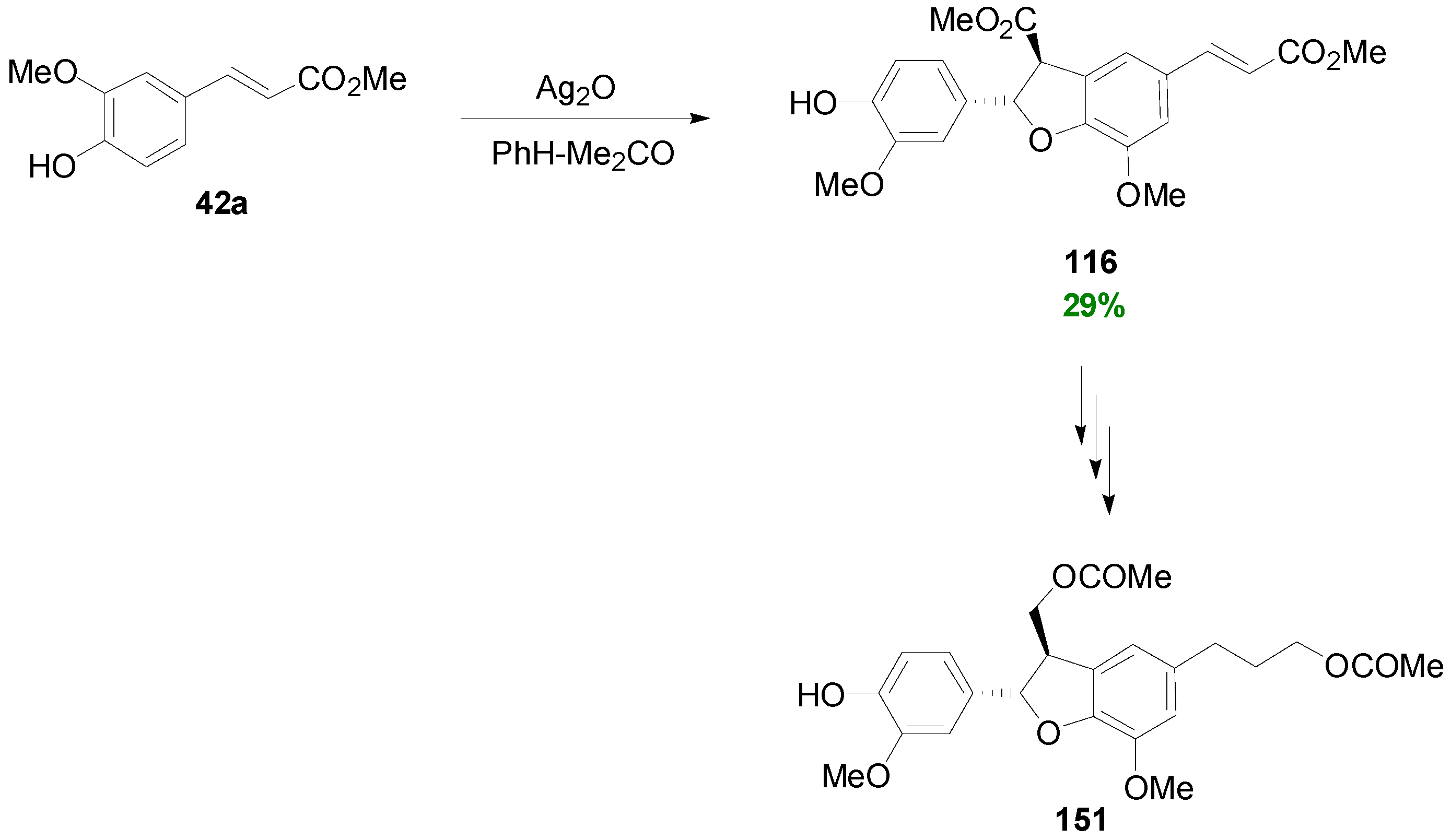
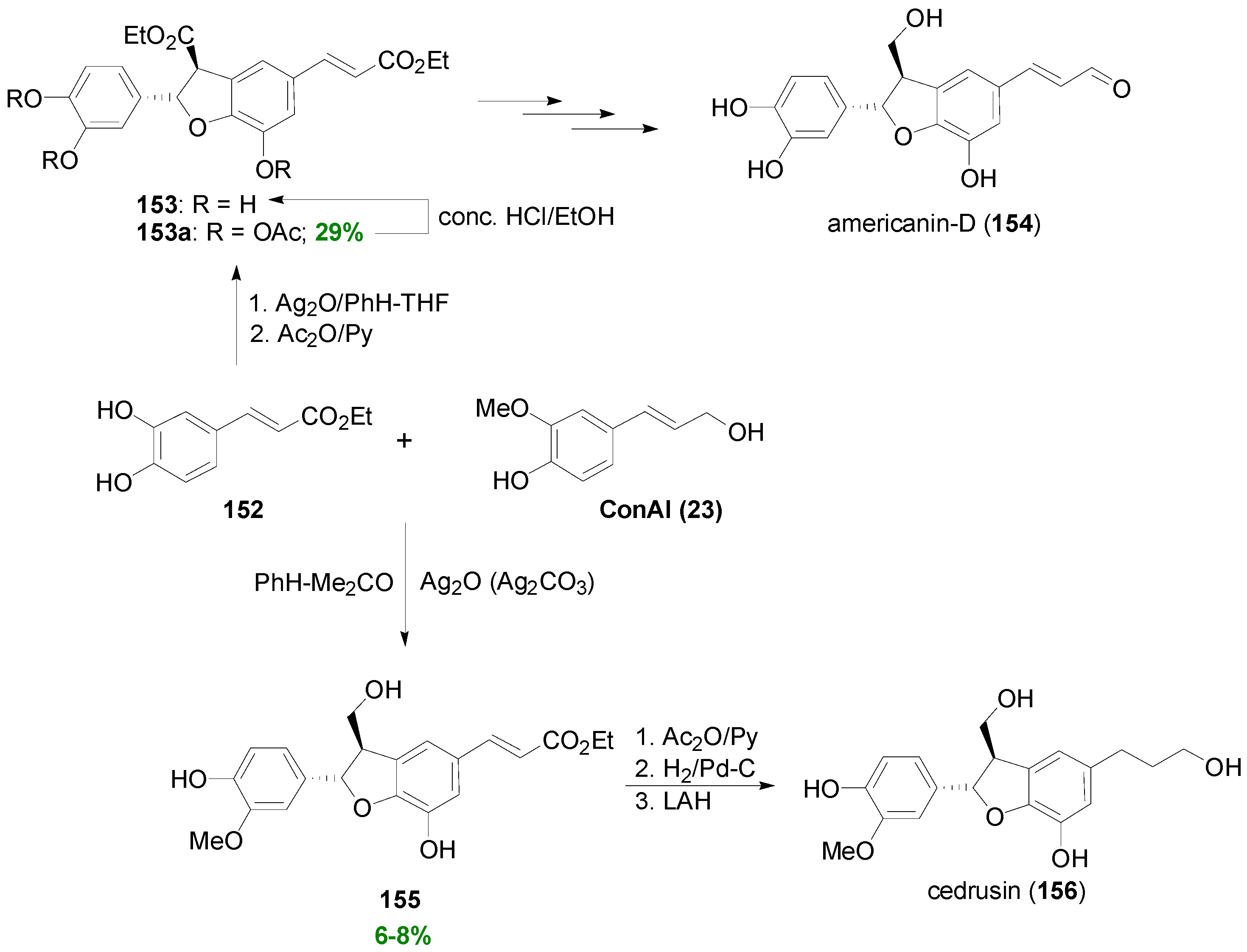



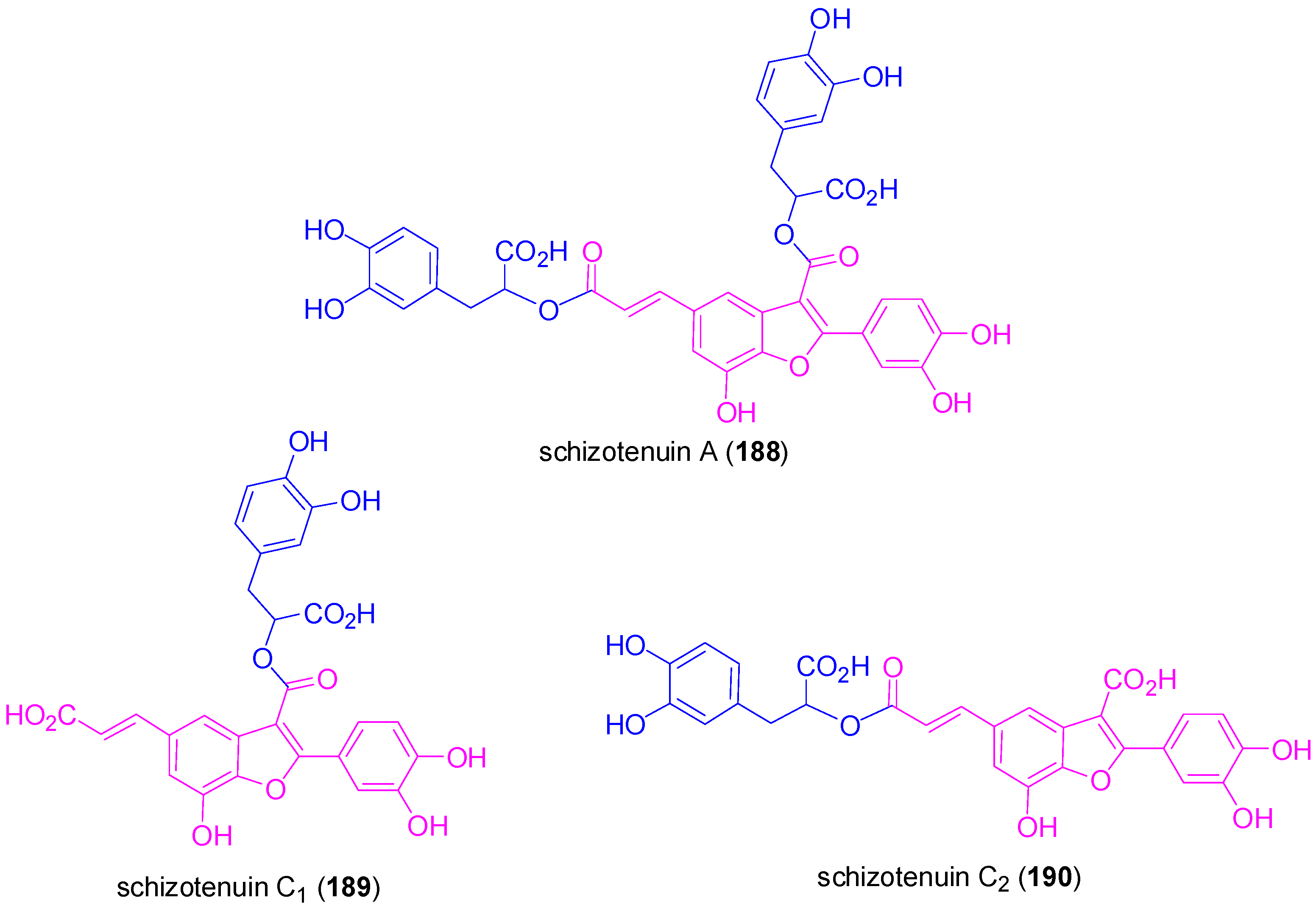
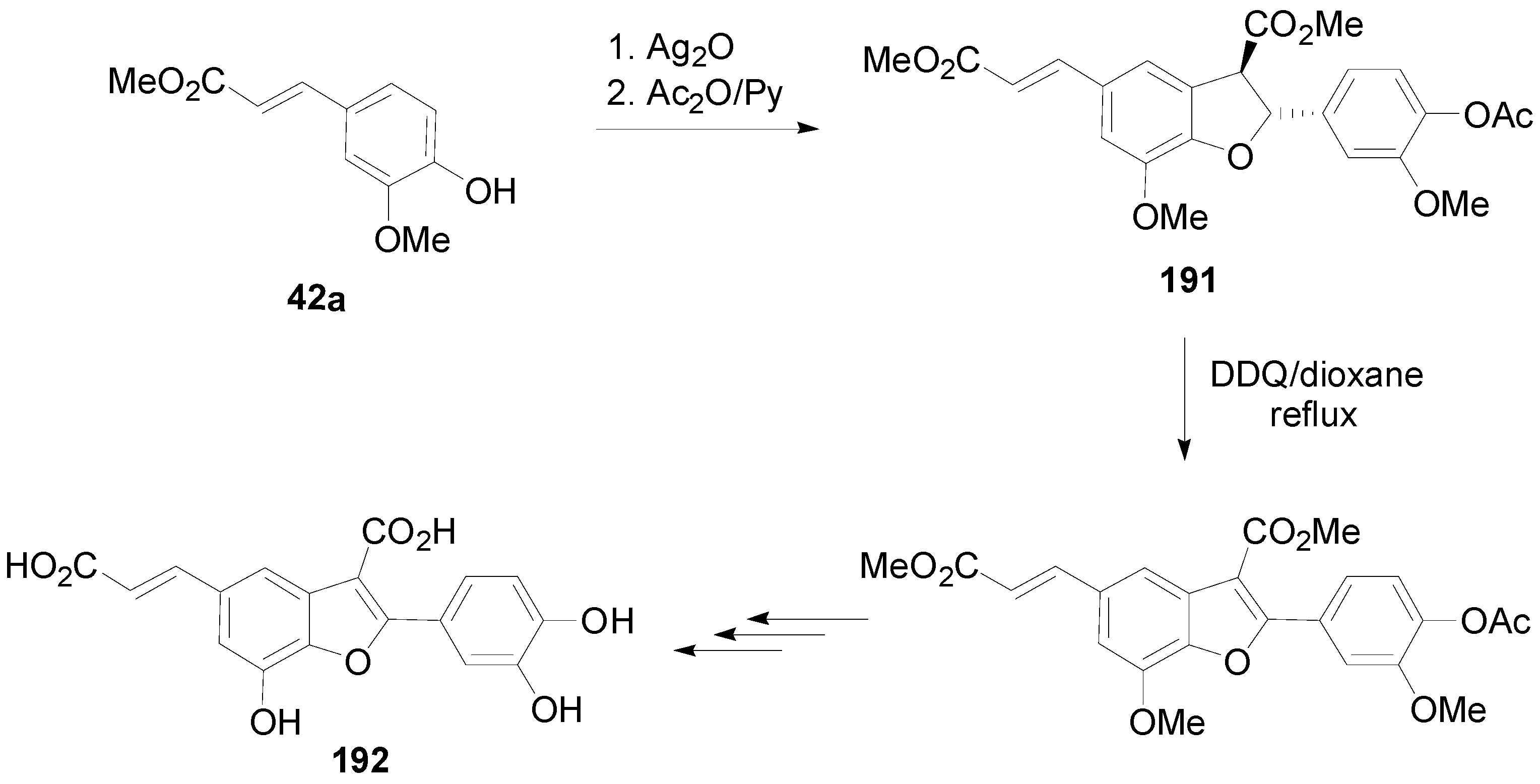
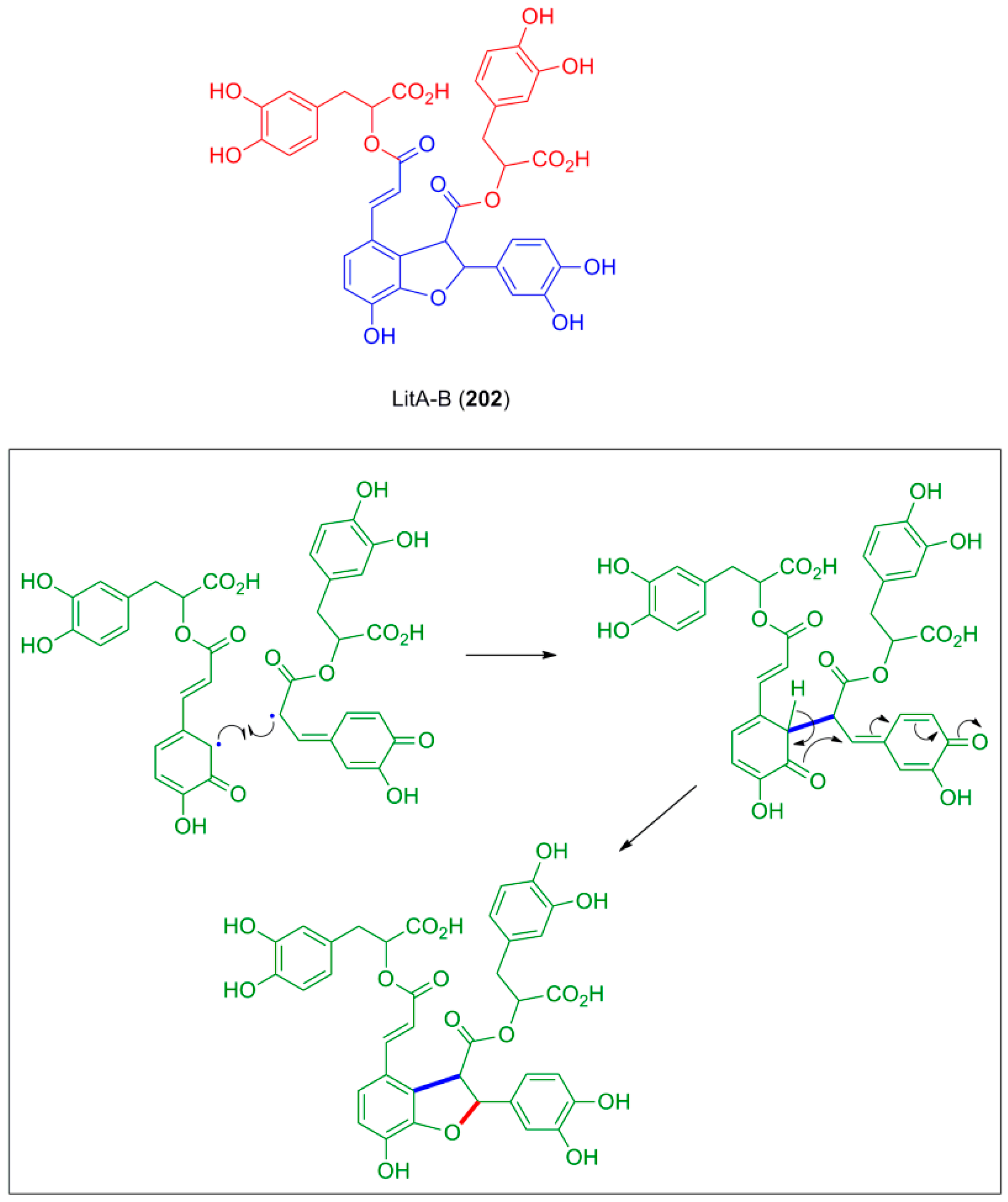
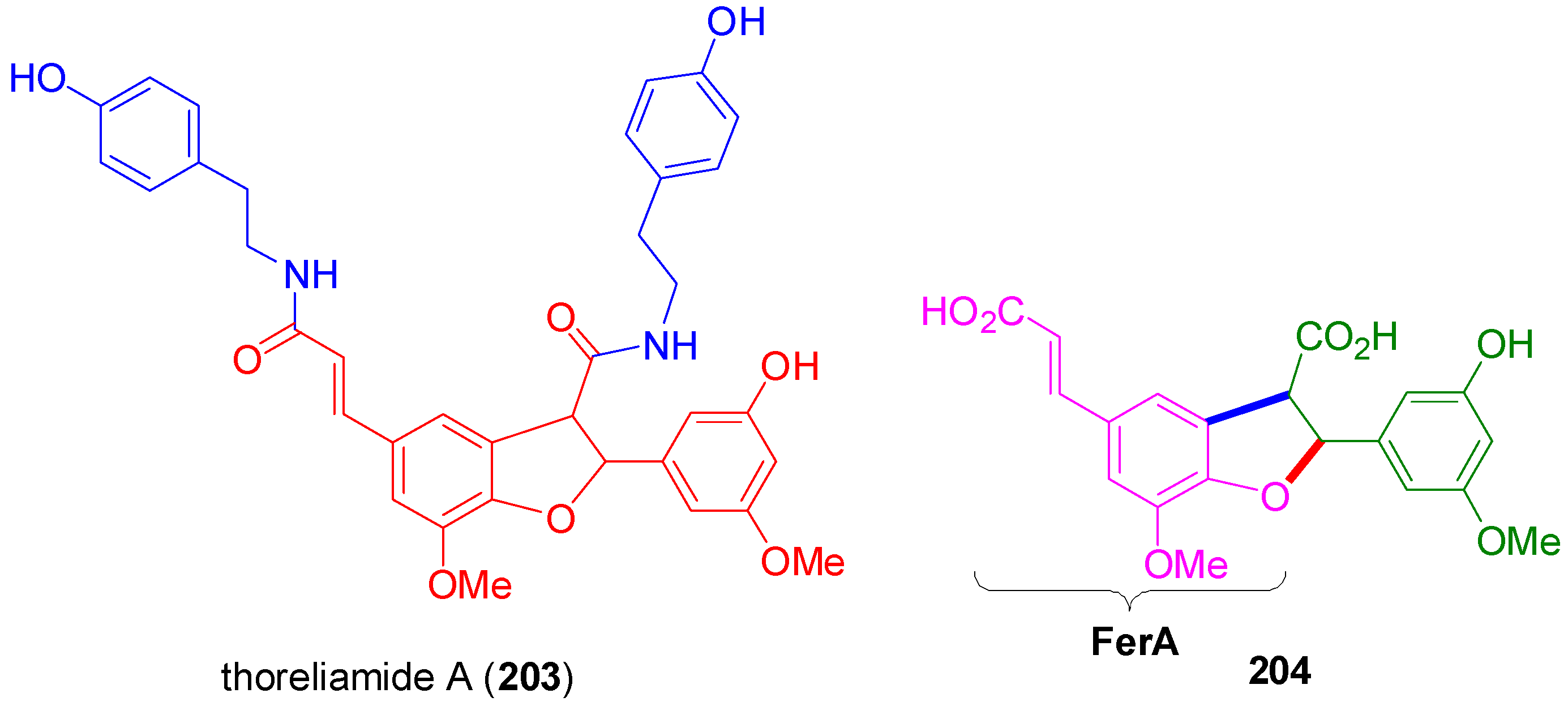
3.2. Synthesis of Oxyneolignans of the Ether Type—Formation of β-O-4 Bond as the Key Step

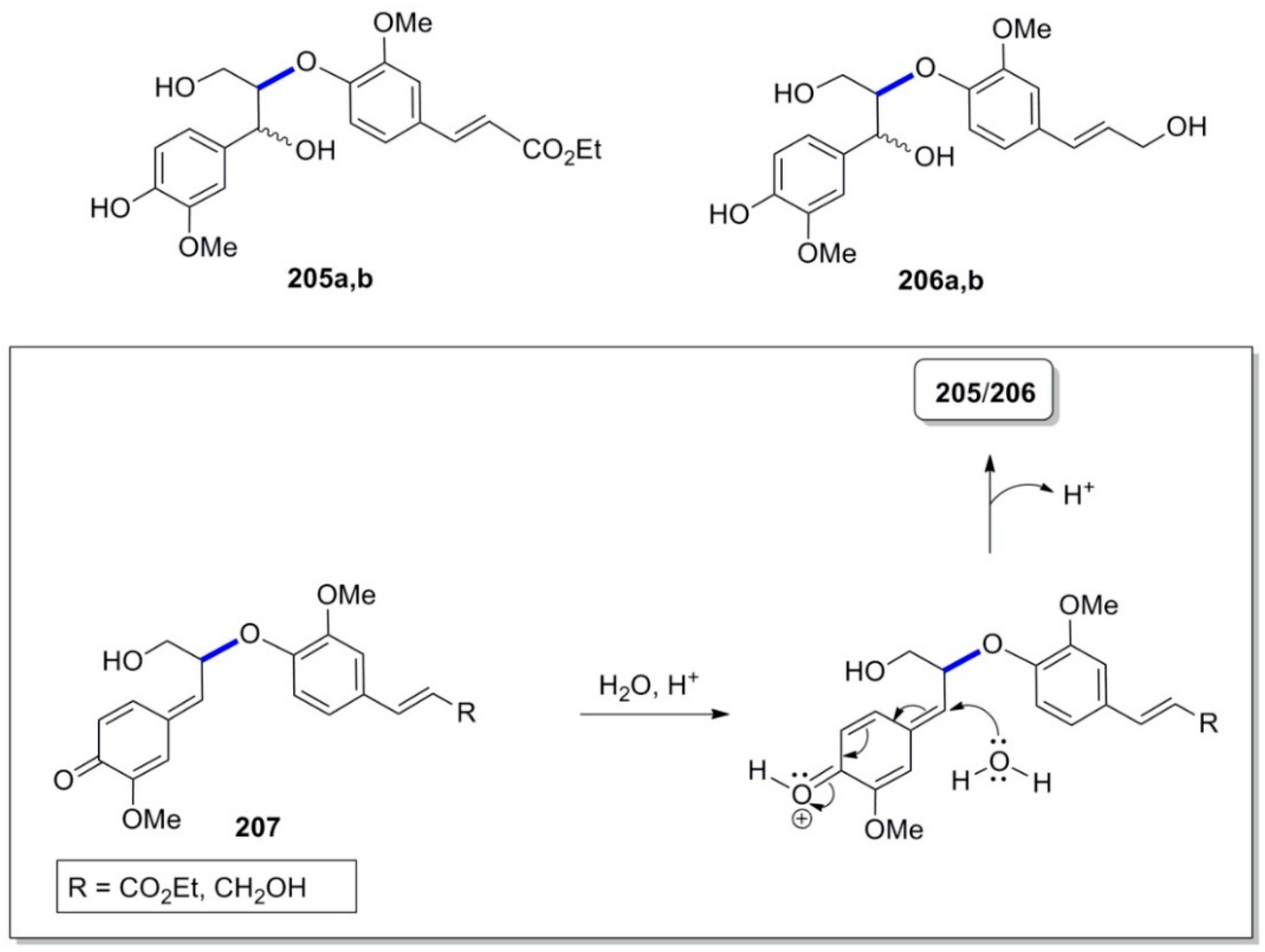
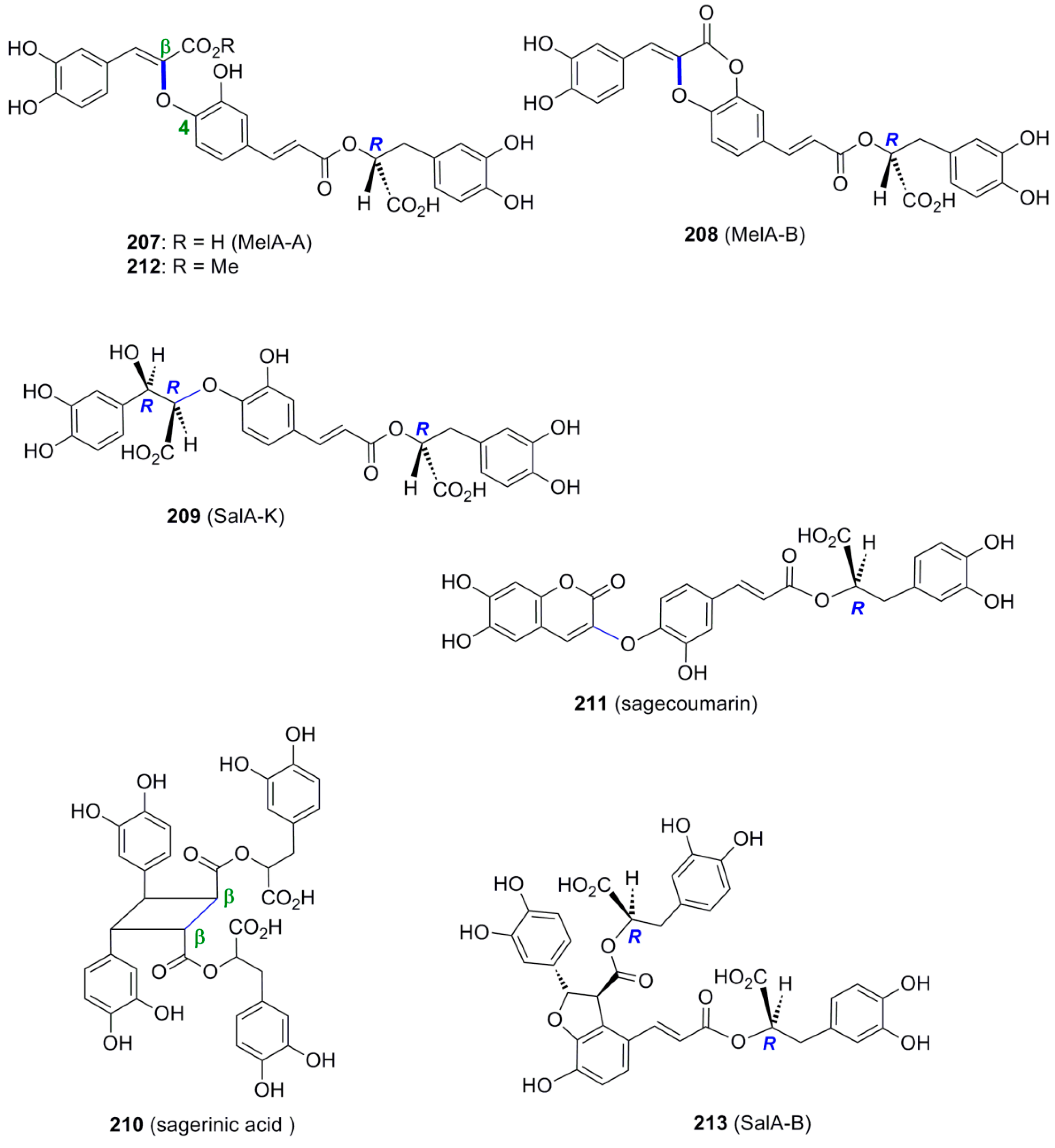

3.3. Synthesis of Oxyneolignans of the 1,4-Benzodioxane Type—Formation of β-O-4 Bond as the Key Step


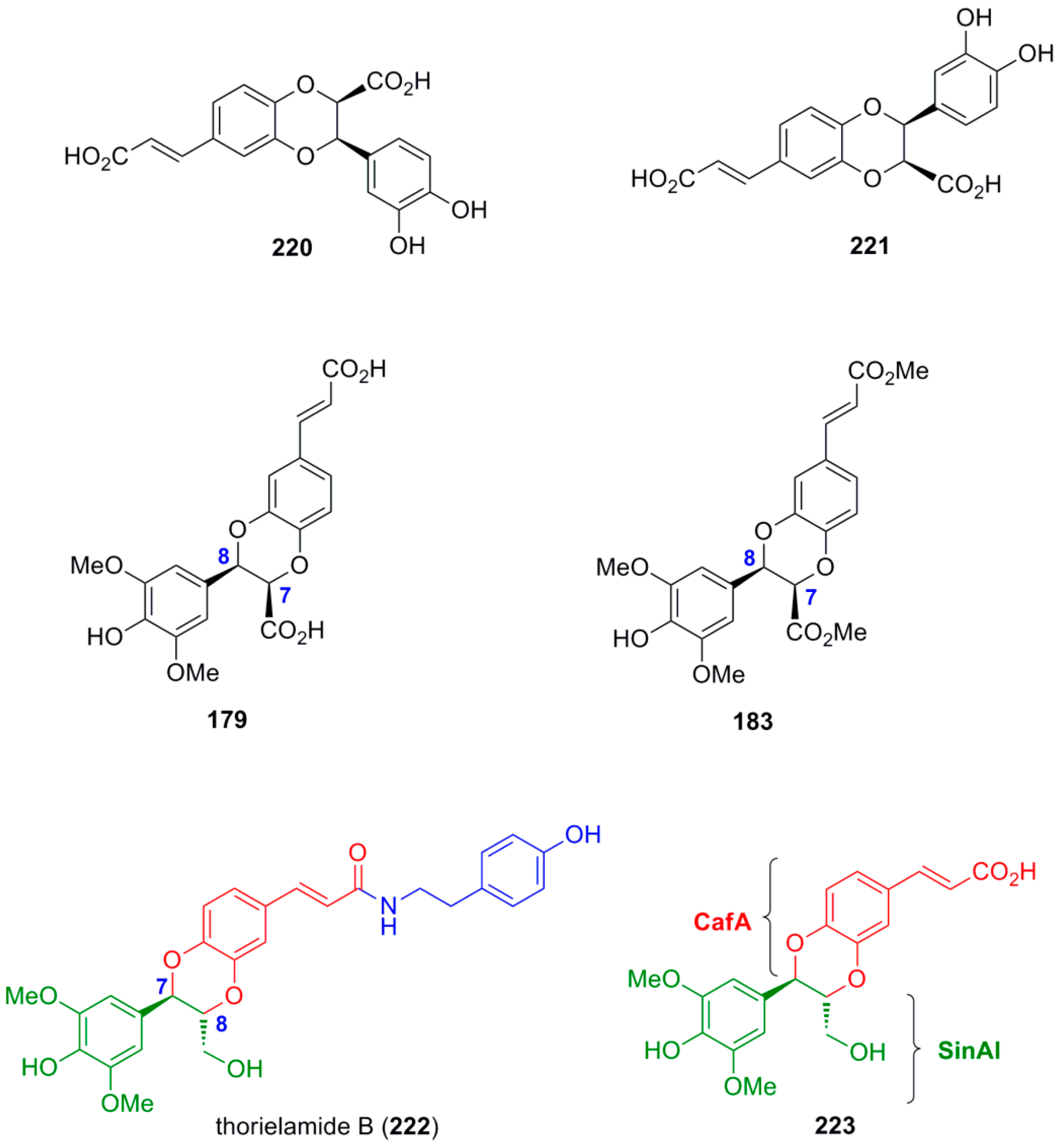
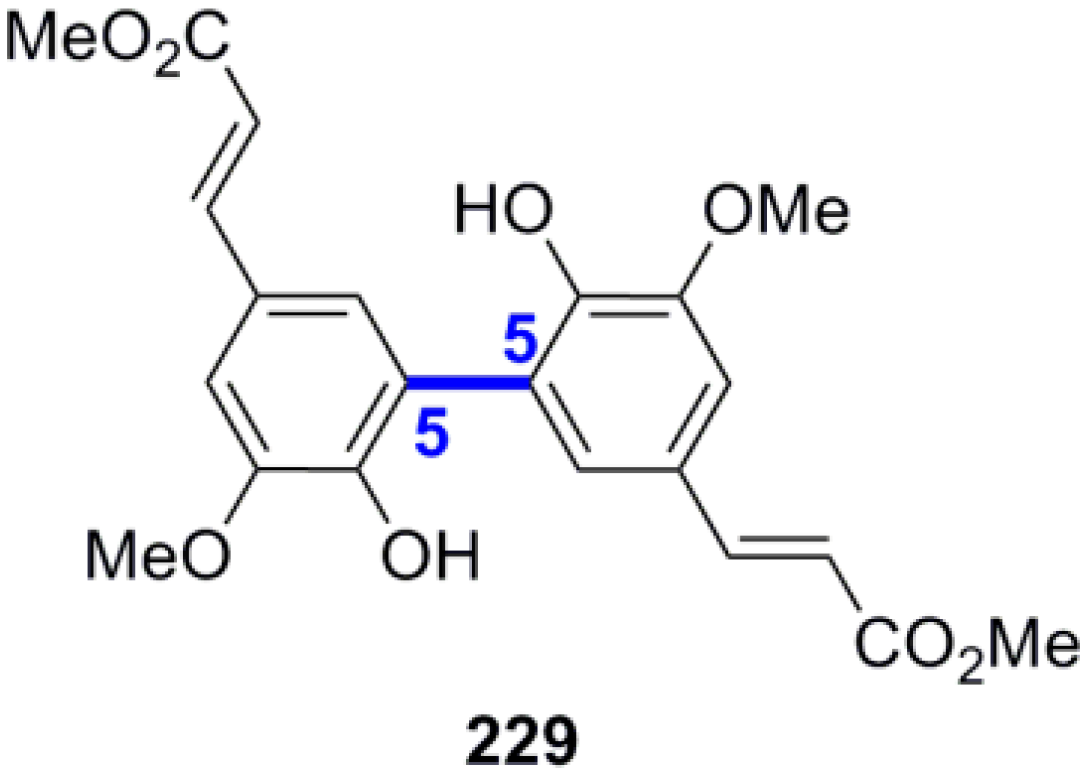
3.4. Synthesis of Other NLs—Formation of β-1 or 5-5 Bond as the Key Step

4. Conclusions
Abbreviation
| ACAs | alkoxycinnamic acids |
| APP | anionic potato peroxidase |
| CafA | caffeic acid |
| CAN | ceric ammonium nitrate |
| CAPE | phenethyl caffeate |
| CC | column chromatography |
| CinA | cinnamic acid |
| CL | classical lignan |
| ConAl | coniferyl alcohol |
| CouA | p-coumaric acid |
| DBU | 1,8-diazabicyclo[5.4.0]undec-7-ene |
| DCC | N,N'-dicyclohexylcarbodiimide |
| DDQ | 2,3-dichloro-5,6-dicyano-p-benzoquinone |
| DIBAL | diisobutylaluminium hydride |
| DMAP | 4-(dimethylamino)pyridine |
| DplA | 3-(3,4-dihydroxyphenyl)lactic acid |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| Et-Caf | ethyl caffeate |
| Et-Fer | ethyl ferulate |
| Et-Sin | ethyl sinapate |
| FCC | flash column chromatography |
| FerA | ferulic acid |
| Gly | glycine |
| HCAs | 4-hydroxycinnamic acids |
| HCAls | 4-hydroxycinnamyl alcohols |
| HFeA | 5-hydroxyferulic acid |
| HRP | horse radish peroxidase |
| LAH | lithium aluminium hydride |
| LitA | lithospermic acid |
| Me-Caf | methyl caffeate |
| Me-Fer | methyl ferulate |
| MelA | melitric acid |
| Me-Sin | methyl sinapate |
| Mn(ACAC)2 | manganese(II) acetylacetonate |
| Mn(III)TPPOAc | tetraphenylporphyrinatomanganese(III) acetate |
| NL | neolignan |
| non-POC | “non-phenolic” oxidative coupling |
| PA | polyamine |
| Phe | Phenylalanine |
| PIDA | phenyliodonium diacetate |
| PIFA | phenyliodonium bis(trifluoroacetate) |
| POC | phenol oxidative coupling |
| Pro | proline |
| pTSA | p-toluenesulfonic acid |
| RosA | rosmarinic acid |
| SalA | Salvianolic acid |
| SARS | structure-activity relationship studies |
| ShiA | shikimic acid |
| SinA | sinapic acid |
| SinAl | sinapyl alcohol |
| Spm | spermine |
| TBAF | tetrabutylammonium fluoride |
| TBDMS | tert-butyldimethylsilyl |
| ThoA | thomasidioic acid |
| TLC | thin layer chromatography |
| TMEDA | tetramethylethylenediamine |
| Tyr | tyrosine |
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Geissman, T.A.; Crout, D.H.G. Organic Chemistry of Secondary Plant Metabolism; Freeman, Cooper & Company: San Francisco, CA, USA, 1969; Chapter V; pp. 136–166, Chapter XIV, pp. 372–400. [Google Scholar]
- Teixera, J.; Gaspar, A.; Garrido, E.M.; Garrido, J.; Borges, F. Hydroxycinnamic acid antioxidants: An electrochemical overview. BioMed Res. Int. 2013. [Google Scholar] [CrossRef]
- De, P.; Baltas, M.; Bedos-Belval, F. Cinnamic acid derivatives as anticancer agents—A review. Curr. Med. Chem. 2011, 18, 1672–1703. [Google Scholar] [CrossRef] [PubMed]
