Natural Cinnamic Acids, Synthetic Derivatives and Hybrids with Antimicrobial Activity
Abstract
:1. Introduction
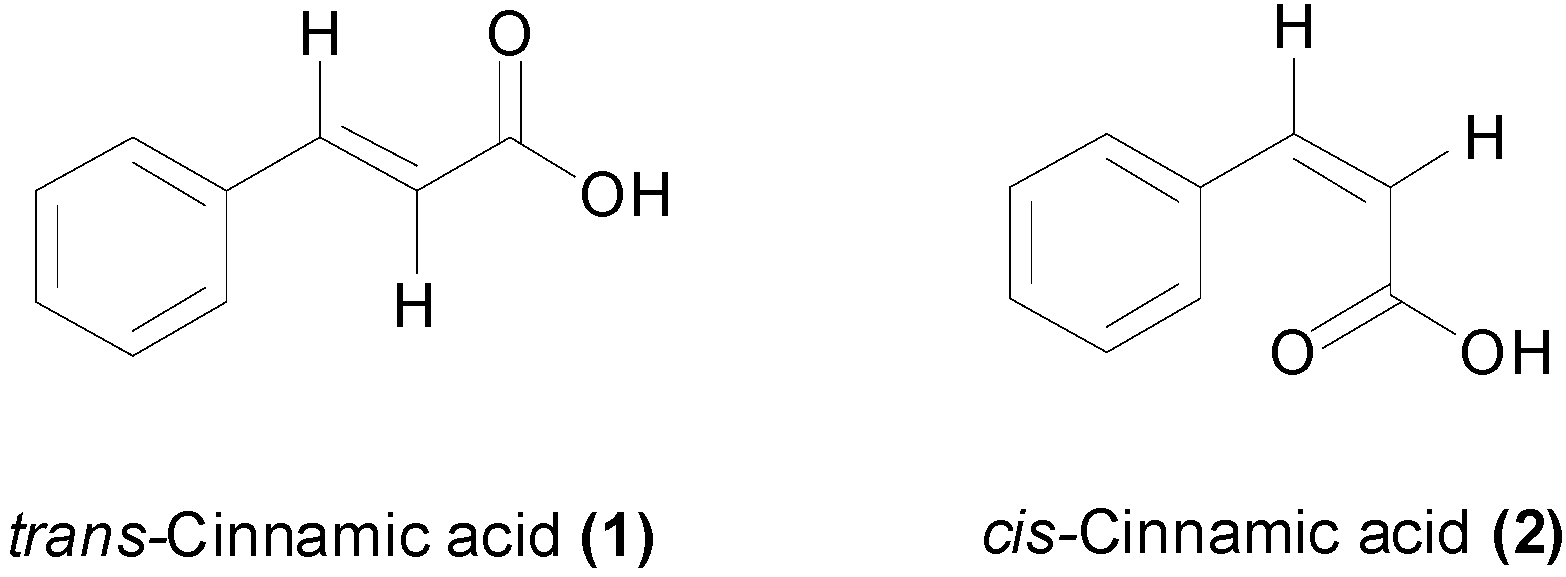
2. Natural and Synthetic Cinnamic Acids
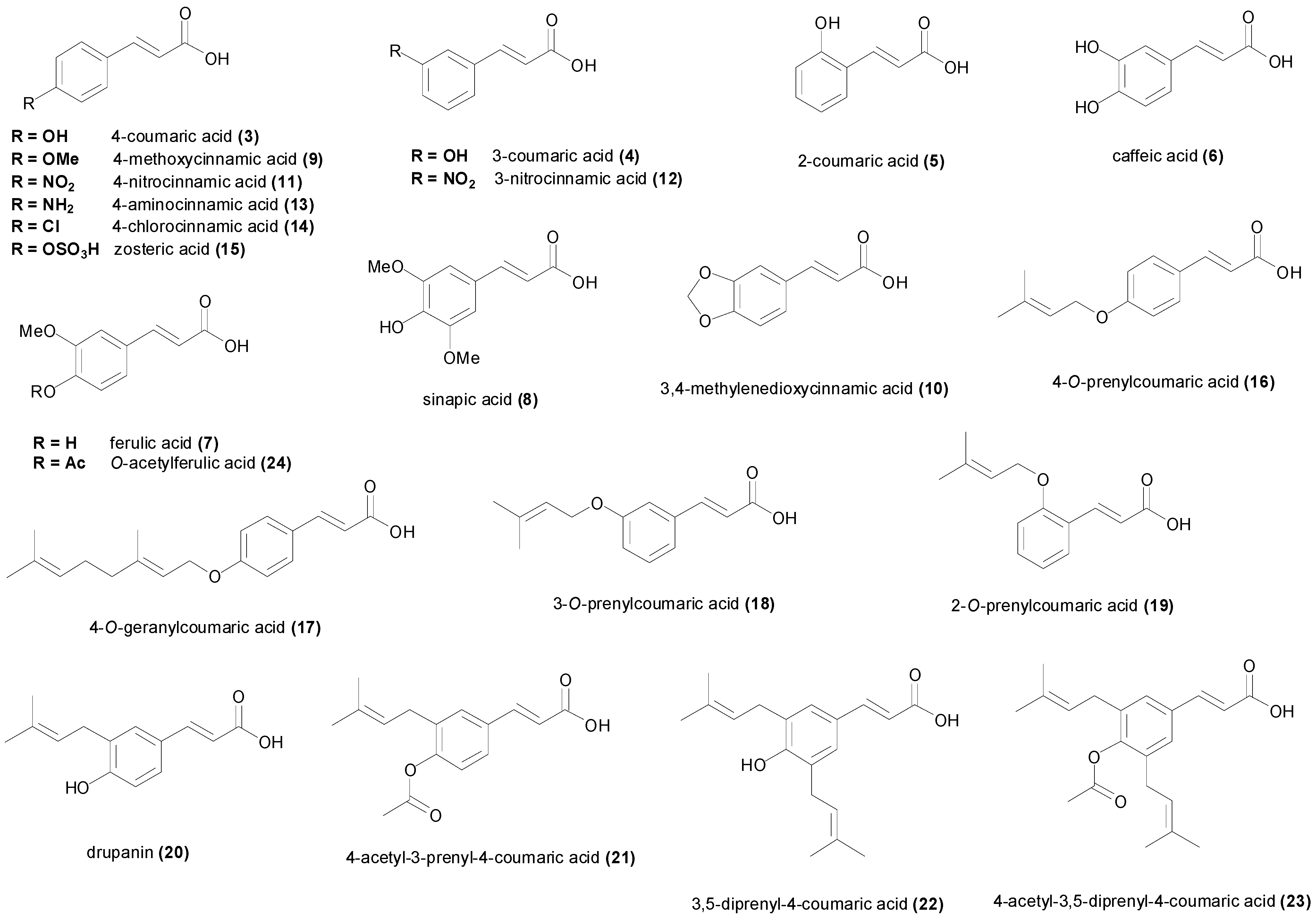
| Compound | Microbial Strain | MIC | Refs. |
|---|---|---|---|
| cinnamic acid (1) | Aeromonas hydrophila MTCC 646 | 7.7 mM | [59] |
| Aeromonas salmonicida MTCC 1522 | 5.6 mM | [59] | |
| Aspergillus flavus UBA294 | 1.7 mM | [65] | |
| Aspergillus niger ATCC 11394 | 844 µM | [65] | |
| Aspergillus terreus INM 031783 | 1.7 mM | [65] | |
| Candida albicans | 405 µM | [66] | |
| Edwardiella tarda MTCC 2400 | 7.0 mM | [59] | |
| Enterococcus faecalis | 6.75 mM | [12] | |
| Escherichia coli | 6.75 mM | [12] | |
| Escherichia coli | 5.0 mM | [56] | |
| Escherichia coli | >6.75 mM | [58] | |
| Escherichia coli ATCC 25922 | 9.0 mM | [57] | |
| Listeria monocytogenes | 13.5 mM | [46] | |
| Morganella morganni | >6.75 mM | [58] | |
| Mycobacterium tuberculosis H37Rv | 270 µM | [60] | |
| Mycobacterium tuberculosis H37Rv | 675 µM | [62] | |
| Neisseria gonorrhoeae | 6.75 mM | [58] | |
| Pasteurella multocida | >6.75 mM | [58] | |
| Proteus mirabilis | >6.75 mM | [58] | |
| Pseudomonas aeruginosa | 6.75 mM | [12] | |
| Salmonella sp. | 6.75 mM | [12] | |
| Salmonella typhimurium LT2 | 7.5 mM | [56] | |
| Staphylococcus aureus | 6.75 mM | [12] | |
| Staphylococcus epidermis | 6.75 mM | [12] | |
| Streptococcus pyogenes 10535 | 844 µM | [77] | |
| Vibrio parahaemolyticus | 6.75 mM | [12] | |
| 4-coumaric acid (3) | Aspergillus flavus UBA294 | 1.5 mM | [65] |
| Aspergillus niger ATCC 11394 | 761 µM | [65] | |
| Aspergillus terreus INM 031783 | 1.5 mM | [65] | |
| Bacillus cereus No-8 | 2.44 mM | [70] | |
| Bacillus subtilis NCIMB 8649 | 2.0 mM | [68] | |
| Bacillus subtilis 9372 | 122 µM | [67] | |
| Escherichia coli NCIMB 12210 | 2.0 mM | [68] | |
| Escherichia coli | 6.09 mM | [58] | |
| Escherichia coli #916 | 3.04 mM | [69] | |
| Escherichia coli O157:H7 | 2.74 mM | [70] | |
| Escherichia coli ATCC 25922 | 490 µM | [67] | |
| Fusarium oxysporum | 3.5 mM | [73] | |
| Fusarium verticillioides | 2.2. mM | [73] | |
| Lactobacillus brevis | 6.09 mM | [72] | |
| Lactobacillus collinoides | 6.09 mM | [72] | |
| Lactobacillus hilgardii IFI-CA 49 | 6.09 mM | [71] | |
| Lactobacillus rhamnosus #299 | 3.04 mM | [69] | |
| Listeria monocytogenes | 13.4 mM | [46] | |
| Morganella morganni | >6.09 mM | [58] | |
| Mycobacterium tuberculosis H37Rv | 244 µM | [60] | |
| Neisseria gonorrhoeae | 6.09 mM | [58] | |
| Pasteurella multocida | 6.09 mM | [58] | |
| Pediococcus pentosaceus IFI-CA 85 | 4.87 mM | [71] | |
| Proteus mirabilis | >6.09 mM | [58] | |
| Pseudomonas syringae NCIMB 649 | 2.0 mM | [68] | |
| Saccharomyces cerevisiae 019 391 | >8.0 mM | [68] | |
| Salmonella typhimurium #450 | 3.04 mM | [69] | |
| Salmonella typhimurium NRRL E4463 | 2.44 mM | [70] | |
| Salmonella typhimurium 50013 | 122 µM | [67] | |
| Schizosaccharomyces pombe 039 917 | 8.0 mM | [68] | |
| Shigella disenteriae 51302 | 61 µM | [67] | |
| Sporobolomyces roseus 043 529 | 8.0 mM | [68] | |
| Staphylococcus aureus # 917 | 761 µM | [69] | |
| Staphylococcus aureus NCTC 10657 | >3.65 mM | [70] | |
| Staphylococcus aureus 6538 | 122 µM | [67] | |
| Streptococcus pneumoniae ATCC 49619 | 122 µM | [67] | |
| Streptococcus pyogenes 10535 | 761 µM | [77] | |
| 3-coumaric acid (4) | Aspergillus flavus UBA294 | 1.5 mM | [65] |
| Aspergillus niger ATCC 11394 | 1.5 mM | [65] | |
| Aspergillus terreus INM 031783 | 1.5 mM | [65] | |
| Mycobacterium tuberculosis H37Rv | 366 µM | [60] | |
| 2-coumaric acid (5) | Bacillus cereus No-8 | 2.44 mM | [70] |
| Escherichia coli #916 | 1.5 mM | [69] | |
| Escherichia coli O157:H7 | 2.74 mM | [70] | |
| Lactobacillus rhamnosus #299 | 1.5 mM | [69] | |
| Morganella morganni | >6.09 mM | [58] | |
| Mycobacterium tuberculosis H37Rv | 122 µM | [60] | |
| Neisseria gonorrhoeae | >6.09 mM | [58] | |
| Pasteurella multocida | >6.09 mM | [58] | |
| Proteus mirabilis | >6.09 mM | [58] | |
| Salmonella typhimurium NRRL E4463 | 2.44 mM | [70] | |
| Salmonella typhimurium #450 | 1.5 mM | [69] | |
| Staphylococcus aureus NCTC 10657 | >3.65 mM | [70] | |
| Staphylococcus aureus # 917 | 760 µM | [69] | |
| caffeic acid (6) | Aspergillus flavus UBA294 | >1.39 mM | [65] |
| Aspergillus flavus | >5.5 mM | [73] | |
| Aspergillus fumigatus | >5.5 mM | [73] | |
| Aspergillus niger ATCC 11394 | >1.39 mM | [65] | |
| Aspergillus terreus INM 031783 | >1.39 mM | [65] | |
| Bacillus cereus No-8 | 1.94 mM | [70] | |
| Bacillus subtilis NCIMB 8649 | 4.0 mM | [68] | |
| Campylobacter jejuni KC40 | >1.0 mM | [93] | |
| Candida albicans 62 | 694 µM | [77] | |
| Candida albicans biofilm | 1.42 mM | [78] | |
| Candida albicans planktonic | 710 µM | [78] | |
| Escherichia coli | >5.5 mM | [58] | |
| Escherichia coli #916 | 2.78 mM | [69] | |
| Escherichia coli | 1.78 mM | [57] | |
| Escherichia coli NCIMB 12210 | 8.0 mM | [68] | |
| Escherichia coli O157:H7 | 1.94 mM | [70] | |
| Fusarium oxysporum | >5.5 mM | [73] | |
| Fusarium verticilioides | >5.5 mM | [73] | |
| Lactobacillus hilgardii IFI-CA 49 | 4.44 mM | [71] | |
| Lactobacillus rhamnosus #299 | <1.39 mM | [69] | |
| Listeria monocytogenes | 16.1 mM | [46] | |
| Morganella morganni | >5.5 mM | [58] | |
| Neisseria gonorrhoeae | >5.5 mM | [58] | |
| Pasteurella multocida | 5.5 mM | [58] | |
| Pediococcus pentosaceus IFI-CA 85 | 3.89 mM | [71] | |
| Penicillium brevicompactum | >5.5 mM | [73] | |
| Penicillium expansum | >5.5 mM | [73] | |
| Proteus mirabilis | >5.5 mM | [58] | |
| Pseudomonas syringae NCIMB 649 | 4.0 mM | [68] | |
| Saccharomyces cerevisiae 019 391 | >8.0 mM | [68] | |
| Salmonella typhimurium #450 | 2.78 mM | [69] | |
| Salmonella typhimurium NRRL E4463 | 1.94 mM | [70] | |
| Schizosaccharomyces pombe 039 917 | >8.0 mM | [68] | |
| Sporobolomyces roseus 043 529 | >8.0 mM | [68] | |
| Staphylococcus aureus 209 | 694 µM | [77] | |
| Staphylococcus aureus # 917 | 694 µM | [69] | |
| Staphylococcus aureus NCTC 10657 | 2.22 mM | [70] | |
| Streptococcus pyogenes 10535 | 694 µM | [77] | |
| ferulic acid (7) | Aspergillus flavus | >5.15 mM | [73] |
| Aspergillus flavus UBA294 | 161 µM | [65] | |
| Aspergillus fumigatus | >5.15 mM | [73] | |
| Aspergillus niger | >10 mM | [80] | |
| Aspergillus niger ATCC 11394 | 322 µM | [65] | |
| Aspergillus terreus INM 031783 | >1.3 mM | [65] | |
| Bacillus cereus No-8 | 2.06 mM | [70] | |
| Bacillus subtilis | 6.0 mM | [80] | |
| Bacillus subtilis NCIMB 8649 | 2.0 mM | [68] | |
| Candida albicans | >10 mM | [80] | |
| Candida albicans ATCC 10231 | 659 µM | [81] | |
| Candida krusei ATCC 6258 | 659 µM | [81] | |
| Enterococcus faecalis ATCC 29212 | 659 µM | [81] | |
| Escherichia coli | >5.15 mM | [58] | |
| Escherichia coli ATCC 25922 | 1.3 mM | [81] | |
| Escherichia coli O157:H7 | 2.32 mM | [70] | |
| Escherichia coli CECT 434 | 515 µM | [79] | |
| Escherichia coli IFO13275 | >5.0 mM | [94] | |
| Escherichia coli NCIMB 12210 | 2.0 mM | [68] | |
| Fusarium oxysporum | >5.15 mM | [73] | |
| Fusarium verticilioides | >5.15 mM | [73] | |
| Klebsiella pneumoniae RSKK 574 | 1.3 mM | [81] | |
| Listeria monocytogenes | 13.9 mM | [46] | |
| Listeria monocytogenes ATCC 15313 | 6.44 mM | [79] | |
| Morganella morganni | >5.15 mM | [58] | |
| Neisseria gonorrhoeae | 5.15 mM | [58] | |
| Pasteurella multocida | 5.15 mM | [58] | |
| Pediococcus pentosaceus IFI-CA 85 | 4.63 mM | [71] | |
| Penicillium brevicompactum | >5.15 mM | [73] | |
| Penicillium expansum | >5.15 mM | [73] | |
| Proteus mirabilis | >5.15 mM | [58] | |
| Pseudomonas aeruginosa ATCC 10145 | 515 µM | [79] | |
| Pseudomonas syringae NCIMB 649 | 2.0 mM | [68] | |
| Saccharomyces cerevisiae | 6.0 mM | [80] | |
| Saccharomyces cerevisiae 019 391 | 4.0 mM | [68] | |
| Salmonella enteriditis IFO3133 | >5.0 mM | [94] | |
| Salmonella typhimurium NRRL E4463 | 2.06 mM | [70] | |
| Schizosaccharomyces pombe 039 917 | 8.0 mM | [68] | |
| Sporobolomyces roseus 043 529 | 2.0 mM | [68] | |
| Staphylococcus aureus | 6.0 mM | [80] | |
| Staphylococcus aureus 209 | 644 µM | [77] | |
| Staphylococcus aureus ATCC 29213 | 1.3 mM | [81] | |
| Staphylococcus aureus CECT 976 | 5.7 mM | [79] | |
| Staphylococcus aureus IFO 12732 | 2.0 mM | [94] | |
| Staphylococcus aureus NCTC 10657 | 3.09 mM | [70] | |
| Streptococcus pyogenes 10535 | 644 µM | [77] | |
| sinapic acid (8) | Bacillus subtilis NCIMB 8649 | 2.0 mM | [68] |
| Bacillus subtilis FAD 110 | 1.3 mM | [83] | |
| Escherichia coli NCIMB 12210 | 2.0 mM | [68] | |
| Escherichia coli IFO13275 | 2.2 mM | [94] | |
| Escherichia coli AW 1.7 | 3.1 mM | [83] | |
| Listeria innocua ATCC 330909 | 1.3 mM | [83] | |
| Listeria monocytogenes ATCC 7644 | 900 µM | [83] | |
| Pseudomonas fluorescens ATCC 13525 | 2.7 mM | [83] | |
| Pseudomonas syringae NCIMB 649 | 4.0 mM | [68] | |
| Saccharomyces cerevisiae 019 391 | >8.0 mM | [68] | |
| Salmonella enteriditis IFO3133 | 2.0 mM | [94] | |
| Schizosaccharomyces pombe 039 917 | >8.0 mM | [68] | |
| Sporobolomyces roseus 043 529 | >8.0 mM | [68] | |
| Staphylococcus aureus 209 | 558 µM | [77] | |
| Staphylococcus aureus IFO 12732 | 1.8 mM | [94] | |
| Staphylococcus aureus ATCC 6538 | 1.3 mM | [83] | |
| Streptococcus pyogenes 10535 | 558 µM | [77] | |
| 4-methoxycinnamic acid (9) | Aspergillus niger | 50.4 µM | [44] |
| Bacillus subtilis | 203 µM | [44] | |
| Candida albicans | 50.4 µM | [44] | |
| Escherichia coli | 164 µM | [44] | |
| Escherichia coli | 281 µM | [84] | |
| Micrococcus luteus | 449 µM | [84] | |
| Salmonella enteriditis | 337 µM | [84] | |
| Staphylococcus aureus | 337 µM | [84] | |
| Staphylococcus aureus | 203 µM | [44] | |
| 3,4-methylenedioxy-cinnamic acid (10) | Mycobacterium tuberculosis H37Rv | 312 µM | [60] |
| Mycobacterium tuberculosis H37Rv | >520 µM | [85] | |
| 4-nitrocinnamic acid (11) | Bacillus subtilis IFO 3009 | 891 µM | [86] |
| Escherichia coli IFO 3301 | 794 µM | [86] | |
| 3-nitrocinnamic acid (12) | Aspergillus niger | 43.5 µM | [44] |
| Bacillus subtilis | 203 µM | [44] | |
| Candida albicans | 43.5 µM | [44] | |
| Escherichia coli | 252 µM | [44] | |
| Staphylococcus aureus | 252 µM | [44] | |
| 4-aminocinnamic acid (13) | Bacillus subtilis IFO 3009 | 602 µM | [86] |
| Escherichia coli IFO 3301 | 708 µM | [86] | |
| 4-chlorocinnamic acid (14) | Bacillus subtilis IFO 3009 | 708 µM | [86] |
| Escherichia coli IFO 3301 | 708 µM | [86] | |
| 4-O-prenylcoumaric acid (16) | Mycobacterium tuberculosis H37Rv | 86.1 µM | [60] |
| 4-O-geranylcoumaric acid (17) | Mycobacterium tuberculosis H37Rv | 66.8 µM | [60] |
| 3-O-prenylcoumaric acid (18) | Mycobacterium tuberculosis H37Rv | 172 µM | [60] |
| 2-O-prenylcoumaric acid (19) | Mycobacterium tuberculosis H37Rv | 258 µM | [60] |
| 3-prenyl-4-coumaric acid (= drupanin) (20) | Aspergillus fumigatus ATCC 26934 | >1.1 mM | [92] |
| Aspergillus flavus ATCC 9170 | >1.1 mM | [92] | |
| Aspergillus niger ATCC 9029 | >1.1 mM | [92] | |
| Candida albicans ATCC 10231 | 1.1 mM | [92] | |
| Candida tropicalis CEREMIC 131 | >1.1 mM | [92] | |
| Cryptococcus neoformans ATCC 32264 | 1.1 mM | [92] | |
| Epidermophyton floccosum C114 | 215 µM | [92] | |
| Escherichia coli ATCC 25922 | >1.1 mM | [92] | |
| Microsporum canis C112 | 431 µM | [92] | |
| Microsporum gypseum C115 | 431 µM | [92] | |
| Staphylococcus aureus LMS | >1.1 mM | [92] | |
| Methicillin-resistant Staphylococcus aureus | >1.1 mM | [92] | |
| Trichophyton mentagrophytes ATCC 9972 | 431 µM | [92] | |
| Trichophyton rubrum C113 | 431 µM | [92] | |
| 4-acetyl-3-prenyl-4-coumaric acid (21) | Aspergillus fumigatus ATCC 26934 | >912 µM | [92] |
| Aspergillus flavus ATCC 9170 | >912 µM | [92] | |
| Aspergillus niger ATCC 9029 | >912 µM | [92] | |
| Candida albicans ATCC 10231 | >912 µM | [92] | |
| Candida tropicalis CEREMIC 131 | >912 µM | [92] | |
| Cryptococcus neoformans ATCC 32264 | >912 µM | [92] | |
| Epidermophyton floccosum C114 | 364 µM | [92] | |
| Escherichia coli ATCC 25922 | >912 µM | [92] | |
| Microsporum canis C112 | >912 µM | [92] | |
| Microsporum gypseum C115 | 912 µM | [92] | |
| Staphylococcus aureus LMS | >912 µM | [92] | |
| Methicillin-resistant Staphylococcus aureus | >912 µM | [92] | |
| Trichophyton mentagrophytes ATCC 9972 | 456 µM | [92] | |
| Trichophyton rubrum C113 | 456 µM | [92] | |
| 3,5-diprenyl-4-coumaric acid (22) | Aspergillus fumigatus ATCC 26934 | >833 µM | [92] |
| Aspergillus flavus ATCC 9170 | >833 µM | [92] | |
| Aspergillus niger ATCC 9029 | >833 µM | [92] | |
| Candida albicans ATCC 10231 | 833 µM | [92] | |
| Candida tropicalis CEREMIC 131 | >833 µM | [92] | |
| Cryptococcus neoformans ATCC 32264 | >833 µM | [92] | |
| Epidermophyton floccosum C114 | 166 µM | [92] | |
| Escherichia coli ATCC 25922 | >833 µM | [92] | |
| Microsporum canis C112 | >833 µM | [92] | |
| Microsporum gypseum C115 | >833 µM | [92] | |
| Staphylococcus aureus LMS | 833 µM | [92] | |
| Methicillin-resistant Staphylococcus aureus | 833 µM | [92] | |
| Trichophyton mentagrophytes ATCC 9972 | >833 µM | [92] | |
| Trichophyton rubrum C113 | 416 µM | [92] | |
| 4-acetyl-3,5-diprenyl-4-coumaric acid (23) | Aspergillus fumigatus ATCC 26934 | >731 µM | [92] |
| Aspergillus flavus ATCC 9170 | >731 µM | [92] | |
| Aspergillus niger ATCC 9029 | >731 µM | [92] | |
| Candida albicans ATCC 10231 | >731 µM | [92] | |
| Candida tropicalis CEREMIC 131 | >731 µM | [92] | |
| Cryptococcus neoformans ATCC 32264 | >731 µM | [92] | |
| Epidermophyton floccosum C114 | 292 µM | [92] | |
| Escherichia coli ATCC 25922 | >731 µM | [92] | |
| Microsporum canis C112 | >731 µM | [92] | |
| Microsporum gypseum C115 | >731 µM | [92] | |
| Staphylococcus aureus LMS | >731 µM | [92] | |
| Methicillin-resistant Staphylococcus aureus | >731 µM | [92] | |
| Trichophyton mentagrophytes ATCC 9972 | 731 µM | [92] | |
| Trichophyton rubrum C113 | 365 µM | [92] | |
| O-acetylferulic acid (24) | Candida albicans ATCC 10231 | 540 µM | [81] |
| Candida krusei ATCC 6258 | 540 µM | [81] | |
| Enterococcus faecalis ATCC 29212 | 540 µM | [81] | |
| Escherichia coli ATCC 25922 | 1.1 mM | [81] | |
| Klebsiella pneumoniae RSKK 574 | 1.1 mM | [81] | |
| Staphylococcus aureus ATCC 29213 | 1.1 mM | [81] |
3. Natural and Synthetic Cinnamic Esters and Amides
3.1. Esters
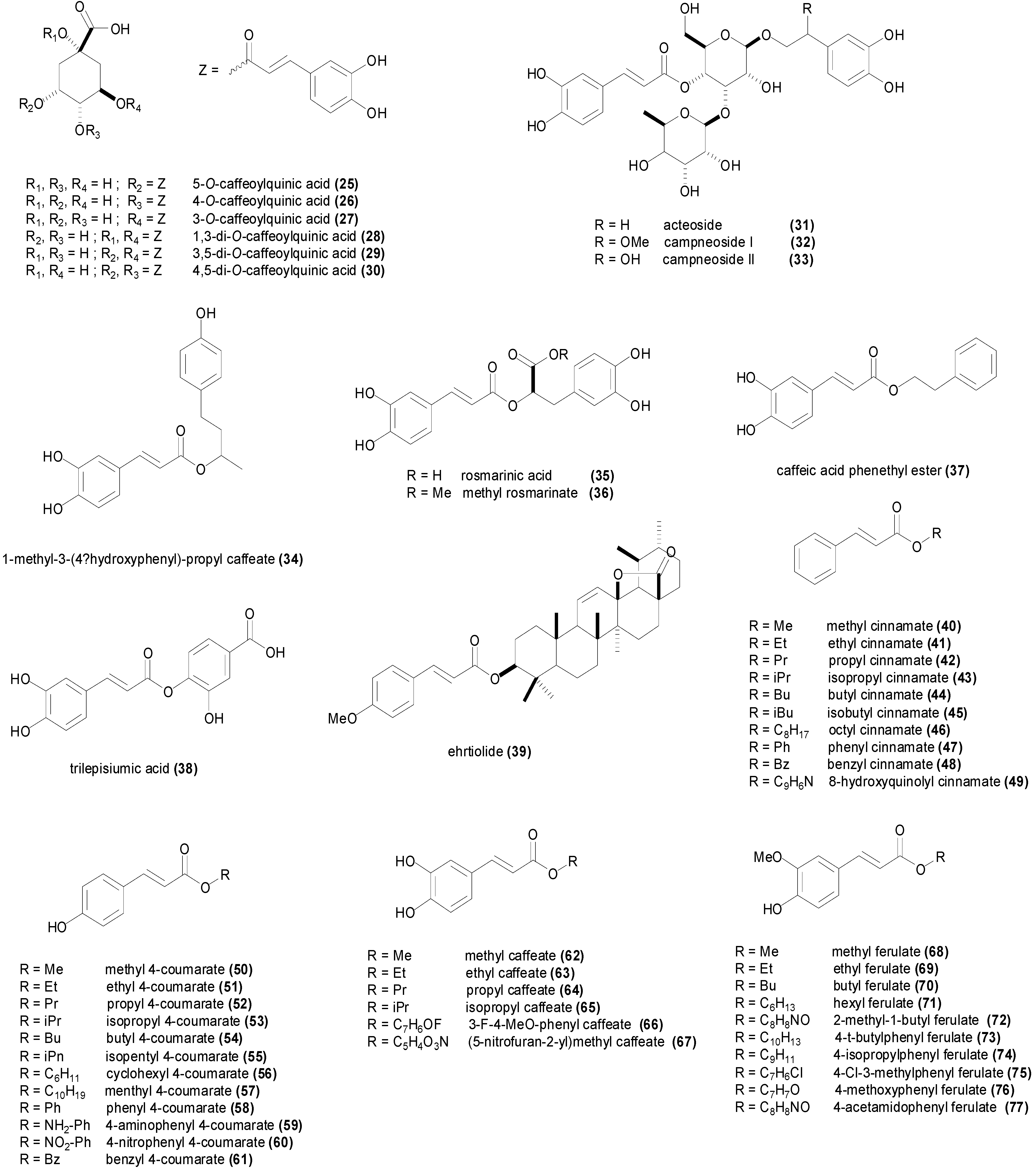
| Compound | Microorganism Strain | MIC | Refs. |
|---|---|---|---|
| 5-O-caffeoylquinic acid (25) | Agrobacterium tumefaciens CGMCC 1.1415 | 282 µM | [96] |
| Aspergillus niger ATCC 10553 | 564 µM | [98] | |
| Aspergillus niger CGMCC 3.316 | 282 µM | [96] | |
| Bacillus subtilis CGMCC 1.1849 | 564 µM | [96] | |
| Bacillus subtilis 9372 | 108 µM | [100] | |
| Candida albicans ATCC 10231 | 141 µM | [96] | |
| Candida albicans ATCC 14053 | 423 µM | [98] | |
| Candida albicans DAY185 | 181 µM | [99] | |
| Candida lusitaniae ATCC 2201 | 141 µM | [96] | |
| Cladosporium cucumerinum ATCC 11279 | 141 µM | [96] | |
| Enterococcus faecalis OGRF1 | 181 µM | [99] | |
| Escherichia coli ATCC 25922 | 216 µM | [100] | |
| Escherichia coli ATCC 25922 | 423 µM | [98] | |
| Escherichia coli CGMCC 1.90 | 564 µM | [96] | |
| Micrococcus luteus CGMCC 1.880 | 282 µM | [96] | |
| Mucor mucedo CGMCC 3.15 | 282 µM | [96] | |
| Penicillium oxalicum CGMCC 3.4022 | 282 µM | [96] | |
| Pseudomonas aeruginosa CG-MCC 1.2031 | 564 µM | [96] | |
| Saccharomyces carlsbergensis ATCC 2166 | 141 µM | [96] | |
| Saccharomyces cerevisiae ATCC 36858 | 564 µM | [98] | |
| Saccharomyces cerevisiae IFFI 1611 | 564 µM | [96] | |
| Salmonella enterica ATCC 13076 | 564 µM | [98] | |
| Salmonella typhimurium CGMCC 1.1190 | 564 µM | [96] | |
| Salmonella typhimurium 50013 | 108 µM | [100] | |
| Shigella disenteriae 51302 | 54 µM | [100] | |
| Staphylococcus aureus | 17.8 mM | [97] | |
| Staphylococcus aureus 8325-4 | 361 µM | [99] | |
| Staphylococcus aureus 6538 | 108 µM | [100] | |
| Staphylococcus aureus ATCC 25923 | 282 µM | [98] | |
| Staphylococcus aureus ATCC 6358P | 564 µM | [96] | |
| Streptococcus mutans | 7.62 mM | [97] | |
| Streptococcus pneumoniae ATCC 49619 | 54 µM | [100] | |
| Vibrio parahaemolyticus ATCC 17802 | 705 µM | [98] | |
| 4-O-caffeoylquinic acid (26) | Aspergillus niger ATCC 10553 | 564 µM | [98] |
| Candida albicans ATCC 14053 | 564 µM | [98] | |
| Escherichia coli ATCC 25922 | 423 µM | [98] | |
| Saccharomyces cerevisiae ATCC 36858 | 705 µM | [98] | |
| Salmonella enterica ATCC 13076 | 705 µM | [98] | |
| Staphylococcus aureus ATCC 25923 | 423 µM | [98] | |
| 3-O-caffeoylquinic acid (27) | Aspergillus niger ATCC 10553 | 423 µM | [98] |
| Candida albicans ATCC 14053 | 423 µM | [98] | |
| Escherichia coli ATCC 25922 | 282 µM | [98] | |
| Saccharomyces cerevisiae ATCC 36858 | 564 µM | [98] | |
| Salmonella enterica ATCC 13076 | 564 µM | [98] | |
| Staphylococcus aureus ATCC 25923 | 282 µM | [98] | |
| Vibrio parahaemolyticus ATCC 17802 | 564 µM | [98] | |
| 1,3-di-O-caffeoylquinic acid (28) | Agrobacterium tumefaciens CGMCC 1.1415 | 194 µM | [96] |
| Aspergillus niger CGMCC 3.316 | 194 µM | [96] | |
| Bacillus subtilis CGMCC 1.1849 | 387 µM | [96] | |
| Candida albicans ATCC 10231 | 194 µM | [96] | |
| Candida lusitaniae ATCC 2201 | 194 µM | [96] | |
| Cladosporium cucumerinum ATCC 11279 | 194 µM | [96] | |
| Escherichia coli CGMCC 1.90 | 194 µM | [96] | |
| Micrococcus luteus CGMCC 1.880 | 194 µM | [96] | |
| Mucor mucedo CGMCC 3.15 | 194 µM | [96] | |
| Penicillium oxalicum CGMCC 3.4022 | 194 µM | [96] | |
| Pseudomonas aeruginosa CG-MCC 1.2031 | 194 µM | [96] | |
| Saccharomyces carlsbergensis ATCC 2166 | 194 µM | [96] | |
| Saccharomyces cerevisiae IFFI 1611 | 387 µM | [96] | |
| Salmonella typhimurium CGMCC 1.1190 | 387 µM | [96] | |
| Staphylococcus aureus ATCC 6358P | 387 µM | [96] | |
| 3,5-di-O-caffeoylquinic acid (29) | Agrobacterium tumefaciens CGMCC 1.1415 | 387 µM | [96] |
| Aspergillus niger CGMCC 3.316 | 194 µM | [96] | |
| Bacillus subtilis CGMCC 1.1849 | 387 µM | [96] | |
| Candida albicans ATCC 10231 | 387 µM | [96] | |
| Candida lusitaniae ATCC 2201 | 387 µM | [96] | |
| Cladosporium cucumerinum ATCC 11279 | 194 µM | [96] | |
| Escherichia coli CGMCC 1.90 | 387 µM | [96] | |
| Micrococcus luteus CGMCC 1.880 | 194 µM | [96] | |
| Mucor mucedo CGMCC 3.15 | 194 µM | [96] | |
| Penicillium oxalicum CGMCC 3.4022 | 194 µM | [96] | |
| Saccharomyces carlsbergensis ATCC 2166 | 387 µM | [96] | |
| Saccharomyces cerevisiae IFFI 1611 | 387 µM | [96] | |
| Salmonella typhimurium CGMCC 1.1190 | >387 µM | [96] | |
| Staphylococcus aureus ATCC 6358P | 387 µM | [96] | |
| 4,5-di-O-caffeoylquinic acid (30) | Agrobacterium tumefaciens CGMCC 1.1415 | 387 µM | [96] |
| Aspergillus niger CGMCC 3.316 | 387 µM | [96] | |
| Bacillus subtilis CGMCC 1.1849 | 387 µM | [96] | |
| Candida lusitaniae ATCC 2201 | 387 µM | [96] | |
| Cladosporium cucumerinum ATCC 11279 | 194 µM | [96] | |
| Escherichia coli CGMCC 1.90 | 194 µM | [96] | |
| Micrococcus luteus CGMCC 1.880 | 97 µM | [96] | |
| Mucor mucedo CGMCC 3.15 | 194 µM | [96] | |
| Penicillium oxalicum CGMCC 3.4022 | 194 µM | [96] | |
| Saccharomyces carlsbergensis ATCC 2166 | 387 µM | [96] | |
| Saccharomyces cerevisiae IFFI 1611 | 387 µM | [96] | |
| Staphylococcus aureus ATCC 6358P | 194 µM | [96] | |
| Acteoside (31) | Enterobacter cloacae P99 | 1.2 mM | [102] |
| Escherichia coli 507E | 4.8 mM | [102] | |
| Klebsiella oxytosa 1082E | 1.2 mM | [102] | |
| Klebsiella aerogenes 1522E | 2.4 mM | [102] | |
| Pseudomonas aeruginosa 9027 | 1.2 mM | [102] | |
| Staphylococcus aureus | 3.2 mM | [102] | |
| Staphylococcus aureus SG511 | 600 µM | [102] | |
| Streptococcus pyogenes A308 | 1.2 mM | [102] | |
| Campneoside I (32) | Enterobacter cloacae P99 | >917 µM | [102] |
| Escherichia coli 507E | >917 µM | [102] | |
| Klebsiella oxytosa 1082E | >917 µM | [102] | |
| Klebsiella aerogenes 1522E | >917 µM | [102] | |
| Pseudomonas aeruginosa 9027 | >917 µM | [102] | |
| Staphylococcus aureus | 200 µM | [102] | |
| Staphylococcus aureus SG511 | 229 µM | [102] | |
| Streptococcus pyogenes A308 | 229 µM | [102] | |
| Campneoside II (33) | Staphylococcus aureus | 2.0 mM | [102] |
| Caffeic acid ester (34) | Phomopsis longicolla | 19 µM | [103] |
| Rosmarinic acid (35) | Aspergillus niger ATCC 16404 | 2.8 mM | [106] |
| Bacillus cereus ATCC 10987 | 1.8 mM | [105] | |
| Bacillus subtilis ATCC 11060 | 1.8 mM | [105] | |
| Candida albicans ATCC 14053 | 694 µM | [106] | |
| Corynebacterium T25-17 | 6.9 mM | [104] | |
| Enterococcus faecalis C159-6 | 833 µM | [104] | |
| Escherichia coli ATCC 8739 | 1.4 mM | [106] | |
| Listeria monocytogenes ATCC 19115 | 1.8 mM | [105] | |
| Mycobacterium smegmatis 5003 | 3.3 mM | [104] | |
| Pseudomonas aeruginosa ATCC 27583 | 6.9 mM | [104] | |
| Pseudomonas aeruginosa ATCC 27853 | 1.8 mM | [105] | |
| Staphylococcus aureus ATCC 29213 | 888 µM | [105] | |
| Staphylococcus aureus ATCC 29737 | 1.4 mM | [106] | |
| Staphylococcus epidermis 5001 | 833 µM | [104] | |
| Staphylococcus lugdunensis T26A3 | 1.6 mM | [104] | |
| Staphylococcus warneri T12A12 | 3.3 mM | [104] | |
| Stenotrophomonas maltophilia | 833 µM | [104] | |
| Methyl rosmarinate (36) | Bacillus cereus ATCC 10987 | >3.4 mM | [105] |
| Bacillus subtilis ATCC 11060 | >3.4 mM | [105] | |
| Corynebacterium T25-17 | 3.2 mM | [104] | |
| Enterococcus faecalis C159-6 | 801 µM | [104] | |
| Listeria monocytogenes ATCC 19115 | >3.4 mM | [105] | |
| Mycobacterium smegmatis 5003 | 1.6 mM | [104] | |
| Pseudomonas aeruginosa ATCC 27583 | 3.2 mM | [104] | |
| Pseudomonas aeruginosa ATCC 27853 | >3.4 mM | [105] | |
| Staphylococcus aureus ATCC 29213 | >3.4 mM | [105] | |
| Staphylococcus epidermis 5001 | 801 µM | [104] | |
| Staphylococcus lugdunensis T26A3 | 1.6 mM | [104] | |
| Staphylococcus warneri T12A12 | 801 µM | [104] | |
| Stenotrophomonas maltophilia | 801 µM | [104] | |
| Caffeic acid phenethyl ester (37) | Escherichia coli ATCC 25922 | >800 µM | [108] |
| Staphylococcus aureus ATCC 6538P | 100 µM | [108] | |
| Enterococcus faecalis ATCC 29212 | 400 µM | [108] | |
| Listeria monocytogenes ATCC 7644 | 400 µM | [108] | |
| Methyl cinnamate (40) | Aspergillus flavus UBA 294 | >1.5 mM | [65] |
| Aspergillus niger | 61 µM | [44] | |
| Aspergillus niger ATCC 11394 | >1.5 mM | [65] | |
| Aspergillus terreus INM 031783 | >1.5 mM | [65] | |
| Bacillus subtilis | 301 µM | [44] | |
| Candida albicans | 50 µM | [44] | |
| Escherichia coli | 164 µM | [44] | |
| Staphylococcus aureus | 252 µM | [44] | |
| Ethyl cinnamate (41) | Aspergillus niger | 61 µM | [44] |
| Bacillus subtilis | 203 µM | [44] | |
| Candida albicans | 50 µM | [44] | |
| Escherichia coli | 203 µM | [44] | |
| Staphylococcus aureus | 252 µM | [44] | |
| Propyl cinnamate (42) | Aspergillus niger | 43 µM | [44] |
| Bacillus subtilis | 203 µM | [44] | |
| Candida albicans | 59 µM | [44] | |
| Escherichia coli | 203 µM | [44] | |
| Staphylococcus aureus | 301 µM | [44] | |
| Isopropyl cinnamate (43) | Aspergillus niger | 43 µM | [44] |
| Bacillus subtilis | 164 µM | [44] | |
| Candida albicans | 43 µM | [44] | |
| Escherichia coli | 139 µM | [44] | |
| Staphylococcus aureus | 139 µM | [44] | |
| Butyl cinnamate (44) | Aspergillus niger | 36 µM | [44] |
| Bacillus subtilis | 203 µM | [44] | |
| Candida albicans | 61 µM | [44] | |
| Escherichia coli | 203 µM | [44] | |
| Staphylococcus aureus | 203 µM | [44] | |
| Isobutyl cinnamate (45) | Aspergillus niger | 12 µM | [44] |
| Bacillus subtilis | 43 µM | [44] | |
| Candida albicans | 14 µM | [44] | |
| Escherichia coli | 43 µM | [44] | |
| Staphylococcus aureus | 50 µM | [44] | |
| Octyl cinnamate (46) | Aspergillus niger | 43 µM | [44] |
| Bacillus subtilis | 203 µM | [44] | |
| Candida albicans | 43 µM | [44] | |
| Escherichia coli | 164 µM | [44] | |
| Staphylococcus aureus | 203 µM | [44] | |
| Phenyl cinnamate (47) | Aspergillus niger | 61 µM | [44] |
| Bacillus subtilis | 164 µM | [44] | |
| Candida albicans | 43 µM | [44] | |
| Escherichia coli | 252 µM | [44] | |
| Staphylococcus aureus | 203 µM | [44] | |
| Benzyl cinnamate (48) | Aspergillus niger | 50 µM | [44] |
| Bacillus subtilis | 203 µM | [44] | |
| Candida albicans | 43 µM | [44] | |
| Escherichia coli | 203 µM | [44] | |
| Staphylococcus aureus | 252 µM | [44] | |
| 8-Hydroxyquinolyl cinnamate (49) | Aspergillus niger | 50 µM | [44] |
| Bacillus subtilis | 252 µM | [44] | |
| Candida albicans | 61 µM | [44] | |
| Escherichia coli | 164 µM | [44] | |
| Staphylococcus aureus | 164 µM | [44] | |
| Methyl 4-coumarate (50) | Aspergillus flavus UBA 294 | 702 µM | [65] |
| Aspergillus niger ATCC 11394 | 702 µM | [65] | |
| Aspergillus niger MTCC 8189 | 247 µM | [111] | |
| Aspergillus terreus INM 031783 | 702 µM | [65] | |