- Lewis, N.G.; Davin, L.B. Lignans: Biosynthesis and function. In Comprehensive Natural Products Chemistry; Barton, Sir D.H.R., Nakanishi, K., Meth-Cohn, O., Eds.; Elsevier: Oxford, UK, 1999; Volume 1, pp. 639–712. [Google Scholar]
- Barton, D.H.R.; Cohen, T. Some biogenetic aspects of phenol oxidation. In Festschrift Arthur Stoll; Birkhauser: Basel, Switzerland, 1957; pp. 117–143. [Google Scholar]
- Barton, D.H.R.; Deflorin, A.M.; Edwards, O.E. The synthesis of Usnic acid. J. Chem. Soc. 1956, 530–534. [Google Scholar]
- Erdtman, H. Dehydrierungen in der coniferylreihe. II. Dehydrodi-isoeugenol. Annalen 1933, 503, 283–294. [Google Scholar] [CrossRef]
- Erdtman, H.; Wachtmeister, C.A. Phenoldehydrogenation as a biosynthetic reaction. In Festschrift Arthur Stoll; Birkhauser: Basel, Switzerland, 1957; pp. 144–165. [Google Scholar]
- Erdtman, H. Recent Advances in Phytochemistry; Aplleton-Century-Crofts: New York, NY, USA, 1968; Volume 1, p. 29. [Google Scholar]
- Gotlieb, O.R. Chemosystematics of the lauraceae. Phytochemistry 1972, 11, 1537–1570. [Google Scholar] [CrossRef]
- Brown, B.R. Biochemical Aspects of Oxidative Coupling of Phenols. In Oxidative Coupling of Phenols; Taylor, W.I., Battersby, A.R., Eds.; Edward Arnold Publishers: London, UK, 1967; pp. 167–201. [Google Scholar]
- Scott, A.I. Oxidative coupling of phenolic compounds. Q. Rev. (Chem. Soc.) 1965, 19, 1–35. [Google Scholar] [CrossRef]
- Lewis, N.G.; Sarkanen, S. (Eds.) Lignin and Lignan Biosynthesis; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1998; Volume 697.
- Moss, G.P. Nomenclature of lignans and neolignans. Pure Appl. Chem. 2000, 72, 1493–1523. [Google Scholar] [CrossRef]
- Setälä, H. Regio- and Stereoselectivity of Oxidative Coupling Reactions of Phenols: Spirodienones as Construction Units in Lignin. Ph.D. Thesis, University of Helsinki, Helsinki, Finland, September 2008. [Google Scholar]
- Umezawa, T.; Okunishi, T.; Shimada, M. Stereochemical diversity of lignan biosynthesis. Wood Res.: Bull. Wood Res. Inst. Kyoto Univ. 1997, 84, 62–75. [Google Scholar]
- Deyama, T.; Nishibe, S. Pharmacological properties of lignans. In Lignins and Lignans: Advances in Chemistry; Heitner, C., Dimmel, D.R., Schmidt, J.A., Eds.; CRC Press; Taylor & Francis Group: Boca Raton, FL, USA, 2010; pp. 585–629. [Google Scholar]
- Ward, R.S. The synthesis of lignans and neolignans. Chem. Soc. Rev. 1982, 11, 75–125. [Google Scholar] [CrossRef]
- Ward, R.S. Different strategies for the chemical synthesis of lignans. Phytochem. Rev. 2003, 2, 391–400. [Google Scholar] [CrossRef]
- Pan, J.-Y.; Chen, S.-L.; Yang, M.-H.; Wu, J.; Sinkkonen, J.; Zou, K. An update on lignans: Natural products and synthesis. Nat. Prod. Rep. 2009, 26, 1251–1292. [Google Scholar] [CrossRef] [PubMed]
- Pal, T.; Pal, A. Oxidative phenol coupling: A key step for the biommimetic synthesis of many important natural products. Curr. Sci. 1996, 71, 106–108. [Google Scholar]
- Erdtman, H.; Phenoldehydrierung, V.I. Dehydrierende Kupplung einiger Guajakoi-derivate. Svensk. Kem. Tidskr. 1935, 47, 223–230. [Google Scholar]
- Cartwright, N.J.; Haworth, R.D. Constituents of natural phenolic resins. XIX. Oxidation of ferulic acid. J. Chem. Soc. 1944, 535–537. [Google Scholar]
- Takei, Y.; Mori, K.; Matsui, M. Synthesis of dl-matairesinol dimethyl ether, dehydrodimethyl conidendrin and dehydrodimethyl retrodendrin from ferulic acid. Agric. Biol. Chem. (Jpn.) 1973, 37, 637–641. [Google Scholar] [CrossRef]
- Pelter, A.; Ward, R.S.; Watson, D.J.; Collins, P.; Kay, I.T. Synthesis of 2,6-diaryl-4,8-dihydroxy-3,7-dioxabicyclo[3.3.0]octanes. J. Chem. Soc. Perkin Trans. 1 1982, 175–181. [Google Scholar]
- Freudenberg, K.; Schraube, H. Synthese des syringaresinols und versuche mit sinapinalkohol. Chem. Ber. 1955, 88, 16–23. [Google Scholar] [CrossRef]
- Ahmed, R.; Lehrer, M.; Stevenson, R. Synthesis of thomasidioic acid. Tetrahedron Lett. 1973, 14, 747–750. [Google Scholar] [CrossRef]
- Ahmed, R.; Lehrer, M.; Stevenson, R. Synthesis of thomasic acid. Tetrahedron 1973, 29, 3753–3759. [Google Scholar] [CrossRef]
- Wallis, A.F.A. Oxidation of (E)- and (Z)-2,6-Dimethoxy-4-propenylphenol with ferric chloride—A facile route to the 2-aryl ethers of 1-Arylpropan-1,2-diols. Aust. J. Chem. 1973, 26, 585–594. [Google Scholar] [CrossRef]
- Wallis, A.F.A. Oxidative dimerization of methyl (E)-sinapate. Aust. J. Chem. 1973, 26, 1571–1576. [Google Scholar] [CrossRef]
- Kumada, Y.; Naganawa, H.; Takeuchi, T.; Umezawa, H.; Yamashita, K.; Watanabe, K. Biochemical activities of the derivatives of dehydrodicaffeic acid dilactone. J. Antibiot. 1978, 31, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.; Schreiber, F.G.; Stevenson, R.; Williams, J.R.; Yeo, H.M. Oxidative coupling of bromo- and iodo-ferulic acid derivatives: synthesis of (±)-veraguensin. Tetrahedron 1976, 32, 1339–1344. [Google Scholar] [CrossRef]
- Stevenson, R.; Williams, J.R. Synthesis of tetrahydrofuran lignans, (±)-galbelgin and (±)-grandisin. Tetrahedron 1977, 33, 285–288. [Google Scholar] [CrossRef]
- Ralph, J.; Quideau, S.; Grabber, J.H.; Hatfield, R.D. Identification and synthesis of new ferulic acid dehydrodimers present in grass cell walls. J. Chem. Soc. Perkin Trans. 1 1994, 3485–3498. [Google Scholar]
- Kumada, Y.; Takeuchi, T.; Umezawa, H. Characterization of the dehydrodicaffeic acid dilactone-forming enzyme and the enzymic and chemical synthesis of this mushroom product. Agric. Biol. Chem. 1977, 41, 877–885. [Google Scholar] [CrossRef]
- Jin, X.L.; Yang, R.T.; Shang, Y.J.; Dai, F.; Qan, Y.P.; Cheng, L.X.; Zhou, B.; Liu, Z.L. Oxidative coupling of cinnamic acid derivatives and their radical-scavenging activities. Chin. Sci. Bull. 2010, 55, 2885–2890. [Google Scholar] [CrossRef]
- Iguchi, M.; Nishiyama, A.; Eto, H.; Terada, Y.; Yamamura, S. Anodic oxidation of 4-hydroxycinnamic acids. Chem. Lett. 1979, 1397–1400. [Google Scholar]
- Cooper, R.; Gottlieb, H.E.; Lavie, D.; Levy, E.C. Lignans from Aegilops ovata L. Synthesis of a 2,4- and 2,6-diaryl monoepoxylignanolide. Tetrahedron 1979, 35, 861–868. [Google Scholar] [CrossRef]
- Taylor, E.C.; Andrade, J.G.; Rall, G.J.H.; Steliou, K.; Jagdmann, G.E., Jr.; McKillop, A. Thallium in Organic Synthesis. 60. 2,6-diaryl-3,7-dioxabicyclo[3.3.0]octane-4,8-dione lignans by oxidative dimerization of 4-alkoxycinnamic acids with thallium(III) trifluroacetate or cobalt(III) trifluoride. J. Org. Chem. 1981, 46, 3078–3081. [Google Scholar] [CrossRef]