| Bacillus subtilis MTCC 2063 | 247 µM | [111] | |
| Candida albicans MTCC 227 | 247 µM | [111] | |
| Escherichia coli MTCC 1652 | 247 µM | [111] | |
| Staphylococcus aureus MTCC 2901 | 247 µM | [111] | |
| Ethyl 4-coumarate (51) | Aspergillus niger MTCC 8189 | 176 µM | [111] |
| Bacillus subtilis MTCC 2063 | 176 µM | [111] | |
| Candida albicans MTCC 227 | 176 µM | [111] | |
| Escherichia coli MTCC 1652 | 176 µM | [111] | |
| Staphylococcus aureus MTCC 2901 | 176 µM | [111] | |
| Propyl 4-coumarate (52) | Aspergillus niger MTCC 8189 | 2.3 mM | [111] |
| Bacillus subtilis MTCC 2063 | 137 µM | [111] | |
| Candida albicans MTCC 227 | 137 µM | [111] | |
| Escherichia coli MTCC 1652 | 137 µM | [111] | |
| Staphylococcus aureus MTCC 2901 | 137 µM | [111] | |
| Isopropyl 4-coumarate (53) | Aspergillus niger MTCC 8189 | 2.3 mM | [111] |
| Bacillus subtilis MTCC 2063 | 137 µM | [111] | |
| Candida albicans MTCC 227 | 137 µM | [111] | |
| Escherichia coli MTCC 1652 | 2.3 mM | [111] | |
| Staphylococcus aureus MTCC 2901 | 137 µM | [111] | |
| Butyl 4-coumarate (54) | Aspergillus niger MTCC 8189 | 1.9 mM | [111] |
| Bacillus subtilis MTCC 2063 | 107 µM | [111] | |
| Candida albicans MTCC 227 | 107 µM | [111] | |
| Escherichia coli MTCC 1652 | 1.9 mM | [111] | |
| Staphylococcus aureus MTCC 2901 | 107 µM | [111] | |
| Isopentyl 4-coumarate (55) | Aspergillus niger MTCC 8189 | 92 µM | [111] |
| Bacillus subtilis MTCC 2063 | 92 µM | [111] | |
| Candida albicans MTCC 227 | 92 µM | [111] | |
| Escherichia coli MTCC 1652 | 1.4 mM | [111] | |
| Staphylococcus aureus MTCC 2901 | 92 µM | [111] | |
| Cyclohexyl 4-coumarate (56) | Aspergillus niger MTCC 8189 | 78 µM | [111] |
| Bacillus subtilis MTCC 2063 | 9.1 µM | [111] | |
| Candida albicans MTCC 227 | 1.1 mM | [111] | |
| Escherichia coli MTCC 1652 | 41 mM | [111] | |
| Staphylococcus aureus MTCC 2901 | 78 µM | [111] | |
| Menthyl 4-coumarate (57) | Aspergillus niger MTCC 8189 | 1.9 mM | [111] |
| Bacillus subtilis MTCC 2063 | 107 µM | [111] | |
| Candida albicans MTCC 227 | 107 µM | [111] | |
| Escherichia coli MTCC 1652 | 1.9 mM | [111] | |
| Staphylococcus aureus MTCC 2901 | 107 µM | [111] | |
| Phenyl 4-coumarate (58) | Aspergillus niger MTCC 8189 | 1.2 mM | [111] |
| Bacillus subtilis MTCC 2063 | 85 µM | [111] | |
| Candida albicans MTCC 227 | 10 µM | [111] | |
| Escherichia coli MTCC 1652 | 47 mM | [111] | |
| Staphylococcus aureus MTCC 2901 | 85 µM | [111] | |
| 4-aminophenyl 4-coumarate (59) | Aspergillus niger MTCC 8189 | 67 µM | [111] |
| Bacillus subtilis MTCC 2063 | 8.5 µM | [111] | |
| Candida albicans MTCC 227 | 67 µM | [111] | |
| Escherichia coli MTCC 1652 | 905 µM | [111] | |
| Staphylococcus aureus MTCC 2901 | 67 µM | [111] | |
| 4-nitrophenyl 4-coumarate (60) | Aspergillus niger MTCC 8189 | 558 µM | [111] |
| Bacillus subtilis MTCC 2063 | 46 µM | [111] | |
| Candida albicans MTCC 227 | 46 µM | [111] | |
| Escherichia coli MTCC 1652 | 46 µM | [111] | |
| Staphylococcus aureus MTCC 2901 | 46 µM | [111] | |
| Benzyl 4-coumarate (61) | Aspergillus niger MTCC 8189 | 905 µM | [111] |
| Bacillus subtilis MTCC 2063 | 67 µM | [111] | |
| Candida albicans MTCC 227 | 67 µM | [111] | |
| Escherichia coli MTCC 1652 | 905 µM | [111] | |
| Staphylococcus aureus MTCC 2901 | 67 µM | [111] | |
| Methyl caffeate (62) | Aspergillus flavus UBA 294 | >1.3 mM | [65] |
| Aspergillus niger ATCC 11394 | >1.3 mM | [65] | |
| Aspergillus terreus INM 031783 | >1.3 mM | [65] | |
| Candida albicans ATCC 10231 | MIC50 = 659 µM | [78] | |
| Ethyl caffeate (63) | Candida albicans ATCC 10231 | MIC50 = 615 µM | [78] |
| Propyl caffeate (64) | Candida albicans ATCC 10231 | MIC50 = 576 µM | [78] |
| Isopropyl caffeate (65) | Candida albicans ATCC 10231 | MIC50 = 576 µM | [78] |
| 3-fluoro-4-methoxyphenyl caffeate (66) | Candida albicans ATCC 10231 | MIC50 = 421 µM | [78] |
| (5-nitrofuran-2-yl)methyl caffeate (67) | Candida albicans ATCC 10231 | MIC50 = 52 µM | [78] |
| Methyl ferulate (68) | Aspergillus flavus UBA 294 | >1.2 mM | [65] |
| Aspergillus niger | 4.0 mM | [80] | |
| Aspergillus niger ATCC 11394 | >1.2 mM | [65] | |
| Aspergillus terreus INM 031783 | >1.2 mM | [65] | |
| Bacillus subtilis | 6.0 mM | [80] | |
| Candida albicans | 4.0 mM | [80] | |
| Saccharomyces cerevisiae | 4.0 mM | [80] | |
| Staphylococcus aureus | 6.0 mM | [80] | |
| Ethyl ferulate (69) | Aspergillus niger | 4.0 mM | [80] |
| Bacillus subtilis | 2.0 mM | [80] | |
| Candida albicans | 4.0 mM | [80] | |
| Saccharomyces cerevisiae | 4.0 mM | [80] | |
| Staphylococcus aureus | 4.0 mM | [80] | |
| Butyl ferulate (70) | Aspergillus niger | >10 mM | [80] |
| Bacillus subtilis | 500 µM | [80] | |
| Candida albicans | 10 mM | [80] | |
| Saccharomyces cerevisiae | 500 µM | [80] | |
| Staphylococcus aureus | 500 µM | [80] | |
| Hexyl ferulate (71) | Aspergillus niger | >10 mM | [80] |
| Bacillus subtilis | 63 µM | [80] | |
| Candida albicans | >10 mM | [80] | |
| Saccharomyces cerevisiae | >10 mM | [80] | |
| Staphylococcus aureus | 125 µM | [80] | |
| 2-methyl-1-butyl ferulate (72) | Aspergillus niger | >10 mM | [80] |
| Bacillus subtilis | 125 µM | [80] | |
| Candida albicans | >10 mM | [80] | |
| Saccharomyces cerevisiae | 250 µM | [80] | |
| Staphylococcus aureus | 125 µM | [80] | |
| 4-t-butylphenyl ferulate (73) | Candida albicans ATCC 10231 | 391 µM | [81] |
| Candida krusei ATCC 6258 | 391 µM | [81] | |
| Enterococcus faecalis ATCC 29212 | 391 µM | [81] | |
| Escherichia coli ATCC 25922 | 782 µM | [81] | |
| Klebsiella pneumoniae RSKK 574 | 782 µM | [81] | |
| Staphylococcus aureus ATCC 29213 | 782 µM | [81] | |
| 4-isopropylphenyl ferulate (74) | Candida albicans ATCC 10231 | 204 µM | [81] |
| Candida krusei ATCC 6258 | 204 µM | [81] | |
| Enterococcus faecalis ATCC 29212 | 51 µM | [81] | |
| Escherichia coli ATCC 25922 | 818 µM | [81] | |
| Klebsiella pneumoniae RSKK 574 | 409 µM | [81] | |
| Staphylococcus aureus ATCC 29213 | 409 µM | [81] | |
| 4-chloro-3-methylphenyl ferulate (75) | Candida albicans ATCC 10231 | 201 µM | [81] |
| Candida krusei ATCC 6258 | 201 µM | [81] | |
| Enterococcus faecalis ATCC 29212 | 50 µM | [81] | |
| Escherichia coli ATCC 25922 | 812 µM | [81] | |
| Klebsiella pneumoniae RSKK 574 | 401 µM | [81] | |
| Staphylococcus aureus ATCC 29213 | <25 µM | [81] | |
| 4-methoxyphenyl ferulate (76) | Candida albicans ATCC 10231 | 425 µM | [81] |
| Candida krusei ATCC 6258 | 425 µM | [81] | |
| Enterococcus faecalis ATCC 29212 | 850 µM | [81] | |
| Escherichia coli ATCC 25922 | 850 µM | [81] | |
| Klebsiella pneumoniae RSKK 574 | 850 µM | [81] | |
| Staphylococcus aureus ATCC 29213 | 850 µM | [81] | |
| 4-acetamidophenyl ferulate (77) | Candida albicans ATCC 10231 | 390 µM | [81] |
| Candida krusei ATCC 6258 | 390 µM | [81] | |
| Enterococcus faecalis ATCC 29212 | 390 µM | [81] | |
| Escherichia coli ATCC 25922 | 780 µM | [81] | |
| Klebsiella pneumoniae RSKK 574 | 780 µM | [81] | |
| Staphylococcus aureus ATCC 29213 | 780 µM | [81] |
3.2. Amides
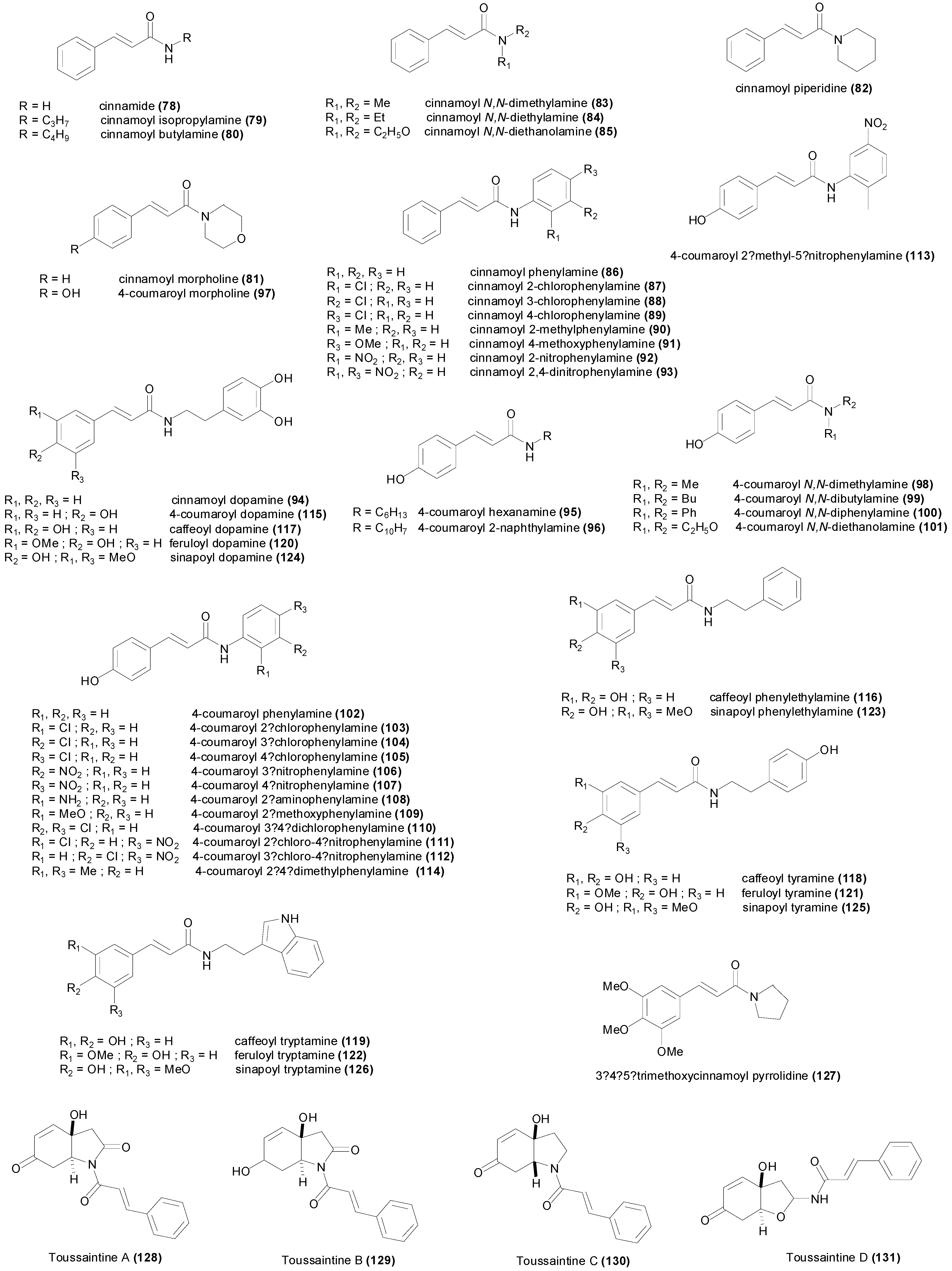
| Compound | Microorganism Strain | MIC | Refs. |
|---|---|---|---|
| Cinnamide (78) | Aspergillus niger | 60.8 µM | [44] |
| Bacillus subtilis | 252 µM | [44] | |
| Candida albicans | 60.8 µM | [44] | |
| Escherichia coli | 252 µM | [44] | |
| Staphylococcus aureus | 252 µM | [44] | |
| Cinnamoyl isopropylamine (79) | Aspergillus niger | 60.8 µM | [44] |
| Bacillus subtilis | 164 µM | [44] | |
| Candida albicans | 50.4 µM | [44] | |
| Escherichia coli | 164 µM | [44] | |
| Staphylococcus aureus | 252 µM | [44] | |
| Cinnamoyl butylamine (80) | Aspergillus niger | 43.5 µM | [44] |
| Bacillus subtilis | 203 µM | [44] | |
| Candida albicans | 43.5 µM | [44] | |
| Escherichia coli | 203 µM | [44] | |
| Staphylococcus aureus | 164 µM | [44] | |
| Cinnamoyl morpholine (81) | Aspergillus niger | 60.8 µM | [44] |
| Bacillus subtilis | 164 µM | [44] | |
| Candida albicans | 60.8 µM | [44] | |
| Escherichia coli | 203 µM | [44] | |
| Staphylococcus aureus | 203 µM | [44] | |
| Cinnamoyl piperidine (82) | Aspergillus niger | 43.5 µM | [44] |
| Bacillus subtilis | 164 µM | [44] | |
| Candida albicans | 50.4 µM | [44] | |
| Escherichia coli | 164 µM | [44] | |
| Staphylococcus aureus | 139 µM | [44] | |
| Cinnamoyl N,N-dimethylamine (83) | Aspergillus niger | 50.4 µM | [44] |
| Bacillus subtilis | 139 µM | [44] | |
| Candida albicans | 43.5 µM | [44] | |
| Escherichia coli | 139 µM | [44] | |
| Staphylococcus aureus | 139 µM | [44] | |
| Cinnamoyl N,N-diethylamine (84) | Aspergillus niger | 14.3 µM | [44] |
| Bacillus subtilis | 203 µM | [44] | |
| Candida albicans | 36.3 µM | [44] | |
| Escherichia coli | 203 µM | [44] | |
| Staphylococcus aureus | 252 µM | [44] | |
| Cinnamoyl N,N-diethanolamine (85) | Aspergillus niger | 60.8 µM | [44] |
| Bacillus subtilis | 301 µM | [44] | |
| Candida albicans | 86 µM | [44] | |
| Escherichia coli | 301 µM | [44] | |
| Staphylococcus aureus | 301 µM | [44] | |
| Cinnamoyl phenylamine (86) | Aspergillus niger | 73.6 µM | [44] |
| Bacillus subtilis | 301 µM | [44] | |
| Candida albicans | 86 µM | [44] | |
| Escherichia coli | 301 µM | [44] | |
| Staphylococcus aureus | 203 µM | [44] | |
| Cinnamoyl 2-chlorophenylamine (87) | Aspergillus niger | 50.4 µM | [44] |
| Bacillus subtilis | 301 µM | [44] | |
| Candida albicans | 60.8 µM | [44] | |
| Escherichia coli | 301 µM | [44] | |
| Staphylococcus aureus | 301 µM | [44] | |
| Cinnamoyl 3-chlorophenylamine (88) | Aspergillus niger | 43.5 µM | [44] |
| Bacillus subtilis | 203 µM | [44] | |
| Candida albicans | 60.8 µM | [44] | |
| Escherichia coli | 164 µM | [44] | |
| Staphylococcus aureus | 203 µM | [44] | |
| Cinnamoyl 4-chlorophenylamine (89) | Aspergillus niger | 50.4 µM | [44] |
| Bacillus subtilis | 301 µM | [44] | |
| Candida albicans | 60.8 µM | [44] | |
| Escherichia coli | 301 µM | [44] | |
| Staphylococcus aureus | 301 µM | [44] | |
| Cinnamoyl 2-methylphenylamine (90) | Aspergillus niger | 73.6 µM | [44] |
| Bacillus subtilis | 114 µM | [44] | |
| Candida albicans | 86 µM | [44] | |
| Escherichia coli | 139 µM | [44] | |
| Staphylococcus aureus | 139 µM | [44] | |
| Cinnamoyl 4-methoxyphenylamine (91) | Aspergillus niger | 50.4 µM | [44] |
| Bacillus subtilis | 203 µM | [44] | |
| Candida albicans | 50.4 µM | [44] | |
| Escherichia coli | 203 µM | [44] | |
| Staphylococcus aureus | 164 µM | [44] | |
| Cinnamoyl 2-nitrophenylamine (92) | Aspergillus niger | 86 µM | [44] |
| Bacillus subtilis | 164 µM | [44] | |
| Candida albicans | 73.6 µM | [44] | |
| Escherichia coli | 203 µM | [44] | |
| Staphylococcus aureus | 139 µM | [44] | |
| Cinnamoyl 2,4-dinitrophenylamine (93) | Aspergillus niger | 50.4 µM | [44] |
| Bacillus subtilis | 164 µM | [44] | |
| Candida albicans | 86 µM | [44] | |
| Escherichia coli | 164 µM | [44] | |
| Staphylococcus aureus | 164 µM | [44] | |
| Cinnamoyl dopamine (94) | Bacillus subtilis 1A95 | 1.76 mM | [77] |
| Candida albicans 62 | 1.76 mM | [77] | |
| Listeria monocytogenes C12 | 1.76 mM | [77] | |
| Staphylococcus aureus 209 | 1.76 mM | [77] | |
| Streptococcus pyogenes 10535 | 441 µM | [77] | |
| 4-coumaroyl hexanamine (95) | Aspergillus niger MTCC 8189 | 72.5 µM | [111] |
| Bacillus subtilis MTCC 2063 | 9.1 µM | [111] | |
| Candida albicans MTCC 227 | 72.5 µM | [111] | |
| Escherichia coli MTCC 1652 | 72.5 µM | [111] | |
| Staphylococcus aureus MTCC 2901 | 72.5 µM | [111] | |
| 4-coumaroyl 2-naphtylamine (96) | Aspergillus niger MTCC 8189 | 558 µM | [111] |
| Bacillus subtilis MTCC 2063 | 46.2 µM | [111] | |
| Candida albicans MTCC 227 | 15.5 mM | [111] | |
| Escherichia coli MTCC 1652 | 46.2 µM | [111] | |
| Staphylococcus aureus MTCC 2901 | 5.9 µM | [111] | |
| 4-coumaroyl morpholine (97) | Aspergillus niger MTCC 8189 | 1.4 mM | [111] |
| Bacillus subtilis MTCC 2063 | 91.6 µM | [111] | |
| Candida albicans MTCC 227 | 91.6 µM | [111] | |
| Escherichia coli MTCC 1652 | 91.6 µM | [111] | |
| Staphylococcus aureus MTCC 2901 | 1.4 mM | [111] | |
| 4-coumaroyl N,N-dimethylamine (98) | Aspergillus niger MTCC 8189 | 191 µM | [111] |
| Bacillus subtilis MTCC 2063 | 191 µM | [111] | |
| Candida albicans MTCC 227 | 191 µM | [111] | |
| Escherichia coli MTCC 1652 | 3.6 mM | [111] | |
| Staphylococcus aureus MTCC 2901 | 3.6 mM | [111] | |
| 4-coumaroyl N,N-dibutylamine (99) | Aspergillus niger MTCC 8189 | 676 µM | [111] |
| Bacillus subtilis MTCC 2063 | 53.6 µM | [111] | |
| Candida albicans MTCC 227 | 53.6 µM | [111] | |
| Escherichia coli MTCC 1652 | 676 µM | [111] | |
| Staphylococcus aureus MTCC 2901 | 53.6 µM | [111] | |
| 4-coumaroyl N,N-diphenylamine (100) | Aspergillus niger MTCC 8189 | 386 µM | [111] |
| Bacillus subtilis MTCC 2063 | 5.0 µM | [111] | |
| Candida albicans MTCC 227 | 34.6 µM | [111] | |
| Escherichia coli MTCC 1652 | 5.0 µM | [111] | |
| Staphylococcus aureus MTCC 2901 | 34.6 µM | [111] | |
| 4-coumaroyl N,N-diethanolamine (101) | Aspergillus niger MTCC 8189 | 72.5 µM | [111] |
| Bacillus subtilis MTCC 2063 | 9.1 µM | [111] | |
| Candida albicans MTCC 227 | 72.5 µM | [111] | |
| Escherichia coli MTCC 1652 | 72.5 µM | [111] | |
| Staphylococcus aureus MTCC 2901 | 72.5 µM | [111] | |
| 4-coumaroyl phenylamine (102) | Aspergillus niger MTCC 8189 | 1.2 mM | [111] |
| Bacillus subtilis MTCC 2063 | 84.7 µM | [111] | |
| Candida albicans MTCC 227 | 84.7 µM | [111] | |
| Escherichia coli MTCC 1652 | 84.7 µM | [111] | |
| Staphylococcus aureus MTCC 2901 | 10.3 µM | [111] | |
| 4-coumaroyl 2'-nitrophenylamine (103) | Aspergillus niger MTCC 8189 | 46.2 µM | [111] |
| Bacillus subtilis MTCC 2063 | 46.2 µM | [111] | |
| Candida albicans MTCC 227 | 6.3 µM | [111] | |
| Escherichia coli MTCC 1652 | 46.2 µM | [111] | |
| Staphylococcus aureus MTCC 2901 | 46.2 µM | [111] | |
| 4-coumaroyl 3'-chlorophenylamine (104) | Aspergillus niger MTCC 8189 | 676 µM | [111] |
| Bacillus subtilis MTCC 2063 | 7.1 µM | [111] | |
| Candida albicans MTCC 227 | 53.6 µM | [111] | |
| Escherichia coli MTCC 1652 | 53.6 µM | [111] | |
| Staphylococcus aureus MTCC 2901 | 7.1 µM | [111] | |
| 4-coumaroyl 4'-chlorophenylamine (105) | Aspergillus niger MTCC 8189 | 676 µM | [111] |
| Bacillus subtilis MTCC 2063 | 7.1 µM | [111] | |
| Candida albicans MTCC 227 | 53.6 µM | [111] | |
| Escherichia coli MTCC 1652 | 53.6 µM | [111] | |
| Staphylococcus aureus MTCC 2901 | 53.6 µM | [111] | |
| 4-coumaroyl 3'-nitrophenylamine (106) | Aspergillus niger MTCC 8189 | 46.2 µM | [111] |
| Bacillus subtilis MTCC 2063 | 46.2 µM | [111] | |
| Candida albicans MTCC 227 | 46.2 µM | [111] | |
| Escherichia coli MTCC 1652 | 558 µM | [111] | |
| Staphylococcus aureus MTCC 2901 | 46.2 µM | [111] | |
| 4-coumaroyl 4'-nitrophenylamine (107) | Aspergillus niger MTCC 8189 | 18 mM | [111] |
| Bacillus subtilis MTCC 2063 | 46.2 µM | [111] | |
| Candida albicans MTCC 227 | 46.2 µM | [111] | |
| Escherichia coli MTCC 1652 | 46.2 µM | [111] | |
| Staphylococcus aureus MTCC 2901 | 46.2 µM | [111] | |
| 4-coumaroyl 2'-aminophenylamine (108) | Aspergillus niger MTCC 8189 | 905 µM | [111] |
| Bacillus subtilis MTCC 2063 | 67.2 µM | [111] | |
| Candida albicans MTCC 227 | 67.2 µM | [111] | |
| Escherichia coli MTCC 1652 | 67.2 µM | [111] | |
| Staphylococcus aureus MTCC 2901 | 67.2 µM | [111] | |
| 4-coumaroyl 2'-methoxyphenylamine (109) | Aspergillus niger MTCC 8189 | 744 µM | [111] |
| Bacillus subtilis MTCC 2063 | 7.5 µM | [111] | |
| Candida albicans MTCC 227 | 57.7 µM | [111] | |
| Escherichia coli MTCC 1652 | 57.7 µM | [111] | |
| Staphylococcus aureus MTCC 2901 | 57.7 µM | [111] | |
| 4-coumaroyl 3',4'-dichlorophenylamine (110) | Aspergillus niger MTCC 8189 | 422 µM | [111] |
| Bacillus subtilis MTCC 2063 | 37.1 µM | [111] | |
| Candida albicans MTCC 227 | 37.1 µM | [111] | |
| Escherichia coli MTCC 1652 | 37.1 µM | [111] | |
| Staphylococcus aureus MTCC 2901 | 37.1 µM | [111] | |
| 4-coumaroyl 2'-chloro-4'-nitrophenylamine (111) | Aspergillus niger MTCC 8189 | 352 µM | [111] |
| Bacillus subtilis MTCC 2063 | 929 nM | [111] | |
| Candida albicans MTCC 227 | 32.2 µM | [111] | |
| Escherichia coli MTCC 1652 | 32.2 µM | [111] | |
| Staphylococcus aureus MTCC 2901 | 352 µM | [111] | |
| 4-coumaroyl 3'-chloro-4'-nitrophenylamine (112) | Aspergillus niger MTCC 8189 | 32.2 µM | [111] |
| Bacillus subtilis MTCC 2063 | 4.7 µM | [111] | |
| Candida albicans MTCC 227 | 32.2 µM | [111] | |
| Escherichia coli MTCC 1652 | 4.7 µM | [111] | |
| Staphylococcus aureus MTCC 2901 | 32.2 µM | [111] | |
| 4-coumaroyl 2'-methyl-5'-nitrophenylamine (113) | Aspergillus niger MTCC 8189 | 39.9 µM | [111] |
| Bacillus subtilis MTCC 2063 | 39.9 µM | [111] | |
| Candida albicans MTCC 227 | 39.9 µM | [111] | |
| Escherichia coli MTCC 1652 | 463 µM | [111] | |
| Staphylococcus aureus MTCC 2901 | 39.9 µM | [111] | |
| 4-coumaroyl 2',4'-dimethylphenylamine (114) | Aspergillus niger MTCC 8189 | 744 µM | [111] |
| Bacillus subtilis MTCC 2063 | 7.55 µM | [111] | |
| Candida albicans MTCC 227 | 57.7 µM | [111] | |
| Escherichia coli MTCC 1652 | 744 µM | [111] | |
| Staphylococcus aureus MTCC 2901 | 57.7 µM | [111] | |
| 4-coumaroyl dopamine (115) | Bacillus subtilis 1A95 | 1.67 mM | [77] |
| Candida albicans 62 | 1.67 mM | [77] | |
| Listeria monocytogenes C12 | 1.67 mM | [77] | |
| Staphylococcus aureus 209 | 418 µM | [77] | |
| Streptococcus pyogenes 10535 | 418 µM | [77] | |
| Caffeoyl phenylethylamine (116) | Bacillus subtilis 1A95 | 1.76 mM | [77] |
| Candida albicans 62 | 882 µM | [77] | |
| Listeria monocytogenes C12 | 441 µM | [77] | |
| Staphylococcus aureus 209 | 882 µM | [77] | |
| Streptococcus pyogenes 10535 | 882 µM | [77] | |
| Caffeoyl dopamine (117) | Bacillus subtilis 1A95 | 793 µM | [77] |
| Candida albicans 62 | 396 µM | [77] | |
| Listeria monocytogenes C12 | 793 µM | [77] | |
| Staphylococcus aureus 209 | 793 µM | [77] | |
| Streptococcus pyogenes 10535 | 793 µM | [77] | |
| Caffeoyl tyramine (118) | Bacillus subtilis 1A95 | 836 µM | [77] |
| Candida albicans 62 | 418 µM | [77] | |
| Listeria monocytogenes C12 | 836 µM | [77] | |
| Staphylococcus aureus 209 | 836 µM | [77] | |
| Streptococcus pyogenes 10535 | 836 µM | [77] | |
| Caffeoyl tryptamine (119) | Bacillus subtilis 1A95 | 1.55 mM | [77] |
| Candida albicans 62 | 1.55 mM | [77] | |
| Listeria monocytogenes C12 | 1.55 mM | [77] | |
| Staphylococcus aureus 209 | 388 µM | [77] | |
| Streptococcus pyogenes 10535 | 194 µM | [77] | |
| Feruloyl dopamine (120) | Bacillus subtilis 1A95 | 759 µM | [77] |
| Candida albicans 62 | 1.52 mM | [77] | |
| Listeria monocytogenes C12 | 1.52 mM | [77] | |
| Staphylococcus aureus 209 | 190 µM | [77] | |
| Streptococcus pyogenes 10535 | 380 µM | [77] | |
| Feruloyl tyramine (121) | Bacillus subtilis 1A95 | 798 µM | [77] |
| Candida albicans 62 | 1.59 mM | [77] | |
| Listeria monocytogenes C12 | 1.59 mM | [77] | |
| Staphylococcus aureus 209 | 199 µM | [77] | |
| Streptococcus pyogenes 10535 | 399 µM | [77] | |
| Feruloyl tryptamine (122) | Bacillus subtilis 1A95 | 743 µM | [77] |
| Candida albicans 62 | 1.49 mM | [77] | |
| Listeria monocytogenes C12 | 1.49 mM | [77] | |
| Staphylococcus aureus 209 | 372 µM | [77] | |
| Streptococcus pyogenes 10535 | 372 µM | [77] | |
| Sinapoyl phenylethylamine (123) | Bacillus subtilis 1A95 | 1.53 mM | [77] |
| Candida albicans 62 | 1.53 mM | [77] | |
| Listeria monocytogenes C12 | 1.53 mM | [77] | |
| Staphylococcus aureus 209 | 382 µM | [77] | |
| Streptococcus pyogenes 10535 | 382 µM | [77] | |
| Sinapoyl dopamine (124) | Bacillus subtilis 1A95 | 1.39 mM | [77] |
| Candida albicans 62 | 1.39 mM | [77] | |
| Listeria monocytogenes C12 | 1.39 mM | [77] | |
| Staphylococcus aureus 209 | 696 µM | [77] | |
| Streptococcus pyogenes 10535 | 696 µM | [77] | |
| Sinapoyl tyramine (125) | Bacillus subtilis 1A95 | 1.46 mM | [77] |
| Candida albicans 62 | 1.46 mM | [77] | |
| Listeria monocytogenes C12 | 1.46 mM | [77] | |
| Staphylococcus aureus 209 | 182 µM | [77] | |
| Streptococcus pyogenes 10535 | 182 µM | [77] | |
| Sinapoyl tryptamine (126) | Bacillus subtilis 1A95 | 1.36 mM | [77] |
| Candida albicans 62 | 1.36 mM | [77] | |
| Listeria monocytogenes C12 | 1.36 mM | [77] | |
| Staphylococcus aureus 209 | 682 µM | [77] | |
| Streptococcus pyogenes 10535 | 171 µM | [77] | |
| 3',4',5'-trimethoxycinnamoyl pyrrolidine (127) | Mycobacterium tuberculosis H37Ra | 600 µM | [118] |
| Toussaintine A (128) | Escherichia coli DSM 1103 | 34 µM | [119] |
| Staphylococcus aureus ATCC 25923 | >136 µM | [119] | |
| Toussaintine B (129) | Escherichia coli DSM 1103 | 67 µM | [119] |
| Staphylococcus aureus ATCC 25923 | 67 µM | [119] | |
| Toussaintine C (130) | Escherichia coli DSM 1103 | 34 µM | [119] |
| Staphylococcus aureus ATCC 25923 | >136 µM | [119] | |
| Toussaintine D (131) | Escherichia coli DSM 1103 | >136 µM | [119] |
| Staphylococcus aureus ATCC 25923 | 17 µM | [119] |
4. Natural and Synthetic Cinnamic Aldehydes and Alcohols
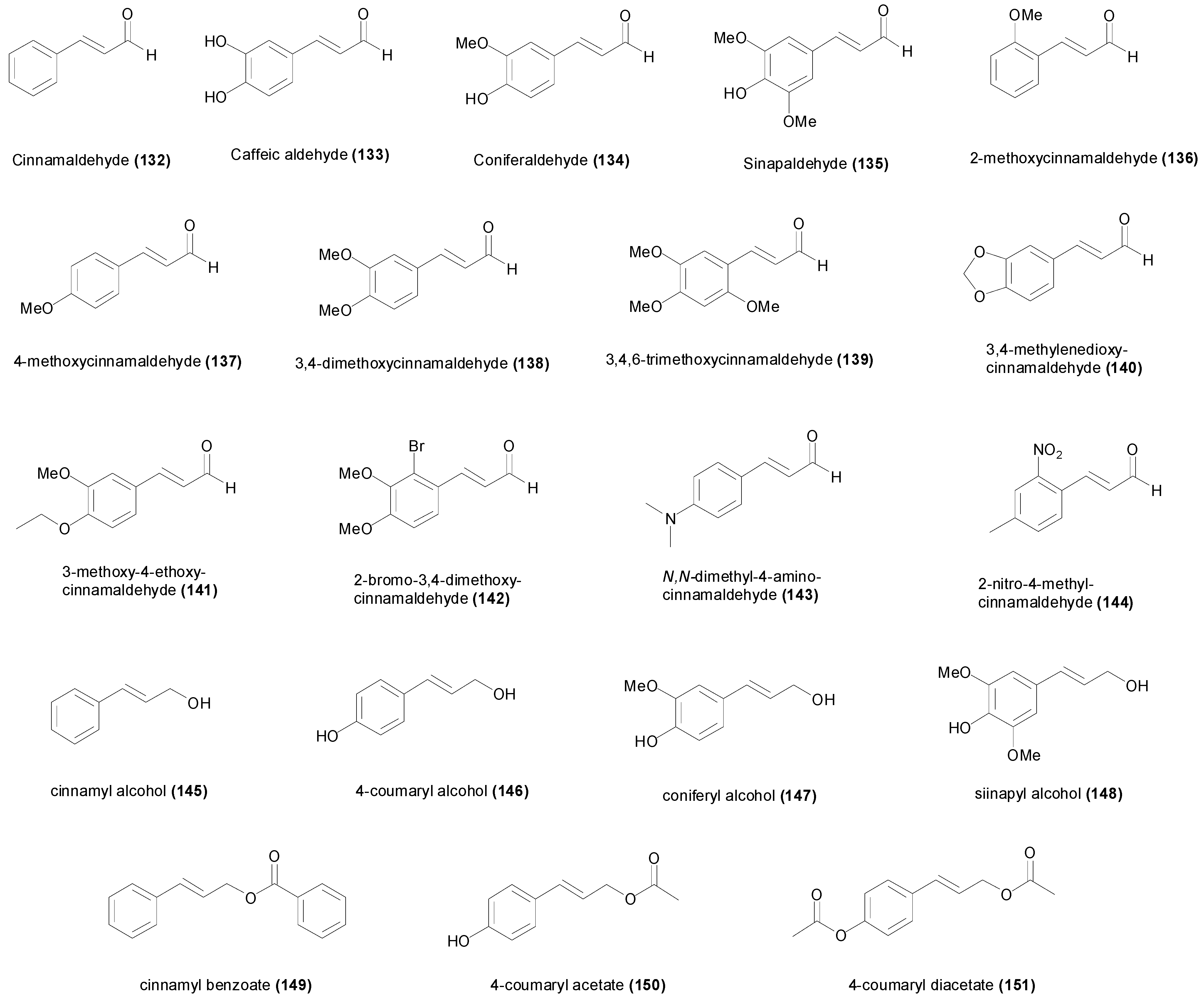
| Compound | Microorganism Strain | MIC | Refs. |
|---|---|---|---|
| Cinnamaldehyde (132) | Aspergillus niger MTCC 404 | 1.89 mM | [139] |
| Aspergillus sydowii MTCC 4335 | 3.78 mM | [139] | |
| Aspergillus parasiticus MTCC 2797 | 3.78 mM | [139] | |
| Bacillus cereus ATCC 11778 | 3.2 mM | [127] | |
| Bacillus subtilis | 7.6 mM | [124] | |
| Bacillus subtilis MTCC 121 | 1.89 mM | [139] | |
| Burkholderia cepacea MTCC 438 | 1.89 mM | [139] | |
| Candida albicans ATCC 2091 | 1.1 mM | [131] | |
| Candida albicans ATCC 90028 | 459 µM | [132] | |
| Candida albicans MTCC 3017 | 470 µM | [139] | |
| Candida albicans STD-1141 | 3.0 mM | [130] | |
| Candida tropicalis STD-1118 | 3.8 mM | [130] | |
| Clostridium difficile PHTCC 107 | 605 µM | [13] | |
| Enterobacter cloacae MTCC 509 | 1.89 mM | [139] | |
| Enterococcus faecalis | 1.89 mM | [12] | |
| Escherichia coli | 3.78 mM | [124] | |
| Escherichia coli | 3.78 mM | [12] | |
| Escherichia coli ATCC 11105 | 3.78 mM | [125] | |
| Escherichia coli CGMCC 1.487 | 2.0 mM | [123] | |
| Escherichia coli MTCC 43 | 1.89 mM | [139] | |
| Escherichia coli NCIM-2089 | 7.6 µM | [121] | |
| Escherichia coli NTCT 8196 | < 1.5 µM | [122] | |
| Escherichia coli O157:H7 | 1.89 mM | [125] | |
| Fusarium verticillioides | 358 µM | [129] | |
| Gloeophyllum trabeum BCRC 31614 | 2.3 mM | [134] | |
| Helicobacter pylori ATCC 26695 | 15 µM | [121] | |
| Issatchenkia orientalis MTCC 231 | 470 µM | [139] | |
| Klebsiella pneumoniae MTCC 109 | 3.78 mM | [139] | |
| Laetiporus betulina | 750 µM | [133] | |
| Legionella pneumophila JCM 7571 | 620 µM | [126] | |
| Lenzites betulina BCRC 35296 | 757 µM | [134] | |
| Laetiporus sulphureus | 700 µM | [133] | |
| Laetiporus sulphurous BCRC 35305 | 757 µM | [134] | |
| Malassezia furfur IP305 | 757 µM | [131] | |
| Micrococcus luteus MTCC 2470 | 3.78 mM | [139] | |
| Pseudomonas aeruginosa | 7.57 mM | [12] | |
| Pseudomonas aeruginosa MTCC 424 | 1.89 mM | [139] | |
| Pseudomonas aeruginosa NCTC 9027 | 10.6 µM | [122] | |
| Sclerotinia sclerotiorum | 1.94 mM | [146] | |
| Staphylococcus aureus | 1.89 mM | [12] | |
| Staphylococcus aureus MTCC 121 | 1.89 mM | [139] | |
| Methicillin-resistant Staphylococcus aureus | 1.89 mM | [124] | |
| Tametes versicolor BCRC 35253 | 2.3 mM | [134] | |
| Trychophyton rubrum MTCC 296 | 470 µM | [139] | |
| Caffeic aldehyde (133) | Mycobacterium tuberculosis H37Rv | 154 µM | [135] |
| Coniferaldehyde (134) | Streptococcus mitis ATCC 49456T | 351 µM | [137] |
| Streptococcus mutans DMST 26095 | 1.4 mM | [137] | |
| Streptococcus pyogenes DMST 17020 | 351 µM | [137] | |
| Sinapaldehyde (135) | Streptococcus mitis ATCC 49456T | 601 µM | [137] |
| Streptococcus mutans DMST 26095 | 601 µM | [137] | |
| Streptococcus pyogenes DMST 17020 | 150 µM | [137] | |
| 2-Methoxycinnamaldehyde (136) | Aspergillus fumigatus Kuboyama | 617 µM | [138] |
| Aspergillus niger stA-2 | 1.2 mM | [138] | |
| Candida albicans stT-1 | 308 µM | [138] | |
| Cryptococcus neoformans stY-8 | 77 µM | [138] | |
| Escherichia coli E-2602 | >1.2 mM | [138] | |
| Microsporum canis stT-6 | 19 µM | [138] | |
| Salmonella typhimurium 75-276 | >1.2 mM | [138] | |
| Staphylococcus aureus 209P | 1.2 mM | [138] | |
| 4-Methoxycinnamaldehyde (137) | Aspergillus niger MTCC 404 | 3.08 mM | [139] |
| Aspergillus sydowii MTCC 4335 | 3.08 mM | [139] | |
| Aspergillus parasiticus MTCC 2797 | 3.08 mM | [139] | |
| Bacillus subtilis MTCC 121 | 6.16 mM | [139] | |
| Candida albicans MTCC 3017 | 770 µM | [139] | |
| Enterobacter cloacae MTCC 509 | 3.08 mM | [139] | |
| Escherichia coli MTCC 43 | 3.08 mM | [139] | |
| Issatchenkia orientalis MTCC 231 | 770 µM | [139] | |
| Klebsiella pneumoniae MTCC 109 | 3.08 mM | [139] | |
| Micrococcus luteus MTCC 2470 | 3.08 mM | [139] | |