- Mori, N.; Watanabe, H.; Kitahara, T. Simple and efficient asymmetric synthesis of furofuran lignans yangambin and caruilignan A. Synthesis 2006, 400–404. [Google Scholar]
- Setälä, H.; Pajunen, A.; Kilpelläinen, I.; Brunow, G. Horse radish peroxidase-catalysed oxidative coupling of methyl sinapate to give diastereomeric spiro dimers. J. Chem. Soc. Perkin Trans. 1 1994, 1163–1165. [Google Scholar]
- Bunzel, M.; Ralph, J.; Kim, H.; Lu, F.; Ralph, S.A.; Marita, J.M.; Hatfield, R.D.; Steinhart, H. Sinapate dehydrodimers and sinapate-ferulate heterodimers in cereal dietary fiber. J. Agric. Food Chem. 2003, 51, 1427–1434. [Google Scholar] [CrossRef] [PubMed]
- Neudorffer, A.; Deguin, B.; el Ha, C.; Fleury, M.-B.; Largeron, M. Electrochemical oxidative coupling of 4-hydroxycinnamic ester derivatives: A convenient methodology for the biomimetic synthesis of lignin precursors. Collect. Czechoslov. Chem. Commun. 2003, 68, 1515–1530. [Google Scholar] [CrossRef]
- Zoia, L.; Bruschi, M.; Orlandi, M.; Tolppa, E.-L.; Rindone, B. Assymetric biomimetic oxidations of phenols: the mechanism of the diastereo- and enantioselective synthesis of thomasidioic acid. Molecules 2008, 13, 129–148. [Google Scholar] [CrossRef] [PubMed]
- Cilliers, J.J.L.; Singleton, V.L. Characterization of the products of non-enzymic autoxidative phenolic reactions in a caffeic acid model system. J. Agric. Food Chem. 1991, 39, 1298–1303. [Google Scholar] [CrossRef]
- Maeda, S.; Masuda, H.; Tokoroyama, T. Studies on the preparation of bioactive lignans by oxidative coupling reaction. IV. Oxidative coupling reaction of methyl (E)-3-(3,4-dihydroxy-2-methoxyphenyl)propenoate and lipid peroxidation inhibitory effects of the produced lignans. Chem. Pharm. Bull. 1995, 43, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Maeda, S.; Masuda, H.; Tokoroyama, T. Studies on the preparation of bioactive lignans by oxidative coupling reaction. IΙ. Oxidative coupling reaction of methyl (E)-3-(4,5-dihydroxy-2-methoxyphenyl)propenoate and lipid peroxidation inhibitory effects of the produced lignans. Chem. Pharm. Bull. 1994, 42, 2506–2513. [Google Scholar] [CrossRef] [PubMed]
- Agata, I.; Hatano, T.; Nishibe, S.; Okuda, T. Rabdosiin, a new rosmarinic acid dimer with a lignan skeleton from Rabdosia japonica. Chem. Pharm. Bull. 1988, 36, 3223–3225. [Google Scholar] [CrossRef]
- Agata, I.; Hatano, T.; Nishibe, S.; Okuda, T. A tetrameric derivative of caffeic acid from Rabdosia japonica. Phytochemistry 1989, 28, 2447–2450. [Google Scholar] [CrossRef]
- Kashiwada, Y.; Nishizawa, M.; Yamagishi, T.; Tanaka, T.; Nonaka, G.; Cosentino, L.M.; Snider, J.V.; Lee, K. Anti-AIDS agents, 18. Sodium and potassium salts of caffeic acid tetramers from Arnebia euchroma as anti-HIV agents. J. Nat. Prod. 1995, 58, 392–400. [Google Scholar] [CrossRef] [PubMed]
- </b>Kashiwada, Y.; Bastow, K.F.; Lee, K. Novel lignan derivatives as selective inhibitors of DNA topoisomerase II. Bioorg. Med. Chem. Lett. 1995, 5, 905–908. [Google Scholar] [CrossRef]
- Bogucki, D.E.; Charlton, J.L. A non-enzymatic synthesis of (S)-(−)-rosmarinic acid and a study of a biomimetic route to (+)-rabdosiin. Can. J. Chem. 1997, 75, 1783–1794. [Google Scholar] [CrossRef]
- Reimann, E.; Pflug, T. Synthese der enantiomeren (+)- and (−)-rosmarinsäuremethylester. Monatsch. Chem. 1998, 129, 187–193. [Google Scholar]