| Pseudomonas aeruginosa MTCC 424 | 770 µM | [139] | |
| Staphylococcus aureus MTCC 121 | 3.08 mM | [139] | |
| Trychophyton rubrum MTCC 296 | 770 µM | [139] | |
| 3,4-dimethoxy-cinnamaldehyde (138) | Aspergillus niger MTCC 404 | 5.20 mM | [139] |
| Aspergillus sydowii MTCC 4335 | 5.20 mM | [139] | |
| Aspergillus parasiticus MTCC 2797 | 2.60 mM | [139] | |
| Bacillus subtilis MTCC 121 | 5.20 mM | [139] | |
| Burkholderia cepacea MTCC 438 | 5.20 mM | [139] | |
| Candida albicans MTCC 3017 | 2.60 mM | [139] | |
| Enterobacter cloacae MTCC 509 | 10.4 mM | [139] | |
| Escherichia coli MTCC 43 | 10.4 mM | [139] | |
| Issatchenkia orientalis MTCC 231 | 2.60 mM | [139] | |
| Klebsiella pneumoniae MTCC 109 | 10.4 mM | [139] | |
| Micrococcus luteus MTCC 2470 | 2.60 mM | [139] | |
| Pseudomonas aeruginosa MTCC 424 | 2.60 mM | [139] | |
| Staphylococcus aureus MTCC 121 | 5.20 mM | [139] | |
| Trychophyton rubrum MTCC 296 | 160 µM | [139] | |
| 3,4,6-trimethoxy-cinnamaldehyde (139) | Aspergillus sydowii MTCC 4335 | 4.50 mM | [139] |
| Candida albicans MTCC 3017 | 8.99 mM | [139] | |
| Issatchenkia orientalis MTCC 231 | 8.99 mM | [139] | |
| Micrococcus luteus MTCC 2470 | 2.25 mM | [139] | |
| Trychophyton rubrum MTCC 296 | 280 µM | [139] | |
| 3,4-methylenedioxy-cinnamaldehyde (140) | Aspergillus niger MTCC 404 | 1.42 mM | [139] |
| Aspergillus sydowii MTCC 4335 | 350 µM | [139] | |
| Aspergillus parasiticus MTCC 2797 | 710 µM | [139] | |
| Bacillus subtilis MTCC 121 | 2.84 mM | [139] | |
| Burkholderia cepacea MTCC 438 | 710 µM | [139] | |
| Candida albicans MTCC 3017 | 350 µM | [139] | |
| Enterobacter cloacae MTCC 509 | 1.42 mM | [139] | |
| Escherichia coli MTCC 43 | 2.84 mM | [139] | |
| Issatchenkia orientalis MTCC 231 | 350 µM | [139] | |
| Klebsiella pneumoniae MTCC 109 | 1.42 mM | [139] | |
| Micrococcus luteus MTCC 2470 | 2.84 mM | [139] | |
| Pseudomonas aeruginosa MTCC 424 | 350 µM | [139] | |
| Staphylococcus aureus MTCC 121 | 2.84 mM | [139] | |
| Trychophyton rubrum MTCC 296 | 40 µM | [139] | |
| 3-methoxy-4-ethoxy-cinnamaldehyde (141) | Aspergillus niger MTCC 404 | 4.85 mM | [139] |
| Aspergillus sydowii MTCC 4335 | 4.85 mM | [139] | |
| Aspergillus parasiticus MTCC 2797 | 2.42 mM | [139] | |
| Bacillus subtilis MTCC 121 | 4.85 mM | [139] | |
| Burkholderia cepacea MTCC 438 | 4.85 mM | [139] | |
| Candida albicans MTCC 3017 | 2.42 mM | [139] | |
| Enterobacter cloacae MTCC 509 | 9.70 mM | [139] | |
| Escherichia coli MTCC 43 | 9.70 mM | [139] | |
| Issatchenkia orientalis MTCC 231 | 4.85 mM | [139] | |
| Klebsiella pneumoniae MTCC 109 | 9.70 mM | [139] | |
| Micrococcus luteus MTCC 2470 | 4.85 mM | [139] | |
| Pseudomonas aeruginosa MTCC 424 | 4.85 mM | [139] | |
| Staphylococcus aureus MTCC 121 | 2.42 mM | [139] | |
| Trychophyton rubrum MTCC 296 | 600 µM | [139] | |
| 2-bromo-3,4-dimethoxy-cinnamaldehyde (142) | Trychophyton rubrum MTCC 296 | 460 µM | [139] |
| N,N-dimethyl-4-amino-cinnamaldehyde (143) | Enterobacter cloacae MTCC 509 | 11.4 mM | [139] |
| Micrococcus luteus MTCC 2470 | 11.4 mM | [139] | |
| Trychophyton rubrum MTCC 296 | 180 µM | [139] | |
| 2-nitro-4-methyl-cinnamaldehyde (144) | Bacillus subtilis MTCC 121 | 2.61 mM | [139] |
| Burkholderia cepacea MTCC 438 | 2.61 mM | [139] | |
| Enterobacter cloacae MTCC 509 | 10.5 mM | [139] | |
| Escherichia coli MTCC 43 | 2.61 mM | [139] | |
| Klebsiella pneumoniae MTCC 109 | 10.5 mM | [139] | |
| Micrococcus luteus MTCC 2470 | 2.61 mM | [139] | |
| Pseudomonas aeruginosa MTCC 424 | 2.61 mM | [139] | |
| Staphylococcus aureus MTCC 121 | 10.5 mM | [139] | |
| Trychophyton rubrum MTCC 296 | 160 µM | [139] | |
| Cinnamyl alcohol (145) | Aspergillus niger 101 | 7 mM | [143] |
| Aspergillus oryzae 102 | 8 mM | [143] | |
| Bacillus subtilis IFO 13721 | 10 mM | [143] | |
| Candida albicans IFO 0597 | 7 mM | [143] | |
| Epidermophyton floccosum IFO 9045 | 3 mM | [143] | |
| Escherichia coli | 7.5 mM | [12] | |
| Escherichia coli IFO 3545 | 9 mM | [143] | |
| Enterococcus faecalis | 7.5 mM | [12] | |
| Klebsiella pneumonia IFO 13541 | 10 mM | [143] | |
| Legionella pneumophila JCM 7571 | 7.6 mM | [126] | |
| Pseudomonas aeruginosa | 7.5 mM | [12] | |
| Rhizopus sp. 103 | 3 mM | [143] | |
| Saccharomyces cerevisiae Kyokai no. 8 | 8 mM | [143] | |
| Staphylococcus aureus | 3.7 mM | [12] | |
| Trichophyton rubrum IFO 9185 | 4 mM | [143] | |
| Trichophyton violaceum IFO 31064 | 4 mM | [143] | |
| 4-Coumaryl alcohol (146) | Bacillus subtilis 8649 | 8.0 mM | [68] |
| Escherichia coli 12210 | >8.0 mM | [68] | |
| Pseudomonas syringae 649 | >8.0 mM | [68] | |
| Saccharomyces cerevisiae 019391 | >8.0 mM | [68] | |
| Schizosaccharomyces pombe 039917 | >8.0 mM | [68] | |
| Sporobolomyces roseus 043529 | 8.0 mM | [68] | |
| Coniferyl alcohol (147) | Bacillus subtilis 8649 | >8.0 mM | [68] |
| Escherichia coli 12210 | >8.0 mM | [68] | |
| Pseudomonas syringae 649 | >8.0 mM | [68] | |
| Saccharomyces cerevisiae 019391 | >8.0 mM | [68] | |
| Schizosaccharomyces pombe 039917 | >8.0 mM | [68] | |
| Sporobolomyces roseus 043529 | >8.0 mM | [68] | |
| Sinapyl alcohol (148) | Bacillus subtilis 8649 | >8.0 mM | [68] |
| Escherichia coli 12210 | >8.0 mM | [68] | |
| Pseudomonas syringae 649 | >8.0 mM | [68] | |
| Saccharomyces cerevisiae 019391 | >8.0 mM | [68] | |
| Schizosaccharomyces pombe 039917 | >8.0 mM | [68] | |
| Sporobolomyces roseus 043529 | >8.0 mM | [68] | |
| Cinnamyl benzoate (149) | Aspergillus niger 101 | >10 mM | [143] |
| Aspergillus oryzae 102 | >10 mM | [143] | |
| Bacillus subtilis IFO 13721 | >10 mM | [143] | |
| Candida albicans IFO 0597 | >10 mM | [143] | |
| Epidermophyton floccosum IFO 9045 | 20 µM | [143] | |
| Escherichia coli IFO 3545 | >10 mM | [143] | |
| Klebsiella pneumoniae IFO 13541 | >10 mM | [143] | |
| Rhizopus sp. 103 | >10 mM | [143] | |
| Saccharomyces cerevisiae Kyokai no. 8 | >10 mM | [143] | |
| Trichophyton rubrum IFO 9185 | 20 µM | [143] | |
| Trichophyton violaceum IFO 31064 | 20 µM | [143] | |
| 4-coumaryl acetate (150) | Candida albicans ATCC 10231 | 5.2 mM | [144] |
| Microsporum canis ATCC 36299 | 10.4 mM | [144] | |
| Staphylococcus aureus ATCC 25923 | 6.5 mM | [144] | |
| Staphylococcus aureus VISA 24 | 203 µM | [144] | |
| Tricophyton rubrum ATCC 28188 | 10.4 mM | [144] | |
| 4-coumaryl diacetate (151) | Candida albicans ATCC 10231 | 2.7 mM | [144] |
| Microsporum canis ATCC 36299 | 2.7 mM | [144] | |
| Mycobacterium smegmatis mc2 155 | 215 µM | [145] | |
| Staphylococcus aureus ATCC 25923 | 2.7 mM | [144] | |
| Staphylococcus aureus VISA 24 | 672 µM | [144] | |
| Tricophyton rubrum ATCC 28188 | 2.7 mM | [144] |
5. Synthetic Cinnamic Hybrids
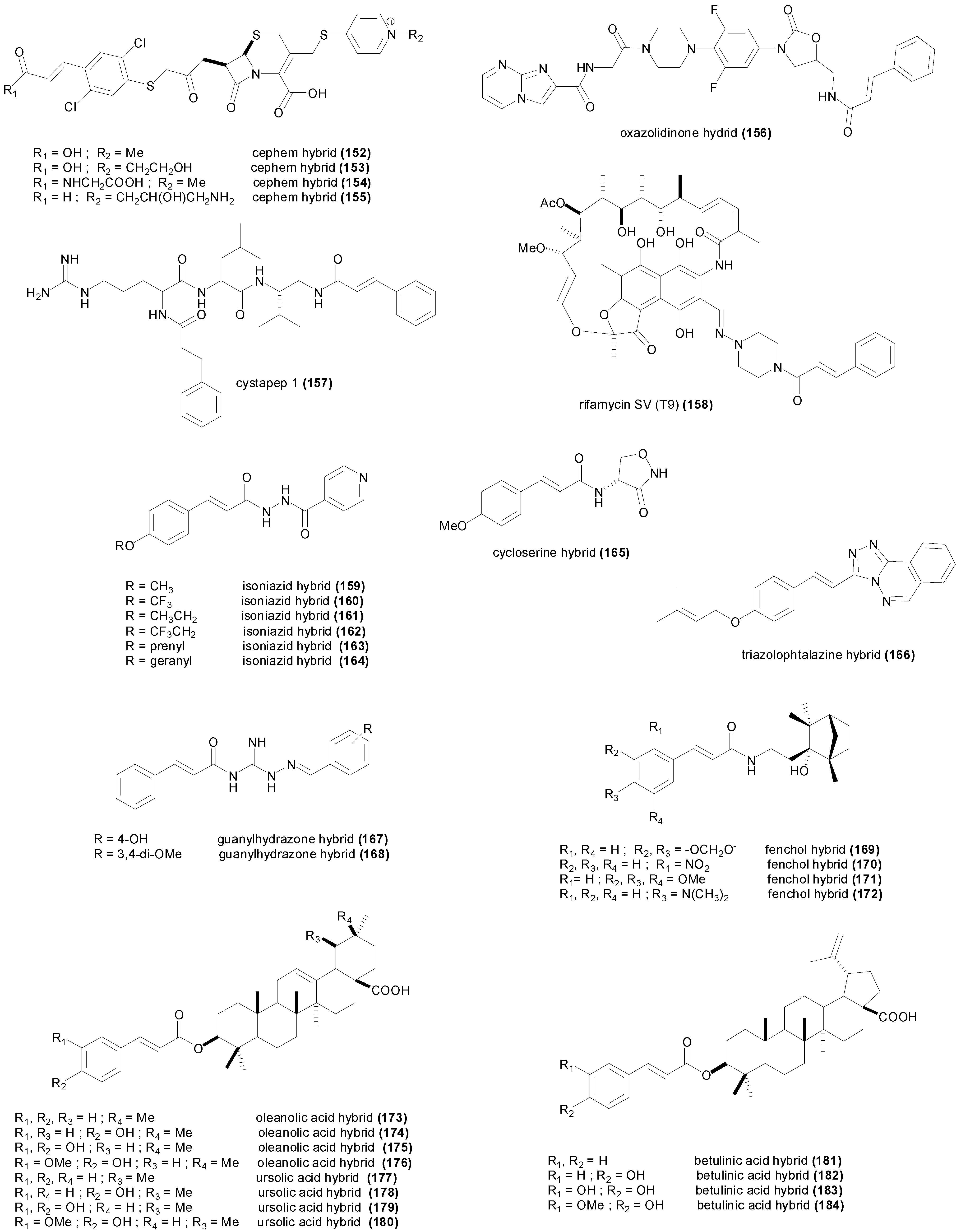
| Compound | Microorganism Strain | MIC | Refs. |
|---|---|---|---|
| cephem (152) | Streptococcus pneumoniae A28272 | 200 nM | [154] |
| Enterococcus faecalis A20688 | 1.6 µM | [154] | |
| Staphylococcus aureus MRSA A27223 | 3.2 µM | [154] | |
| Staphylococcus epidermidis A24548 | 95 nM | [154] | |
| Staphylococcus haemolyticus A21638 | 200 nM | [154] | |
| cephem (153) | Streptococcus pneumoniae A28272 | 760 nM | [154] |
| Enterococcus faecalis A20688 | 1.5 µM | [154] | |
| Staphylococcus aureus MRSA A27223 | 3.0 µM | [154] | |
| Staphylococcus epidermidis A24548 | 91 nM | [154] | |
| Staphylococcus haemolyticus A21638 | 190 nM | [154] | |
| cephem (154) | Streptococcus pneumoniae A28272 | 350 nM | [154] |
| Enterococcus faecalis A20688 | 1.4 mM | [154] | |
| Staphylococcus aureus MRSA A27223 | 2.8 mM | [154] | |
| Staphylococcus epidermidis A24548 | 42 nM | [154] | |
| Staphylococcus haemolyticus A21638 | 175 nM | [154] | |
| cephem (155) | Streptococcus pneumoniae A28272 | 727 nM | [154] |
| Enterococcus faecalis A20688 | 364 nM | [154] | |
| Staphylococcus aureus MRSA A27223 | 364 nM | [154] | |
| Staphylococcus epidermidis A24548 | 22 nM | [154] | |
| Staphylococcus haemolyticus A21638 | 87 nM | [154] | |
| oxazolidinone hybrid (156) | Enterococcus faecalis ATCC 29212 | 194 nM | [155] |
| Enterococcus faecium ATTC 700221 (VRE) | 388 nM | [155] | |
| Staphylococcus aureus ATCC 29213 | 194 nM | [155] | |
| Staphylococcus aureus ATCC 33591 (MRSA) | 1.6 µM | [155] | |
| cystapep 1 (157) | Staphylococcus aureus ATCC 29213 | 25.2 µM | [156] |
| Streptococcus agalactiae NTCC 8181 | 50.5 µM | [156] | |
| Streptococcus anginosus CCUG 27298 | 50.5 µM | [156] | |
| Streptococcus pneumoniae ATCC 49619 | 50.5 µM | [156] | |
| Streptococcus pyogenes type M1 | 25.2 µM | [156] | |
| rifamycin T9 (158) | Mycobacterium avium 101 | 15.9 nM | [159] |
| Mycobacterium avium N-260 | 831 nM | [157] | |
| Mycobacterium tuberculosis H37Rv | 31.9 nM | [159] | |
| Mycobacterium tuberculosis H37Rv | 106 nM | [157] | |
| Mycobacterium tuberculosis MTB9 (RIF-R) | 8.5 µM | [159] | |
| isoniazid hybrid (159) | Mycobacterium tuberculosis H37Rv | 300 nM | [161] |
| isoniazid hybrid (160) | Mycobacterium tuberculosis H37Rv | 1.1 µM | [161] |
| isoniazid hybrid (161) | Mycobacterium tuberculosis H37Rv | 1.3 µM | [161] |
| isoniazid hybrid (162) | Mycobacterium tuberculosis H37Rv | 2.2 µM | [161] |
| isoniazid hybrid (163) | Mycobacterium tuberculosis H37Rv | 2.3 µM | [161] |
| isoniazid hybrid (164) | Mycobacterium tuberculosis H37Rv | 1.9 µM | [161] |
| cycloserine hybrid (165) | Mycobacterium tuberculosis H37Rv | 950 µM | [161] |
| triazophtalazine hybrid (166) | Mycobacterium tuberculosis H37Rv | 1.4 µM | [161] |
| guanylhydrazone hybrid (167) | Mycobacterium tuberculosis H37Rv | 40.5 µM | [164] |
| guanylhydrazone hybrid (168) | Mycobacterium tuberculosis H37Rv | 8.9 µM | [164] |
| Fenchol hybrid (169) | Mycobacterium tuberculosis H37Rv | 6.7 µM | [165] |
| Fenchol hybrid (170) | Mycobacterium tuberculosis H37Rv | 6.7 µM | [165] |
| Fenchol hybrid (171) | Mycobacterium tuberculosis H37Rv | 2.4 µM | [165] |
| Fenchol hybrid (172) | Mycobacterium tuberculosis H37Rv | 540 nM | [165] |
| oleanolic acid hybrid (173) | Mycobacterium tuberculosis H37Rv | 85.2 µM | [166] |
| oleanolic acid hybrid (174) | Mycobacterium tuberculosis H37Rv | 10.4 µM | [166] |
| oleanolic acid hybrid (175) | Mycobacterium tuberculosis H37Rv | 323 µM | [166] |
| oleanolic acid hybrid (176) | Mycobacterium tuberculosis H37Rv | 19.8 µM | [166] |
| ursolic acid hybrid (177) | Mycobacterium tuberculosis H37Rv | >341 µM | [166] |
| ursolic acid hybrid (178) | Mycobacterium tuberculosis H37Rv | 10.4 µM | [166] |
| ursolic acid hybrid (179) | Mycobacterium tuberculosis H37Rv | 323 µM | [166] |
| ursolic acid hybrid (180) | Mycobacterium tuberculosis H37Rv | 4.95 µM | [166] |
| betulinic acid hybrid (181) | Mycobacterium tuberculosis H37Rv | >341 µM | [166] |
| betulinic acid hybrid (182) | Mycobacterium tuberculosis H37Rv | 10.4 µM | [166] |
| betulinic acid hybrid (183) | Mycobacterium tuberculosis H37Rv | 323 µM | [166] |
| betulinic acid hybrid (184) | Mycobacterium tuberculosis H37Rv | 316 µM | [166] |
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Xu, Z.; Zhang, D.; Hu, J.; Zhou, X.; Ye, X.; Reichel, K.; Stewart, N.; Syrenne, R.; Yang, X.; Gao, P.; et al. Comparative genome analysis of lignin biosynthesis gene families across the plant kingdom. BMC Bioinform. 2009, 10, S3. [Google Scholar]
- Lichtenthaler, H.K.; Schweiger, J. Cell wall bound ferulic acid, the major substance of the blue-green fluorescence emission of plants. J. Plant Physiol. 1998, 152, 272–282. [Google Scholar] [CrossRef]
- Kroon, P.A.; Williamson, G. Hydroxycinnamates in plants and food: current and future perspectives. J. Sci. Food Agric. 1999, 79, 355–361. [Google Scholar] [CrossRef]
- Martin-Tanguy, J.; Cabanne, F.; Perdrizet, E.; Martin, C. The distribution of hydroxycinnamic acid amides in flowering plants. Phytochemistry 1978, 17, 1927–1928. [Google Scholar] [CrossRef]
- Vogt, T. Phenylpropanoid biosynthesis. Mol. Plant 2010, 3, 2–20. [Google Scholar] [CrossRef] [PubMed]
- Ververidis, F.; Trantas, E.; Douglas, C.; Vollmer, G.; Kretzschmar, G.; Panopoulos, N. Biotechnology of flavonoids and other phenylpropanoid-derived natural products. Part I: Chemical diversity, impacts on plant biology and human health. Biotech. J. 2007, 2, 1214–1234. [Google Scholar] [CrossRef]
- Solecka, D. Role of phenylpropanoid compounds in plant responses to different stress factors. Acta Physiol. Plant 1997, 19, 257–268. [Google Scholar] [CrossRef]
- Hyun, M.W.; Yun, Y.H.; Kim, J.Y.; Kim, S.H. Fungal and plant phenylalanine ammonia-lyase. Mycobiology 2011, 39, 257–65. [Google Scholar] [CrossRef]
- Pittman, S. Cinnamon: It’s not just for making cinnamon rolls. Ethnobot. Leafl. 2011, 2000, 11. [Google Scholar]
- Wang, R.; Wang, R.; Yang, B. Extraction of essential oils from five cinnamon leaves and identification of their volatile compound compositions. Innov. Food Sci. Emerg. 2009, 10, 289–292. [Google Scholar] [CrossRef]
- Ooi, L.S.M.; Li, Y.; Kam, S.-L.; Wang, H.; Wong, E.Y.L.; Ooi, V.E.C. Antimicrobial Activities of cinnamon oil and cinnamaldehyde from the Chinese medicinal herb Cinnamomum cassia Blume. Am. J. Chin. Med. 2006, 34, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-T.; Chen, P.-F.; Chang, S.-C. Antibacterial activity of leaf essential oils and their constituents from Cinnamomum osmophloeum. J. Ethnopharmacol. 2001, 77, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Shahverdi, A.R.; Monsef-Esfahani, H.R.; Tavasoli, F.; Zaheri, A.; Mirjani, R. Trans-cinnamaldehyde from Cinnamomum zeylanicum bark essential oil reduces the clindamycin resistance of Clostridium difficile in vitro. J. Food Sci. 2007, 72, S055–S058. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N. Chlorogenic acids and other cinnamates—nature, occurrence and dietary burden. J. Sci. Food Agric. 1999, 79, 362–372. [Google Scholar] [CrossRef]
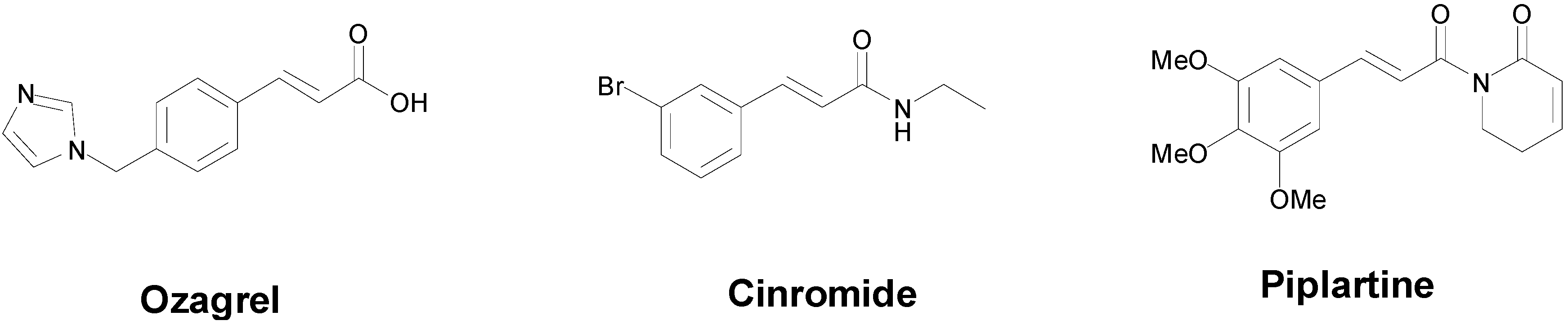
- Zhang, J.; Yang, J.; Chang, X.; Zhang, C.; Zhou, H.; Liu, M. Ozagrel for acute ischemic stroke: A meta-analysis of data from randomized controlled trials. Neurol. Res. 2012, 34, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Wilensky, A.J.; Ojemann, L.M.; Friel, P.N.; Almes, M.J.; Levy, R.H.; Dodrill, C.B. Cinromide in epilepsy: A pilot study. Epilepsia 1983, 24, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, D.P.; Pessoa, C.; de Moraes, M.O.; Saker-Neto, N.; Silveira, E.R.; Costa-Lotufo, L.V. Overview of the therapeutic potential of piplartine (piperlongumine). Eur. J. Pharm. Sci. 2012, 48, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Filho, R.B.; De Souza, M.P.; Mattos, M.E.O. Piplartine-dimer A, a new alkaloid from Piper tuberculatum. Phytochemistry 1981, 20, 345–346. [Google Scholar] [CrossRef]
- Bezerra, D.P.; Pessoa, C.; de Moraes, M.O.; Silveira, E.R.; Lima, M.A.; Elmiro, F.J.; Costa-Lotufo, L.V. Antiproliferative effects of two amides, piperine and piplartine, from Piper species. Z. Naturforsch. C Biol. Sci. 2005, 60, 539–543. [Google Scholar]
- Bezerra, D.P.; Militao, G.C.; de Castro, F.O.; Pessoa, C.; de Moraes, M.O.; Silveira, E.R.; Lima, M.A.; Elmiro, F.J.; Costa-Lotufo, L.V. Piplartine induces inhibition of leukemia cell proliferation triggering both apoptosis and necrosis pathways. Toxicol. In Vitro 2007, 21, 1–8. [Google Scholar] [CrossRef] [PubMed]
- De, P.; Baltas, M.; Bedos-Belval, F. Cinnamic acid derivatives as anticancer agents-a review. Curr. Med. Chem. 2011, 18, 1672–1703. [Google Scholar] [CrossRef] [PubMed]
- De, P.; Veau, D.; Bedos-Belval, F.; Chassaing, S.; Baltas, M. Cinnamic derivatives in tuberculosis. In Understanding Tuberculosis—New Approaches to Fighting against Drug Resistance; Cardona, P.-J., Ed.; InTech Publishing: Rijeka, Croatia, 2012; Chapter 15. [Google Scholar]
- Wiesner, J.; Mitsch, A.; Wißner, P.; Jomaa, H.; Schlitzer, M. Structure–activity relationships of novel anti-malarial agents. Part 2: Cinnamic acid derivatives. Bioorg. Med. Chem. Lett. 2001, 11, 423–424. [Google Scholar] [CrossRef] [PubMed]
- Tawata, S.; Taira, S.; Kobamoto, N.; Zhu, J.; Ishihara, M.; Toyama, S. Synthesis and antifungal activity of cinnamic acid esters. Biosci. Biotechnol. Biochem. 1996, 60, 909–910. [Google Scholar] [CrossRef] [PubMed]
- Sova, M. Antioxidant and antimicrobial activities of cinnamic acid derivatives. Mini Rev. Med. Chem. 2012, 12, 749–767. [Google Scholar] [CrossRef] [PubMed]
- Lapeyre, C.; Delomenède, M.; Bedos-Belval, F.; Duran, H.; Nègre-Salvayre, A.; Baltas, M. Design, synthesis, and evaluation of pharmacological properties of cinnamic derivatives as antiatherogenic agents. J. Med. Chem. 2005, 48, 8115–8124. [Google Scholar] [CrossRef]
- Simonyan, A. Activity of cinnamic acid derivatives and new methods for their synthesis (review). Pharm. Chem. J. 1993, 27, 92–100. [Google Scholar] [CrossRef]
- Wall, V.M.; Eisenstadt, A.; Ager, D.J.; Laneman, S.A. The Heck reaction and cinnamic acid synthesis by heterogeneous catalysis. Platin. Metals Rev. 1999, 43, 138–144. [Google Scholar]
- Sharma, P. Cinnamic acid derivatives: A new chapter of various pharmacological activities. J. Chem. Pharm. Res. 2011, 3, 403–423. [Google Scholar]
- Bygbjerg, I.C. Double burden of noncommunicable and infectious diseases in developing countries. Science 2012, 337, 1499–1501. [Google Scholar] [CrossRef] [PubMed]
- Coker, R.J.; Hunter, B.M.; Rudge, J.W.; Liverani, M.; Hanvoravongchai, P. Emerging infectious diseases in southeast Asia: Regional challenges to control. Lancet 2011, 377, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Mabey, D.; Peeling, R.W.; Ustianowski, A.; Perkins, M.D. Tropical infectious diseases: Diagnostics for the developing world. Nat. Rev. Microbiol. 2004, 2, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Fauci, A.S.; Morens, D.M. The perpetual challenge of infectious diseases. N. Eng. J. Med. 2012, 366, 454–461. [Google Scholar] [CrossRef]
- WHO. The Global Burden of Disease: 2004 Update; World Health Organization: Geneva, Switzerland, 2008; pp. 7–22. [Google Scholar]
- Powers, J.H. Antimicrobial drug development—The past, the present, and the future. Clin. Microbiol. Infect. 2004, 10, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Gold, H.S.; Moellering, R.C. Antimicrobial-drug resistance. N. Eng. J. Med. 1996, 335, 1445–1453. [Google Scholar] [CrossRef]
- Cohen, M.L. Epidemiology of drug resistance: Implications for a post—Antimicrobial era. Science 1992, 257, 1050–1055. [Google Scholar] [CrossRef] [PubMed]
- Sotgiu, G.; Centis, R.; D’Ambrosio, L.; Tadolini, M.; Castiglia, P.; Migliori, G.B. Do we need a new Fleming époque: The nightmare of drug-resistant tuberculosis. Int. J. Mycobacteriol. 2013, 2, 123–125. [Google Scholar] [CrossRef]
- David, M.Z.; Daum, R.S. Community-associated methicillin-resistant Staphylococcus aureus: Epidemiology and clinical consequences of an emerging epidemic. Clin. Microbiol. Rev. 2010, 23, 616–687. [Google Scholar] [CrossRef] [PubMed]
- MacGowan, A.; Macnaughton, E. Antibiotic resistance. Medicine 2013, 41, 642–648. [Google Scholar] [CrossRef]
- Hancock, R.E.W. Mechanisms of action of newer antibiotics for Gram-positive pathogens. Lancet Infect. Dis. 2005, 5, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Korošec, B.; Sova, M.; Turk, S.; Kraševec, N.; Novak, M.; Lah, L.; Stojan, J.; Podobnik, B.; Berne, S.; Zupanec, N.; et al. Antifungal activity of cinnamic acid derivatives involves inhibition of benzoate 4-hydroxylase (CYP53). J. Appl. Microbiol. 2014, 116, 955–966. [Google Scholar] [CrossRef] [PubMed]
- Jitareanu, A.; Tataringa, G.; Zbancioc, A.M.; Tuchilus, C.; Stanescu, U. Antimicrobial activity of some cinnamic acid derivatives. Rev. Med. Chir. Soc. Med. Nat. Iasi 2011, 115, 965–971. [Google Scholar] [PubMed]
- Narasimhan, B.; Belsare, D.; Pharande, D.; Mourya, V.; Dhake, A. Esters, amides and substituted derivatives of cinnamic acid: Synthesis, antimicrobial activity and QSAR investigations. Eur. J. Med. Chem. 2004, 39, 827–834. [Google Scholar] [CrossRef]
- Hemaiswarya, S.; Doble, M. Synergistic interaction of phenylpropanoids with antibiotics against bacteria. J. Med. Microbiol. 2010, 59, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Wen, A.; Delaquis, P.; Stanich, K.; Toivonen, P. Antilisterial activity of selected phenolic acids. Food Microbiol. 2003, 20, 305–311. [Google Scholar] [CrossRef]
- Kujumgiev, A.; Tsvetkova, I.; Serkedjieva, Y.; Bankova, V.; Christov, R.; Popov, S. Antibacterial, antifungal and antiviral activity of propolis of different geographic origin. J. Ethnopharmacol. 1999, 64, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Simone-Finstrom, M.; Spivak, M. Propolis and bee health: The natural history and significance of resin use by honey bees. Apidologie 2010, 41, 295–311. [Google Scholar] [CrossRef]
- Bankova, V.; Christov, R.; Kujumgiev, A.; Marcucci, M.C.; Popov, S. Chemical composition and antibacterial activity of Brazilian propolis. Z. Naturforsch. C Bio. Sci. 1995, 50, 167–172. [Google Scholar]
- Bankova, V.S.; Popov, S.S.; Marekov, N.L. Isopentenyl cinnamates from poplar buds and propolis. Phytochemistry 1989, 28, 871–873. [Google Scholar] [CrossRef]
- Bankova, V.S.; de Castro, S.L.; Marcucci, M.C. Propolis: Recent advances in chemistry and plant origin. Apidologie 2000, 31, 3–16. [Google Scholar] [CrossRef]
- Mohammadzadeh, S.; Shariatpanahi, M.; Hamedi, M.; Ahmadkhaniha, R.; Samadi, N.; Ostad, S.N. Chemical composition, oral toxicity and antimicrobial activity of Iranian propolis. Food Chem. 2007, 103, 1097–1103. [Google Scholar] [CrossRef]
- Stepanović, S.; Antić, N.; Dakić, I.; Švabić-Vlahović, M. In vitro antimicrobial activity of propolis and synergism between propolis and antimicrobial drugs. Microbiol. Res. 2003, 158, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.; Gomes, B.P.F.A.; Rosalen, P.L.; Ambrosano, G.M.B.; Park, Y.K.; Cury, J.A. In vitro antimicrobial activity of propolis and Arnica montana against oral pathogens. Arch. Oral Biol. 2000, 45, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, W.; Vincent, S. Theory and Application of Microbiological Assay; Academic Press: San Diego, CA, USA, 1989; pp. 1–37. [Google Scholar]
- Olasupo, N.A.; Fitzgerald, D.J.; Gasson, M.J.; Narbad, A. Activity of natural antimicrobial compounds against Escherichia coli and Salmonella enterica serovar Typhimurium. Lett. Appl. Microbiol. 2003, 37, 448–451. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, N.; Domadia, P.; Shetty, S.; Dasgupta, D. Screening of natural phenolic compounds for potential to inhibit bacterial cell division protein FtsZ. Ind. J. Exp. Biol. 2008, 46, 783–787. [Google Scholar]
- Alves, M.J.; Ferreira, I.C.; Froufe, H.J.; Abreu, R.M.; Martins, A.; Pintado, M. Antimicrobial activity of phenolic compounds identified in wild mushrooms, SAR analysis and docking studies. J. Appl. Microbiol. 2013, 115, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Prasad, V.G.N.V.; Krishna, B.V.; Swamy, P.L.; Rao, T.S.; Rao, G.S. Antibacterial synergy between quercetin and polyphenolic acids against bacterial pathogens of fish. Asian Pac. J. Trop. Dis. 2014, 4 (Suppl. 1), S326–S329. [Google Scholar] [CrossRef]
- Guzman, J.D.; Mortazavi, P.N.; Munshi, T.; Evangelopoulos, D.; McHugh, T.D.; Gibbons, S.; Malkinson, J.; Bhakta, S. 2-Hydroxy-substituted cinnamic acids and acetanilides are selective growth inhibitors of Mycobacterium tuberculosis. MedChemComm 2014, 5, 47–50. [Google Scholar] [CrossRef]
- Guzman, J.D.; Evangelopoulos, D.; Gupta, A.; Birchall, K.; Mwaigwisya, S.; Saxty, B.; McHugh, T.D.; Gibbons, S.; Malkinson, J.; Bhakta, S. Antitubercular specific activity of ibuprofen and the other 2-arylpropanoic acids using the HT-SPOTi whole-cell phenotypic assay. BMJ Open 2013, 3, e002672–e002685. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, N.; Goh, K.S.; Horgen, L.; Barrow, W.W. Synergistic activities of antituberculous drugs with cerulenin and trans-cinnamic acid against Mycobacterium tuberculosis. FEMS Immunol. Med. Microbiol. 1998, 21, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Huang, S.T.; Sun, F.M.; Chiang, Y.L.; Chiang, C.J.; Tsai, C.M.; Weng, C.J. Transformation of cinnamic acid from trans- to cis-form raises a notable bactericidal and synergistic activity against multiple-drug resistant Mycobacterium tuberculosis. Eur. J. Pharm. Sci. 2011, 43, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Warbasse, J.P. Cinnamic acid in the treatment of tuberculosis. Ann. Surg. 1894, 19, 102–111. [Google Scholar] [CrossRef]
- Bisogno, F.; Mascoti, L.; Sanchez, C.; Garibotto, F.; Giannini, F.; Kurina-Sanz, M.; Enriz, R. Structure–antifungal activity relationship of cinnamic acid derivatives. J. Agric. Food Chem. 2007, 55, 10635–10640. [Google Scholar] [PubMed]