- Huang, L.-J.; Li, C.-H.; Lu, Z.-M.; Ma, Z.-B.; Yu, D.-Q. Total synthesis and biological evaluation of (+)- and (−)-butyl ester of rosmarinic acid. J. Asian Nat. Prod. Res. 2006, 8, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Aung, H.T.; Furukawa, T.; Nikai, T.; Niwa, M.; Takaya, Y. Contribution of cinnamic acid analoues in rosmarinic acid to inhibition of snake venom induced hemorrhage. Bioorg. Med. Chem. 2011, 19, 2392–2396. [Google Scholar] [CrossRef] [PubMed]
- Snyder, S.A.; Kontes, F. Explorations into neolignan biosynthesis: Concise total syntheses of Helicterin B, Helisorin, and Helisterculin A from a common intermediate. J. Am. Chem. Soc. 2009, 131, 1745–1752. [Google Scholar] [CrossRef] [PubMed]
- Exarchou, V.; Takis, P.G.; Malouta, M.; Vervoort, J.; Karali, E.; Troganis, A.N. Four new depsides in Origanum dictamnus methanol extract. Phytochem. Lett. 2013, 6, 46–52. [Google Scholar] [CrossRef]
- Ge, F.; Tang, C.-P.; Ye, Y. Lignanamides and sesquiterpenoids from stems of Mitrephora thorelii. Helv. Chim. Acta 2008, 91, 1023–1030. [Google Scholar] [CrossRef]
- Daquino, C.; Rescifina, A.; Spatafora, C.; Tringali, C. Biomimetic synthesis of natural and “unnatural” lignans by oxidative coupling of caffeic esters. Eur. J. Org. Chem. 2009, 2009, 6289–6300. [Google Scholar] [CrossRef]
- Lin, H.-P.; Lin, C.-Y.; Huo, C.; Su, L.-C.; Chuu, C.-P. Anticancer effect of caffeic acid phenethyl ester. Pharmacologia 2012, 3, 26–30. [Google Scholar] [CrossRef]
- Maddaford, S.P.; Charlton, J.L. A general asymmetric synthesis of (−)-α-dimethylretrodendrin and its diastereomers. J. Org. Chem. 1993, 58, 4132–4138. [Google Scholar] [CrossRef]
- Broomhead, A.J.; Rahman, M.M.A.; Dewick, P.M.; Jackson, D.E.; Lucas, J.A. Matairesinol as precursor of Podophyllum lignans. Phytochemistry 1991, 30, 1489–1492. [Google Scholar] [CrossRef]
- Cambie, R.C.; Craw, P.A.; Rutledge, P.S.; Woodgate, P.D. Oxidative coupling of lignans III. Non-phenolic oxidative coupling of deoxypodorhizon and related compounds. Aust. J. Chem. 1988, 41, 897–918. [Google Scholar] [CrossRef]
- Sarkanen, K.V.; Wallis, A.F.A. Oxidative dimerization of methyl (E)-4-hydroxy-3,5-di-t-butylcinnamate with potassium ferricyanide. J. Chem. Soc. Perkin Trans. 1 1973, 1878–1881. [Google Scholar]
- Wang, Q.; Yang, Y.; Li, Y.; Yu, W.; Hou, Z.H. An efficient method for the synthesis of lignans. Tetrahedron 2006, 62, 6107–6112. [Google Scholar] [CrossRef]
- Sugahara, T.; Yamauchi, S.; Kondo, A.; Ohno, F.; Tominaga, S.; Nakashima, Y.; Kishida, T.; Akiyama, K.; Maruyama, M. First stereoselective synthesis of meso-secoisolariciresinol and comparison of its biological activity with (+) and (−) secoisolariciresinol. Biosci. Biotechnol. Biochem. 2007, 71, 2962–2968. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.-S.; Rahman, A.A.; Kim, J.-Y.; Kee, S.-H. Hanultarin, a cytotoxic lignan as an inhibitor of actin cytoskeleton polymerization from the seeds of Trichosanthes kirilowii. Bioorg. Med. Chem. 2008, 16, 7264–7269. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Ahamed, V.S.J.; Kumar, M.S.; Rhee, S.W.; Moon, S.