- Schmidt, E.; Bail, S.; Friedl, S.M.; Jirovetz, L.; Buchbauer, G.; Wanner, J.; Denkova, Z.; Slavchev, A.; Stoyanova, A.; Geissler, M. Antimicrobial activities of single aroma compounds. Nat. Prod. Commun. 2010, 5, 1365–1368. [Google Scholar] [PubMed]
- Lou, Z.; Wang, H.; Rao, S.; Sun, J.; Ma, C.; Li, J. p-Coumaric acid kills bacteria through dual damage mechanisms. Food Control 2012, 25, 550–554. [Google Scholar] [CrossRef]
- Barber, M.S.; McConnell, V.S.; DeCaux, B.S. Antimicrobial intermediates of the general phenylpropanoid and lignin specific pathways. Phytochemistry 2000, 54, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Parkar, S.G.; Stevenson, D.E.; Skinner, M.A. The potential influence of fruit polyphenols on colonic microflora and human gut health. Int. J. Food Microbiol. 2008, 124, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Tunçel, G.; Nergiz, C. Antimicrobial effect of some olive phenols in a laboratory medium. Lett. Appl. Microbiol. 1993, 17, 300–302. [Google Scholar] [CrossRef]
- García-Ruiz, A.; Bartolomé, B.; Cueva, C.; Martín-Álvarez, P.J.; Moreno-Arribas, M.V. Inactivation of oenological lactic acid bacteria (Lactobacillus hilgardii and Pediococcus pentosaceus) by wine phenolic compounds. J. Appl. Microbiol. 2009, 107, 1042–1053. [Google Scholar] [CrossRef] [PubMed]
- Stead, D. The effect of hydroxycinnamic acids on the growth of wine-spoilage lactic acid bacteria. J. Appl. Bacteriol. 1993, 75, 135–141. [Google Scholar] [CrossRef]
- Zabka, M.; Pavela, R. Antifungal efficacy of some natural phenolic compounds against significant pathogenic and toxinogenic filamentous fungi. Chemosphere 2013, 93, 1051–1056. [Google Scholar] [CrossRef] [PubMed]
- Puupponen-Pimiä, R.; Nohynek, L.; Alakomi, H.-L.; Oksman-Caldentey, K.-M. Bioactive berry compounds—Novel tools against human pathogens. Appl. Microbiol. Biotechnol. 2005, 67, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Gasparetto, J.C.; Guimarães de Francisco, T.M.; Campos, F.R.; Pontarolo, R. Development and validation of two methods based on high-performance liquid chromatography-tandem mass spectrometry for determining 1,2-benzopyrone, dihydrocoumarin, o-coumaric acid, syringaldehyde and kaurenoic acid in guaco extracts and pharmaceutical preparations. J. Sep. Sci. 2011, 34, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Herald, P.J.; Davidson, P.M. Antibacterial activity of selected hydroxycinnamic acids. J. Food Sci. 1983, 48, 1378–1379. [Google Scholar] [CrossRef]
- Georgiev, L.; Chochkova, M.; Ivanova, G.; Najdenski, H.; Ninova, M.; Milkova, T. Radical scavenging and antimicrobial activities of cinnamoyl amides of biogenic monoamines. Riv. Ital. Sost. Grasse 2012, 89, 91–102. [Google Scholar]
- De Vita, D.; Friggeri, L.; D’Auria, F.D.; Pandolfi, F.; Piccoli, F.; Panella, S.; Palamara, A.T.; Simonetti, G.; Scipione, L.; di Santo, R.; et al. Activity of caffeic acid derivatives against Candida albicans biofilm. Bioorg. Med. Chem. Lett. 2014, 24, 1502–1505. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.; Ferreira, C.; Saavedra, M.J.; Simoes, M. Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microb. Drug Resist. 2013, 19, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Nakauchi, M.; Ikemoto, S.; Yamanishi, H.; Ozaki, Y.; Tsuno, T.; Nomura, E.; Hosoda, A.; Taniguchi, H. Antimicrobial activities of synthetic ferulic acid derivatives. Food Preserv. Sci. 2002, 28, 183–188. [Google Scholar] [CrossRef]
- Ergün, B.; Çoban, T.; Onurdag, F.; Banoglu, E. Synthesis, antioxidant and antimicrobial evaluation of simple aromatic esters of ferulic acid. Arch. Pharm. Res. 2011, 34, 1251–1261. [Google Scholar] [CrossRef] [PubMed]
- Klančnik, A.; Možina, S.S.; Zhang, Q. Anti-Campylobacter activities and resistance mechanisms of natural phenolic compounds in Campylobacter. PLoS One 2012, 7, e51800. [Google Scholar] [CrossRef] [PubMed]
- Engels, C.; Schieber, A.; Gänzle, M. Sinapic acid derivatives in defatted Oriental mustard (Brassica juncea L.) seed meal extracts using UHPLC-DAD-ESI-MS n and identification of compounds with antibacterial activity. Eur. Food Res. Technol. 2012, 234, 535–542. [Google Scholar] [CrossRef]
- Nakazono, Y.; Watanabe, Y.; Hashinaga, F.; Tadera, K. Studies on antimicrobial and antioxidative substance of Yuzu (Citrus junos hort. ex Tanaka) seed. J. Biol. Sci. 2006, 6, 135–139. [Google Scholar] [CrossRef]
- Cheng, M.-J.; Wu, M.-D.; Yanai, H.; Su, Y.-S.; Chen, I.-S.; Yuan, G.-F.; Hsieh, S.-Y.; Chen, J.-J. Secondary metabolites from the endophytic fungus Biscogniauxia formosana and their antimycobacterial activity. Phytochem. Lett. 2012, 5, 467–472. [Google Scholar] [CrossRef]
- Tonari, K.; Mitsui, K.; Yonemoto, K. Structure and antibacterial activity of cinnamic acid related compounds. J. Oleo Sci. 2002, 51, 271–273. [Google Scholar] [CrossRef]
- Feasley, C.F.; Gwynn, B.H.; Degering, F.; Tetrault, P.A. Bacteriostatic properties of phenylacetic acid. Effect of ortho-substituents on. J. Am. Pharm. Assoc. 1941, 30, 41–45. [Google Scholar] [CrossRef]
- Kanao, S.; Kashino, S.; Haisa, M. Topochemical studies. XIII. Structures of 3-bromocinnamic acid and 3-chlorocinnamic acid. Acta Crystallograph. Sect. C Cryst. Struct. Commun. 1990, 46, 2436–2438. [Google Scholar] [CrossRef]
- Schaldach, B.; Grützmacher, H.F. The fragmentations of substituted cinnamic acids after electron impact. Org. Mass Spectrom. 1980, 15, 175–181. [Google Scholar] [CrossRef]
- Newby, B.M.Z.; Cutright, T.; Barrios, C.; Xu, Q. Zosteric acid—An effective antifoulant for reducing fresh water bacterial attachment on coatings. J. Coat. Technol. Res. 2006, 3, 69–76. [Google Scholar] [CrossRef]
- Villa, F.; Pitts, B.; Stewart, P.; Giussani, B.; Roncoroni, S.; Albanese, D.; Giordano, C.; Tunesi, M.; Cappitelli, F. Efficacy of zosteric acid sodium salt on the yeast biofilm model Candida albicans. Microb. Ecol. 2011, 62, 584–598. [Google Scholar] [CrossRef] [PubMed]
- Feresin, G.E.; Tapia, A.; Gimenez, A.; Ravelo, A.G.; Zacchino, S.; Sortino, M.; Schmeda-Hirschmann, G. Constituents of the Argentinian medicinal plant Baccharis grisebachii and their antimicrobial activity. J. Ethnopharmacol. 2003, 89, 73–80. [Google Scholar] [PubMed]
- Hermans, D.; Martel, A.; van Deun, K.; van Immerseel, F.; Heyndrickx, M.; Haesebrouck, F.; Pasmans, F. The cinnamon-oil ingredient trans-cinnamaldehyde fails to target Campylobacter jejuni strain KC 40 in the broiler chicken cecum despite marked in vitro activity. J. Food Prot. 2011, 74, 1729–1734. [Google Scholar] [CrossRef] [PubMed]
- Tesaki, S.; Tanabe, S.; Ono, H.; Fukushi, E.; Kawabata, J.; Watanabe, M. 4-Hydroxy-3-nitrophenylacetic and sinapic acids as antibacterial compounds from mustard seeds. Biosci. Biotechnol. Biochem. 1998, 62, 998–1000. [Google Scholar] [CrossRef]
- Olthof, M.R.; Hollman, P.C.H.; Katan, M.B. Chlorogenic acid and caffeic acid are absorbed in humans. J. Nutr. 2001, 131, 66–71. [Google Scholar] [PubMed]
- Zhu, X.; Zhang, H.; Lo, R. Phenolic compounds from the leaf extract of artichoke (Cynara scolymus L.) and their antimicrobial activities. J. Agric. Food Chem. 2004, 52, 7272–7278. [Google Scholar] [CrossRef] [PubMed]
- Daglia, M.; Papetti, A.; Grisoli, P.; Aceti, C.; Spini, V.; Dacarro, C.; Gazzani, G. Isolation, identification, and quantification of roasted coffee antibacterial compounds. J. Agric. Food Chem. 2007, 55, 10208–10213. [Google Scholar] [CrossRef] [PubMed]
- Xia, D.; Wu, X.; Shi, J.; Yang, Q.; Zhang, Y. Phenolic compounds from the edible seeds extract of Chinese Mei (Prunus mume Sieb. et Zucc) and their antimicrobial activity. LWT—Food Sci. Technol. 2011, 44, 347–349. [Google Scholar] [CrossRef]
- Fiamegos, Y.C.; Kastritis, P.L.; Exarchou, V.; Han, H.; Bonvin, A.M.J.J.; Vervoort, J.; Lewis, K.; Hamblin, M.R.; Tegos, G.P. Antimicrobial and efflux pump inhibitory activity of caffeoylquinic acids from Artemisia absinthium against Gram-positive pathogenic bacteria. PLoS One 2011, 6, e18127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lou, Z.; Wang, H.; Zhu, S.; Ma, C.; Wang, Z. Antibacterial activity and mechanism of action of chlorogenic acid. J. Food Sci. 2011, 76, M398–M403. [Google Scholar] [PubMed]
- Zhang, M.; Liu, W.-X.; Zheng, M.-F.; Xu, Q.-L.; Wan, F.-H.; Wang, J.; Lei, T.; Zhou, Z.-Y.; Tan, J.-W. Bioactive quinic acid derivatives from Ageratina adenophora. Molecules 2013, 18, 14096–14104. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.; Jang, S.; Kim, B.-K.; Park, M. Antibacterial phenylpropanoid glycosides from Paulownia tomentosa Steud. Arch. Pharm. Res. 1994, 17, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Svetaz, L.; Tapia, A.; López, S.N.; Furlán, R.L.E.; Petenatti, E.; Pioli, R.; Schmeda-Hirschmann, G.; Zacchino, S.A. Antifungal chalcones and new caffeic acid esters from Zuccagnia punctata acting against soybean infecting fungi. J. Agric. Food Chem. 2004, 52, 3297–3300. [Google Scholar] [CrossRef] [PubMed]
- Abedini, A.; Roumy, V.; Mahieux, S.; Biabiany, M.; Standaert-Vitse, A.; Riviere, C.; Sahpaz, S.; Bailleul, F.; Neut, C.; Hennebelle, T. Rosmarinic acid and its methyl ester as antimicrobial components of the hydromethanolic extract of Hyptis atrorubens Poit. (Lamiaceae). Evid. Based Complement. Altern. Med. 2013, 2013, 604536:1–604536:11. [Google Scholar]
- Lin, L.; Zhu, D.; Zou, L.; Yang, B.; Zhao, M. Antibacterial activity-guided purification and identification of a novel C-20 oxygenated ent-kaurane from Rabdosia serra (Maxim.) Hara. Food Chem. 2013, 139, 902–909. [Google Scholar] [CrossRef] [PubMed]
- Gohari, A.R.; Saeidnia, S.; Shahverdi, A.R.; Yassa, N.; Malmir, M.; Mollazade, K.; Naghinejad, A.R. Phytochemistry and antimicrobial compounds of Hymenocrater calycinus. EurAsian J. Biosci. 2009, 3, 64–68. [Google Scholar] [CrossRef]
- Grunberger, D.; Banerjee, R.; Eisinger, K.; Oltz, E.M.; Efros, L.; Caldwell, M.; Estevez, V.; Nakanishi, K. Preferential cytotoxicity on tumor cells by caffeic acid phenethyl ester isolated from propolis. Experientia 1988, 44, 230–232. [Google Scholar] [CrossRef] [PubMed]
- Velazquez, C.; Navarro, M.; Acosta, A.; Angulo, A.; Dominguez, Z.; Robles, R.; Robles-Zepeda, R.; Lugo, E.; Goycoolea, F.M.; Velazquez, E.F.; et al. Antibacterial and free-radical scavenging activities of Sonoran propolis. J. Appl. Microbiol. 2007, 103, 1747–1756. [Google Scholar] [CrossRef] [PubMed]
- Ango, P.Y.; Kapche, D.W.F.G.; Kuete, V.; Ngadjui, B.T.; Bezabih, M.; Abegaz, B.M. Chemical constituents of Trilepisium madagascariense (Moraceae) and their antimicrobial activity. Phytochem. Lett. 2012, 5, 524–528. [Google Scholar] [CrossRef]
- Chien, Y.C.; Lin, C.H.; Chiang, M.Y.; Chang, H.S.; Liao, C.H.; Chen, I.S.; Peng, C.F.; Tsai, I.L. Secondary metabolites from the root of Ehretia longiflora and their biological activities. Phytochemistry 2012, 80, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Khatkar, A.; Nanda, A.; Kumar, P.; Narasimhan, B. Synthesis, antimicrobial evaluation and QSAR studies of p-coumaric acid derivatives. Arab. J. Chem. 2014, in press. [Google Scholar]
- Lakshmanan, D.; Werngren, J.; Jose, L.; Suja, K.P.; Nair, M.S.; Varma, R.L.; Mundayoor, S.; Hoffner, S.; Kumar, R.A. Ethyl p-methoxycinnamate isolated from a traditional anti-tuberculosis medicinal herb inhibits drug resistant strains of Mycobacterium tuberculosis in vitro. Fitoterapia 2011, 82, 757–761. [Google Scholar] [CrossRef] [PubMed]
- Dyer, L.A.; Richards, J.; Dodson, C.D. Isolation, synthesis, and evolutionary ecology of Piper amides. In Piper: A Model Genus for Studies of Phytochemistry, Ecology, and Evolution; Dyer, L.A., Palmer, A.D.N., Eds.; Springer: New York, NY, USA, 2004; pp. 117–139. [Google Scholar]
- Naika, R.; Prasanna, K.; Ganapathy, P. Antibacterial activity of piperlongumine an alkaloid isolated from the methanolic root extract of Piper longum L. Pharmacophore 2010, 1, 141–148. [Google Scholar]
- Navickiene, H.M.D.; Alécio, A.C.; Kato, M.J.; Bolzani, V.D.S.; Young, M.C.M.; Cavalheiro, A.J.; Furlan, M. Antifungal amides from Piper hispidum and Piper tuberculatum. Phytochemistry 2000, 55, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Vasques da Silva, R.; Debonsi Navickiene, H.M.; Kato, M.J.; Bolzani, V.D.S.; Méda, C.I.; Young, M.C.M.; Furlan, M. Antifungal amides from Piper arboreum and Piper tuberculatum. Phytochemistry 2002, 59, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Rukachaisirikul, T.; Siriwattanakit, P.; Sukcharoenphol, K.; Wongvein, C.; Ruttanaweang, P.; Wongwattanavuch, P.; Suksamrarn, A. Chemical constituents and bioactivity of Piper sarmentosum. J. Ethnopharmacol. 2004, 93, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Tuntiwachwuttikul, P.; Phansa, P.; Pootaeng-On, Y.; Taylor, W.C. Chemical constituents of the roots of Piper sarmentosum. Chem. Pharm. Bull. 2006, 54, 149–151. [Google Scholar] [CrossRef] [PubMed]