-S.; Hong, I.S. Synthesis and evaluation of cytotoxic effect of hanultarin and its derivatives. Bioorg. Med. Chem. Lett. 2011, 21, 6245–6248. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.-Y.; Liu, W.K.; Che, C.-T. Lignanamides and nonalkaloidal components of Hyoscyamus niger seeds. J. Nat. Prod. 2002, 65, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Τomosaka, H.; Chin, Y.-W.; Salim, A.A.; Keller, W.J.; Chai, H.; Kingborn, A.D. Antioxidant and cytoprotective compounds from Berberis vulgaris (Barberry). Phytother. Res. 2008, 22, 979–981. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, W.; Wang, Q.; Yang, Z.; Hou, Z. Consize synthesis of cannabisin G. Bioorg. Med. Chem. Lett. 2010, 20, 5095–5098. [Google Scholar] [CrossRef] [PubMed]
- Chioccara, F.; Poli, S.; Rindone, B.; Pilati, T.; Brunow, G.; Pietikäinen, P.; Setälä, H. Regio- and Diastereo-selective synthesis of dimeric lignans using oxidative coupling. Acta Chem. Scand. 1993, 47, 610–616. [Google Scholar] [CrossRef]
- Maeda, S.; Masuda, H.; Tokoroyama, T. Studies on the preparation of bioactive lignans by oxidative coupling reaction. I. Preparation and lipid peroxidation inhibitory effect of benzofuran lignans related to schizotenuins. Chem. Pharm. Bull. 1994, 42, 2500–2505. [Google Scholar] [CrossRef] [PubMed]
- Maeda, S.; Masuda, H.; Tokoroyama, T. Studies on the preparation of bioactive lignans by oxidative coupling reaction. III. Synthesis of polyphenolic benzofuran and coumestan derivatives by oxidative coupling reaction of methyl (E)-3-(4-hydroxy-2-methoxyphenyl)propenoate and their inhibitory effect on lipid peroxidation. Chem. Pharm. Bull. 1994, 42, 2536–2545. [Google Scholar] [CrossRef] [PubMed]
- Bolzacchini, E.; Brunow, G.; Meinardi, S.; Orlandi, M.; Rindone, B.; Rummakko, P.; Setala, H. Enantioselective synthesis of a benzofuranic neolignan by oxidative coupling. Tetrahedron Lett. 1998, 39, 3291–3294. [Google Scholar] [CrossRef]
- Ralph, J.; Garcia Conesa, M.T.; Williamson, G. Simple preparation of 8–5 coupled diferulate. J. Agric. Food Chem. 1998, 46, 2531–2532. [Google Scholar] [CrossRef]
- Pieters, L.; van Dyck, S.; Gao, M.; Bai, R.; Hamel, E.; Vlietinck, A.; Lemière, G. Synthesis and biological evaluation of dihydrobenzofuran lignans and related compounds as potentia antitumor agents that inhibit tubulin polymerization. J. Med. Chem. 1999, 42, 5475–5481. [Google Scholar] [CrossRef] [PubMed]
- Carunchio, F.; Crescenzi, C.; Girelli, A.M.; Messina, A.; Tarola, A.M. Oxidation of ferulic acid by laccase: Identification of the products and inhibitory effect of some dipeptides. Talanta 2001, 55, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.-H.; Wu, C.-H. Synthesis of 5-(3-hydroxypropyl)-7-methoxy-2-(3'-methoxy-4'-hydroxyphenyl)-3-benzo[b]furancarbaldehyde, a novel adenosine A1 receptor ligand from the root of Salvia miltiorrhiza. J. Nat. Prod. 1996, 59, 625–628. [Google Scholar] [CrossRef]
- Chang, J.Y.; Chang, C.-Y.; Kuo, C.-C.; Chen, L.-T.; Wein, Y.-S.; Kuo, Y.-H. Salvinal, a novel microtubule inhibitor isolated from Salvia miltiorrhizae Bunge (Danshen), with antimitotic activity in multi-drug-sensitive and-resistant human tumor cells. Mol. Pharmacol. 2004, 65, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Rakotondramanana, D.L.A.; Delomenede, M.; Baltas, M.; Duran, H.; Bedos-Belval, F.; Rasoanaivo, P.; Negre-Salvayre, A.; Gornitzka, H. Synthesis of ferulic ester dimers, functionalization and biological evaluation as potential antiatherogenic and antiplasmodial agents. Bioorg. Med. Chem. 2007, 15, 6018–6026. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Lu, F.; Sun, R.; Ralph, J. Ferulate-coniferyl alcohol cross-coupled products formed by radical coupling reactions. Planta 2009, 229, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- Subbaraju, G.; Kavitha, J.; Rajasekhar, D.; Hsu, F.-L.; Cheng, K.-T. Justicia lignans: Part 10—Synthesis of tiruneesiin, the first neolignan from Justicia species. Ind. J. Chem. 2007, 46B, 357–359. [Google Scholar]
- Antus, S.; Bauer, R.; Gottsegen, A.; Seligmann, O.; Wagner, H. Synthese von Americanin-D. Liebigs Ann. Chem. 1987, 1987, 357–360. [Google Scholar] [CrossRef]
- Antus, S.; Baitz-Gàcs, E.; Bauer, R.; Gottsegen, A.; Seligmann, O.; Wagner, H. Regioselective synthesis of 2- and 3-aryl-1,4-benzodioxanes. Liebigs Ann. Chem. 1989, 1989, 1147–1151. [Google Scholar] [CrossRef]
- Antus, S.; Baitz-Gàcs, E.; Bauer, R.; Gottsegen, A.; Seligmann, O.; Wagner, H. Total synthesis of cedrusin and its methyl ether. Liebigs Ann. Chem. 1990, 1990, 495–497. [Google Scholar] [CrossRef]
- Antus, S.; Gottsegen, A.; Kolonits, P.; Wagner, H. Total synthesis of two naturally occurring neolignans of potential biological activity. Liebigs Ann. Chem. 1989, 1989, 593–594. [Google Scholar] [CrossRef]
- Bruschi, M.; Orlandi, M.; Rindone, B.; Rummakko, P.; Zoia, L. Asymmetric biomimetic oxidations of phenols using oxazolidines as chiral auxiliaries: the enantioselective synthesis of (+)- and (−)-dehydrodiconiferyl alcohol. J. Phys. Org. Chem. 2006, 19, 592–596. [Google Scholar] [CrossRef]
- Arrieta-Baez, D.; Stark, R.E. Modeling suberization with peroxidase-catalyzed polymerization of hydroxycinnamic acids: Cross-coupling and dimerization reactions. Phytochemistry 2006, 67, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Saliu, F.; Tolppa, E.-L.; Zoia, L.; Orlandi, M. Horseradish peroxidase catalyzed oxidative cross-coupling reactions: The synthesis of “unnatural” dihydrobenzofuran lignans. Tetrahedron Lett. 2011, 52, 3856–3860. [Google Scholar] [CrossRef]
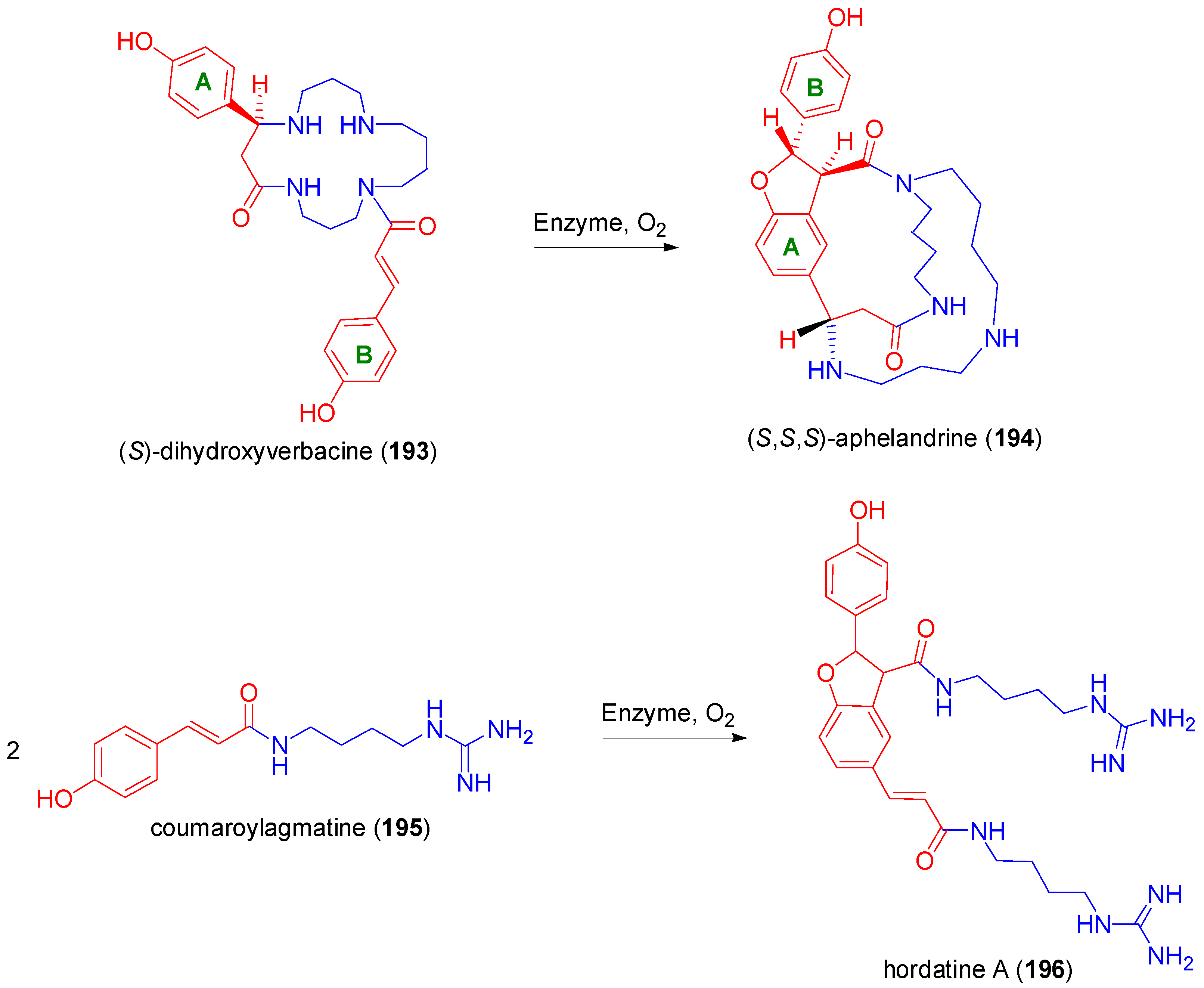
- Nezbedovà, L.; Hesse, M.; Drandarov, K.; Werner, C. New reagent for oxidative phenol coupling. The transformation of the monocyclic spermine base (S)-dihydroverbacine to the bicyclic alkaloid (S,S,S)-aphelandrine by cell free extract of barley seedlings. Tetrahedron Lett. 2001, 42, 4139–4141. [Google Scholar] [CrossRef]
- Bird, C.R.; Smith, T.A. The biosynthesis of coumarylagmantine in barley seedings. Phytochemistry 1981, 20, 2345–2346. [Google Scholar] [CrossRef]
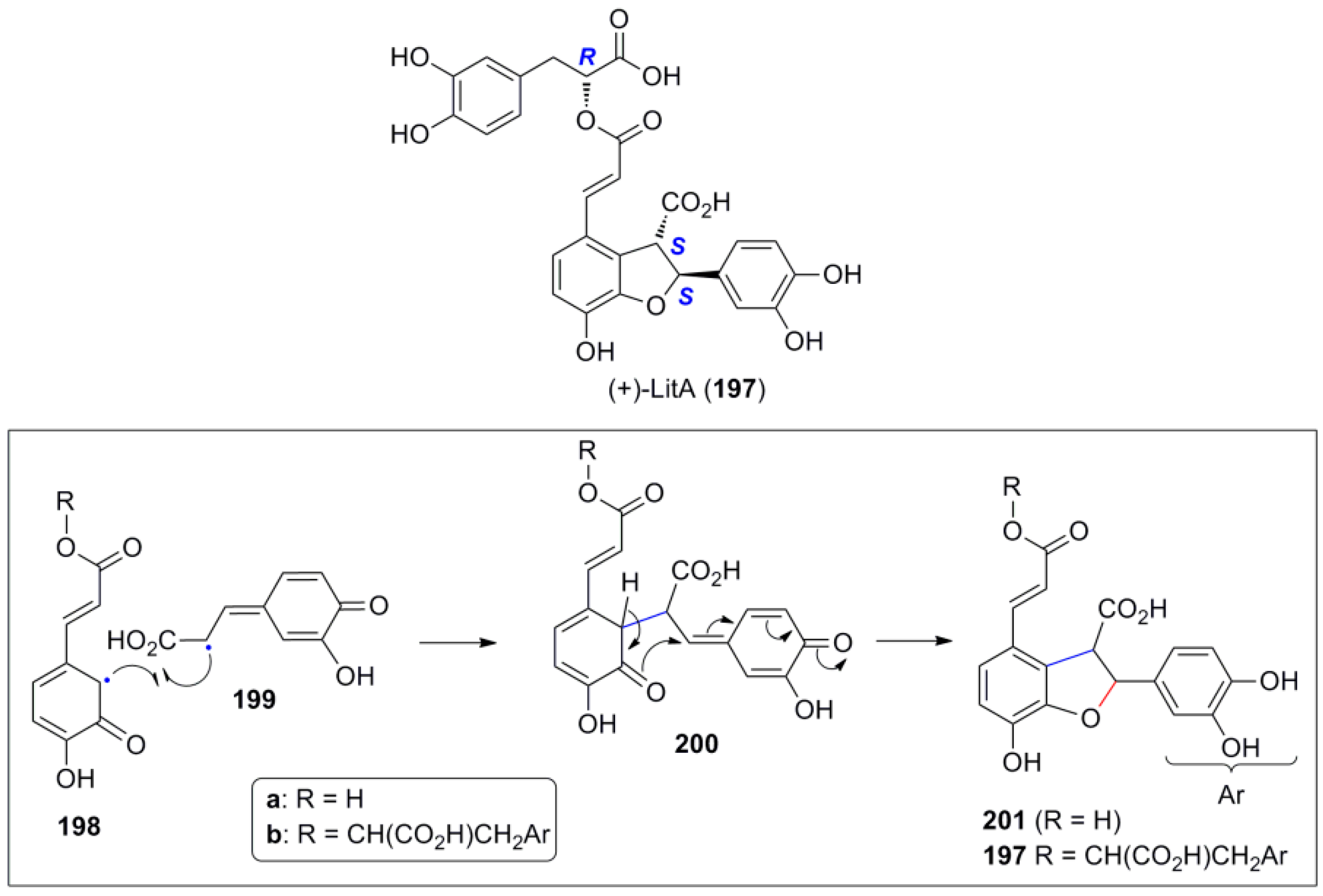
- For a very informative short introduction on the isolation, characterization, biological properties and synthesis of (+)-lithospermic acid see: Varadaraju, T.G.; Hwu, J.R. Synthesis of anti-HIV lithospermic acid by two divergent strategies. Org. Biomol. Chem. 2012, 10, 5456–5465. [Google Scholar]
- Jacobson, R.M.; Raths, R.A. Total synthesis of heptamethyl lithospermate. J. Org. Chem. 1979, 44, 4013–4014. [Google Scholar] [CrossRef]