- Samwel, S.; Odalo, J.O.; Nkunya, M.H.; Joseph, C.C.; Koorbanally, N.A. Toussaintines A-E: Antimicrobial indolidinoids, a cinnamoylhydrobenzofuranoid and a cinnamoylcyclohexenoid from Toussaintia orientalis leaves. Phytochemistry 2011, 72, 1826–1832. [Google Scholar] [CrossRef] [PubMed]
- Jantan, I.B.; Karim Moharam, B.A.; Santhanam, J.; Jamal, J.A. Correlation between chemical composition and antifungal activity of the essential oils of eight Cinnamomum species. Pharm. Biol. 2008, 46, 406–412. [Google Scholar] [CrossRef]
- Ali, S.; Khan, A.; Ahmed, I.; Musaddiq, M.; Ahmed, K.; Polasa, H.; Rao, L.V.; Habibullah, C.; Sechi, L.; Ahmed, N. Antimicrobial activities of eugenol and cinnamaldehyde against the human gastric pathogen Helicobacter pylori. Ann. Clin. Microbiol. Antimicrob. 2005, 4, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Cox, S.D.; Markham, J.L. Susceptibility and intrinsic tolerance of Pseudomonas aeruginosa to selected plant volatile compounds. J. Appl. Microbiol. 2007, 103, 930–936. [Google Scholar] [CrossRef] [PubMed]
- Pei, R.-S.; Zhou, F.; Ji, B.-P.; Xu, J. Evaluation of combined antibacterial effects of eugenol, cinnamaldehyde, thymol, and carvacrol against E. coli with an improved method. J. Food Sci. 2009, 74, M379–M383. [Google Scholar] [CrossRef] [PubMed]
- Domadia, P.; Swarup, S.; Bhunia, A.; Sivaraman, J.; Dasgupta, D. Inhibition of bacterial cell division protein FtsZ by cinnamaldehyde. Biochem. Pharmacol. 2007, 74, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.O.; Park, S.W.; Park, H.D. Inactivation of Escherichia coli O157:H7 by cinnamic aldehyde purified from Cinnamomum cassia shoot. Food Microbiol. 2004, 21, 105–110. [Google Scholar] [CrossRef]
- Shimizu, I.; Isshiki, Y.; Nomura, H.; Sakuda, K.; Sakuma, K.; Kondo, S. The antibacterial activity of fragrance ingredients against Legionella pneumophila. Biol. Pharm. Bull. 2009, 32, 1114–1117. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.A.; Yu, C.B.; Park, H.D. Bacteriocidal effects and inhibition of cell separation of cinnamic aldehyde on Bacillus cereus. Lett. Appl. Microbiol. 2003, 37, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Jia, P.; Xue, Y.J.; Duan, X.J.; Shao, S.H. Effect of cinnamaldehyde on biofilm formation and sarA expression by methicillin-resistant Staphylococcus aureus. Lett. Appl. Microbiol. 2011, 53, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Xing, F.; Hua, H.; Selvaraj, J.N.; Zhao, Y.; Zhou, L.; Liu, X.; Liu, Y. Growth inhibition and morphological alterations of Fusarium verticillioides by cinnamon oil and cinnamaldehyde. Food Control 2014, 46, 343–350. [Google Scholar] [CrossRef]
- Shreaz, S.; Sheikh, R.A.; Rimple, B.; Hashmi, A.A.; Nikhat, M.; Khan, L.A. Anticandidal activity of cinnamaldehyde, its ligand and Ni(II) complex: Effect of increase in ring and side chain. Microb. Pathog. 2010, 49, 75–82. [Google Scholar] [PubMed]
- Ferhout, H.; Bohatier, J.; Guillot, J.; Chalchat, J.C. Antifungal activity of selected essential oils, cinnamaldehyde and carvacrol against Malassezia furfur and Candida albicans. J. Essent. Oil Res. 1999, 11, 119–129. [Google Scholar] [CrossRef]
- Rajput, S.B.; Karuppayil, S.M. Small molecules inhibit growth, viability and ergosterol biosynthesis in Candida albicans. SpringerPlus 2013, 2, 26–28. [Google Scholar] [CrossRef] [PubMed]
- Yen, T.-B.; Chang, S.-T. Synergistic effects of cinnamaldehyde in combination with eugenol against wood decay fungi. Bioresour. Technol. 2008, 99, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Hsu, F.-L.; Chang, H.-T.; Chang, S.-T. Evaluation of antifungal properties of octyl gallate and its synergy with cinnamaldehyde. Bioresour. Technol. 2007, 98, 734–738. [Google Scholar] [PubMed]
- Chen, S.; Huang, H.-Y.; Cheng, M.-J.; Wu, C.-C.; Ishikawa, T.; Peng, C.-F.; Chang, H.-S.; Wang, C.-J.; Wong, S.-L.; Chen, I.-S. Neolignans and phenylpropanoids from the roots of Piper taiwanense and their antiplatelet and antitubercular activities. Phytochemistry 2013, 93, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Shreaz, S.; Bhatia, R.; Khan, N.; Muralidhar, S.; Manzoor, N.; Khan, L.A. Influences of cinnamic aldehydes on H+ extrusion activity and ultrastructure of Candida. J. Med. Microbiol. 2013, 62, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Meerungrueang, W.; Panichayupakaranant, P. Antimicrobial activities of some Thai traditional medical longevity formulations from plants and antibacterial compounds from Ficus foveolata. Pharm. Biol. 2014, 52, 1104–1109. [Google Scholar] [CrossRef] [PubMed]
- Morozumi, S. Isolation, purification, and antibiotic activity of o-methoxycinnamaldehyde from cinnamon. Appl. Environ. Microbiol. 1978, 36, 577–583. [Google Scholar] [PubMed]
- Sharma, U.; Sood, S.; Sharma, N.; Rahi, P.; Kumar, R.; Sinha, A.; Gulati, A. Synthesis and SAR investigation of natural phenylpropene-derived methoxylated cinnamaldehydes and their novel Schiff bases as potent antimicrobial and antioxidant agents. Med. Chem. Res. 2013, 22, 5129–5140. [Google Scholar] [CrossRef]
- Cheng, S.-S.; Liu, J.-Y.; Tsai, K.-H.; Chen, W.-J.; Chang, S.-T. Chemical composition and mosquito larvicidal activity of essential oils from leaves of different Cinnamomum osmophloeum provenances. J. Agric. Food Chem. 2004, 52, 4395–4400. [Google Scholar] [CrossRef] [PubMed]
- Caredda, A.; Marongiu, B.; Porcedda, S.; Soro, C. Supercritical carbon dioxide extraction and characterization of Laurus nobilis essential oil. J. Agric. Food Chem. 2002, 50, 1492–1496. [Google Scholar] [CrossRef] [PubMed]
- Simić, A.; Soković, M.; Ristić, M.; Grujić-Jovanović, S.; Vukojević, J.; Marin, P. The chemical composition of some Lauraceae essential oils and their antifungal activities. Phytother. Res. 2004, 18, 713–717. [Google Scholar] [CrossRef]
- Koga, T.; Matsuse, I.; Teramoto, Y.; Ueda, S. Synthesis and antimycotic activity of cinnamyl benzoate. J. Ferment. Bioengineer. 1993, 76, 524–526. [Google Scholar] [CrossRef]
- Aziz, A.N.; Ibrahim, H.; Rosmy Syamsir, D.; Mohtar, M.; Vejayan, J.; Awang, K. Antimicrobial compounds from Alpinia conchigera. J. Ethnopharmacol. 2013, 145, 798–802. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.K.; Pahwa, S.; Nandanwar, H.; Jachak, S.M. Phenylpropanoids of Alpinia galanga as efflux pump inhibitors in Mycobacterium smegmatis mc2 155. Fitoterapia 2012, 83, 1248–1255. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Jiang, H.; Xie, P. Antifungal activities against Sclerotinia sclerotiorum by Cinnamomum cassia oil and its main components. J. Essent. Oil Res. 2013, 25, 444–451. [Google Scholar]
- Mehta, G.; Singh, V. Hybrid systems through natural product leads: An approach towards new molecular entities. Chem. Soc. Rev. 2002, 31, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Meunier, B. Hybrid molecules with a dual mode of action: Dream or reality? Acc. Chem. Res. 2008, 41, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-J.; Geng, C.-A.; Ma, Y.-B.; Luo, J.; Huang, X.-Y.; Chen, H.; Zhou, N.-J.; Zhang, X.-M.; Chen, J.-J. Design, synthesis, and molecular hybrids of caudatin and cinnamic acids as novel anti-hepatitis B virus agents. Eur. J. Med. Chem. 2012, 54, 352–365. [Google Scholar] [CrossRef] [PubMed]
- Voisin-Chiret, A.S.; Bazin, M.-A.; Lancelot, J.-C.; Rault, S. Synthesis of new L-ascorbic ferulic acid hybrids. Molecules 2007, 12, 2533–2545. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, J.; Silva, T.; Benfeito, S.; Gaspar, A.; Garrido, E.M.; Garrido, J.; Borges, F. Exploring nature profits: Development of novel and potent lipophilic antioxidants based on galloyl–cinnamic hybrids. Eur. J. Med. Chem. 2013, 62, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Pérez, B.; Teixeira, C.; Gut, J.; Rosenthal, P.J.; Gomes, J.R.B.; Gomes, P. Cinnamic acid/chloroquinoline conjugates as potent agents against chloroquine-resistant Plasmodium falciparum. ChemMedChem 2012, 7, 1537–1540. [Google Scholar] [CrossRef] [PubMed]
- Pérez, B.C.; Teixeira, C.; Figueiras, M.; Gut, J.; Rosenthal, P.J.; Gomes, J.R.B.; Gomes, P. Novel cinnamic acid/4-aminoquinoline conjugates bearing non-proteinogenic amino acids: Towards the development of potential dual action antimalarials. Eur. J. Med. Chem. 2012, 54, 887–899. [Google Scholar] [CrossRef] [PubMed]
- Springer, D.M.; Luh, B.-Y.; Goodrich, J.; Bronson, J.J. Anti-MRSA cephems. Part 2: C-7 cinnamic acid derivatives. Bioorg. Med. Chem. 2003, 11, 265–279. [Google Scholar] [CrossRef] [PubMed]
- Michalska, K.; Karpiuk, I.; Król, M.; Tyski, S. Recent development of potent analogues of oxazolidinone antibacterial agents. Bioorg. Med. Chem. 2013, 21, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Jasir, A.; Kasprzykowski, F.; Kasprzykowska, R.; Lindström, V.; Schalén, C.; Grubb, A. New antimicrobial cystatin C-based peptide active against gram-positive bacterial pathogens, including methicillin-resistant Staphylococcus aureus and multiresistant coagulase-negative staphylococci. Acta Pathol. Microbiol. Immunol. Scand. 2003, 111, 1004–1010. [Google Scholar] [CrossRef]
- Sato, K.; Shimizu, T.; Dimova, V.; Tomioka, H. Antimicrobial activities of cinnamyl rifamycin derivatives, T-9 and T-11, against Mycobacterium tuberculosis and Mycobacterium avium complex (MAC) with special reference to the activities against intracellular MAC. Microbiol. Immunol. 2006, 50, 621–623. [Google Scholar] [PubMed]
- Velichka, D.; Ivana, A.; Haruaki, T.; Katsumasa, S.; Venkata, R.; Nadadhur, G.; Donna, D.; Todor, K.; Arvind, D.; Yurii, F.; et al. Experimental and clinical studies on Rifacinna—The new effective antituberculous drug (review). Recent Pat. Antiinfect. Drug Discover. 2010, 5, 76–90. [Google Scholar] [CrossRef]
- Reddy, V.M.; Nadadhur, G.; Daneluzzi, D.; Dimova, V.; Gangadharam, P.R. Antimycobacterial activity of a new rifamycin derivative, 3-(4-cinnamylpiperazinyl iminomethyl) rifamycin SV (T9). Antimicrob. Agents Chemother. 1995, 39, 2320–2324. [Google Scholar] [CrossRef] [PubMed]
- Dhople, A.M. Comparative in vitro activities of rifamycin analogues against Mycobacterium leprae. Indian J. Lepr. 1997, 69, 377–384. [Google Scholar] [PubMed]
- De, P.; Koumba Yoya, G.; Constant, P.; Bedos-Belval, F.; Duran, H.; Saffon, N.; Daffé, M.; Baltas, M. Design, synthesis, and biological evaluation of new cinnamic derivatives as antituberculosis agents. J. Med. Chem. 2011, 54, 1449–1461. [Google Scholar] [CrossRef] [PubMed]
- TBAlliance. Isoniazid. Tuberculosis 2008, 88, 112–116. [Google Scholar]
- TBAlliance. Cycloserine. Tuberculosis 2008, 88, 100–101. [Google Scholar]
- Bairwa, R.; Kakwani, M.; Tawari, N.R.; Lalchandani, J.; Ray, M.K.; Rajan, M.G. R.; Degani, M.S. Novel molecular hybrids of cinnamic acids and guanylhydrazones as potential antitubercular agents. Bioorg. Med. Chem. Lett. 2010, 20, 1623–1625. [Google Scholar] [CrossRef] [PubMed]
- Slavchev, I.; Dobrikov, G.M.; Valcheva, V.; Ugrinova, I.; Pasheva, E.; Dimitrov, V. Antimycobacterial activity generated by the amide coupling of (−)-fenchone derived aminoalcohol with cinnamic acids and analogues. Bioorg. Med. Chem. Lett. 2014, in press. [Google Scholar]
- Tanachatchairatana, T.; Bremner, J.B.; Chokchaisiri, R.; Suksamrarn, A. Antimycobacterial activity of cinnamate-based esters of the triterpenes betulinic, oleanolic and ursolic acids. Chem. Pharm. Bull. 2008, 56, 194–198. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guzman, J.D. Natural Cinnamic Acids, Synthetic Derivatives and Hybrids with Antimicrobial Activity. Molecules 2014, 19, 19292-19349. https://doi.org/10.3390/molecules191219292
Guzman JD. Natural Cinnamic Acids, Synthetic Derivatives and Hybrids with Antimicrobial Activity. Molecules. 2014; 19(12):19292-19349. https://doi.org/10.3390/molecules191219292
Chicago/Turabian StyleGuzman, Juan David. 2014. "Natural Cinnamic Acids, Synthetic Derivatives and Hybrids with Antimicrobial Activity" Molecules 19, no. 12: 19292-19349. https://doi.org/10.3390/molecules191219292