- O’Malley, S.J.; Tan, K.L.; Watzke, A.; Bergman, R.G.; Ellman, J.A. Total synthesis of (+)-lithospermic acid by asymmetric intramolecular alkylation via catalytic C-H bond activation. J. Am. Chem. Soc. 2005, 127, 13496–13497. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-H.; Yu, J.-Q. Highly convergent total synthesis of (+)-lithospermic acid via a late-stage intermolecular C-H olefination. J. Am. Chem. Soc. 2011, 133, 5767–5769. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.; Savage, G.P.; Coster, M.J. A concise route to dehydrobenzo[b]furans: Formal total synthesis of (+)-lithospermic acid. Org. Lett. 2011, 13, 3376–3379. [Google Scholar] [CrossRef] [PubMed]
- Ly, T.N.; Shimoyamada, M.; Yamauchi, R. Isolation and characterization of rosmarinic acid oligomers in Celastrus hindsii Benth leaves and their antioxidative activities. J. Agric. Food Chem. 2006, 54, 3786–3793. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Wei, L.; Azarpira, A.; Ralph, J. Rapid synthesis of dehydrodiferulates via biomometic radical coupling reactions of ethyl ferulate. J. Agric. Food Chem. 2012, 60, 8272–8277. [Google Scholar] [CrossRef] [PubMed]
- Agata, I.; Kusakabe, H.; Hatano, T.; Nishibe, S.; Okuda, T. Melitric acids A and B, new trimeric caffeic acid derivatives from Meliss officinalis. Chem. Pharm. Bull. 1993, 41, 1608–1611. [Google Scholar] [CrossRef]
- Lu, Y.; Foo, L.Y. Rosmarinic acid derivatives from Salvia officinalis. Phytochemistry 1999, 51, 91–94. [Google Scholar] [CrossRef]
- Lu, Y.; Foo, L.Y.; Wong, H. Sagecoumarin, a novel caffeic acid trimer from Salvia officinalis. Phytochemistry 1999, 52, 1149–1152. [Google Scholar] [CrossRef]
- Wang, J.; Xiong, X.; Feng, B. Cardiovascular effects of salvianolic acid B. Evid. Based Complement. Alternat. Med. 2013, 2013. [Google Scholar] [CrossRef]
- Zhao, Y.; Guo, Y.; Gu, X. Salvianolic acid B, a potential chemoprotective agent, for head and necksquamous cell cancer. J. Oncol. 2011, 2011. [Google Scholar] [CrossRef]
- Setälä, H.; Pajunen, A.; Rummakko, P.; Sipilä, J.; Brunow, G. A novel spiro compound formed by oxidative cross coupling of methyl sinapate with a syringyl lignin model compound. A model system for the β-1 pathway in lignin biosynthesis. J. Chem. Soc. Perkin Trans. 1 1999, 461–464. [Google Scholar]
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magoulas, G.E.; Papaioannou, D. Bioinspired Syntheses of Dimeric Hydroxycinnamic Acids (Lignans) and Hybrids, Using Phenol Oxidative Coupling as Key Reaction, and Medicinal Significance Thereof. Molecules 2014, 19, 19769-19835. https://doi.org/10.3390/molecules191219769
Magoulas GE, Papaioannou D. Bioinspired Syntheses of Dimeric Hydroxycinnamic Acids (Lignans) and Hybrids, Using Phenol Oxidative Coupling as Key Reaction, and Medicinal Significance Thereof. Molecules. 2014; 19(12):19769-19835. https://doi.org/10.3390/molecules191219769
Chicago/Turabian StyleMagoulas, George E., and Dionissios Papaioannou. 2014. "Bioinspired Syntheses of Dimeric Hydroxycinnamic Acids (Lignans) and Hybrids, Using Phenol Oxidative Coupling as Key Reaction, and Medicinal Significance Thereof" Molecules 19, no. 12: 19769-19835. https://doi.org/10.3390/molecules191219769