Polylactide Conjugates of Camptothecin with Different Drug Release Abilities
Abstract
:1. Introduction
2. Results and Discussion

| Sample | Spirotox | Protoxkit F | Microtox | |||
|---|---|---|---|---|---|---|
| DCT | EX | DCT | EX | DCT | EX | |
| PLA-1 | 0 | 0 | 1 | 4 | −4 | 0 |
| PLA-2 | 0 | 0 | 6 | 10 | −5 | 1 |
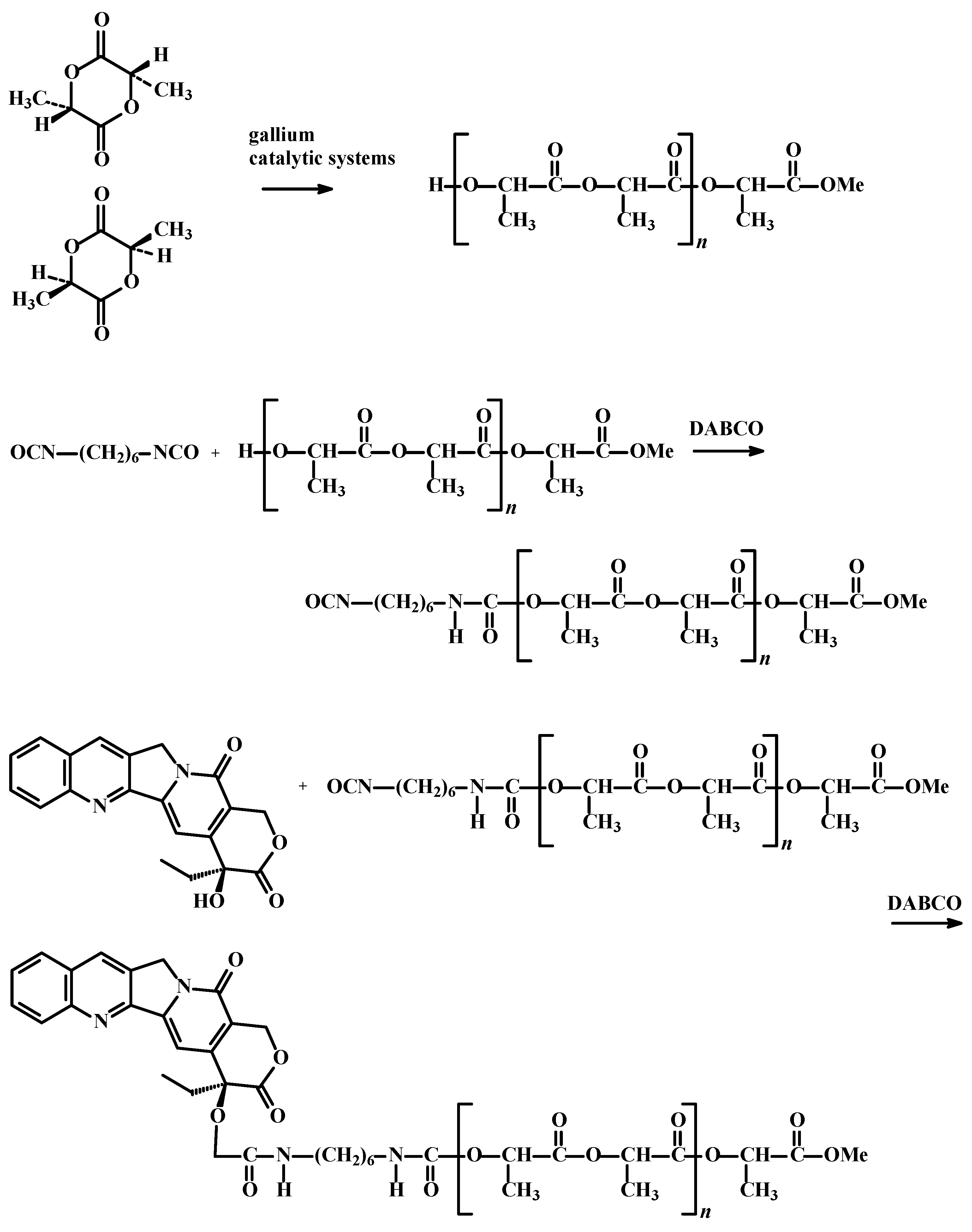

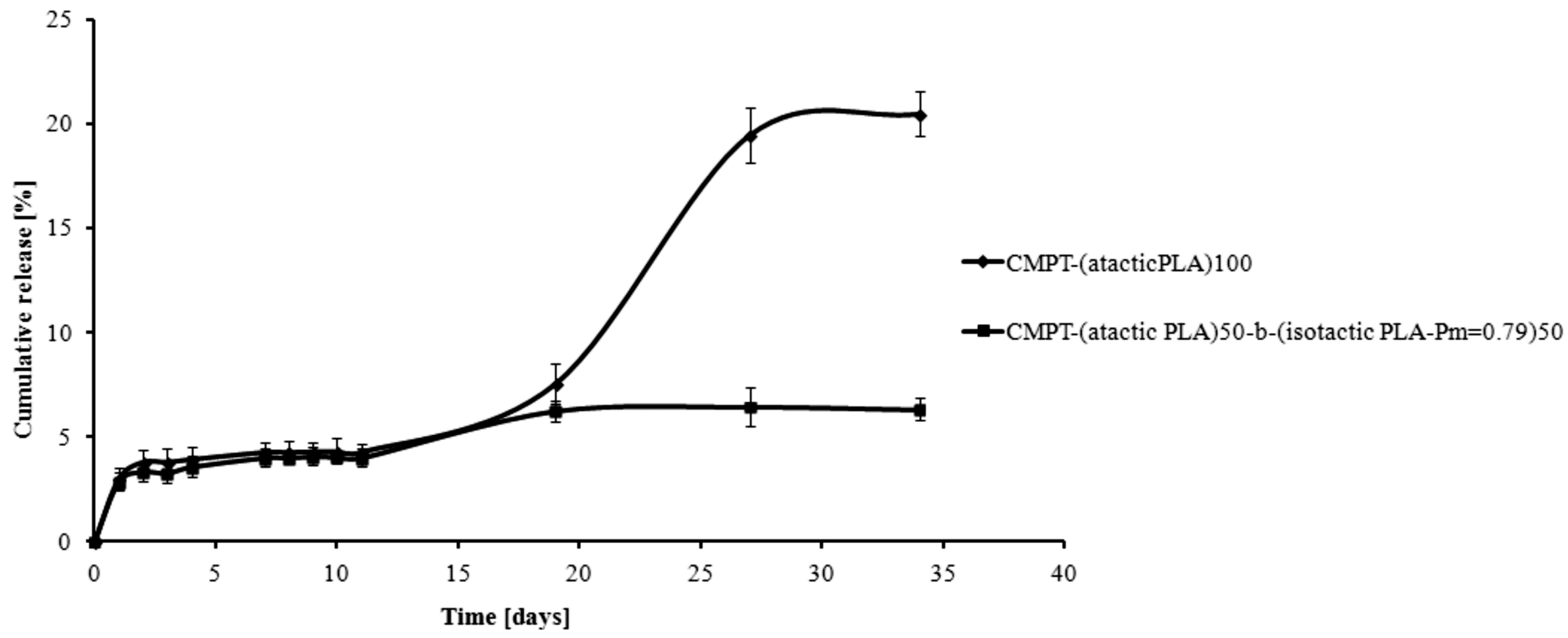
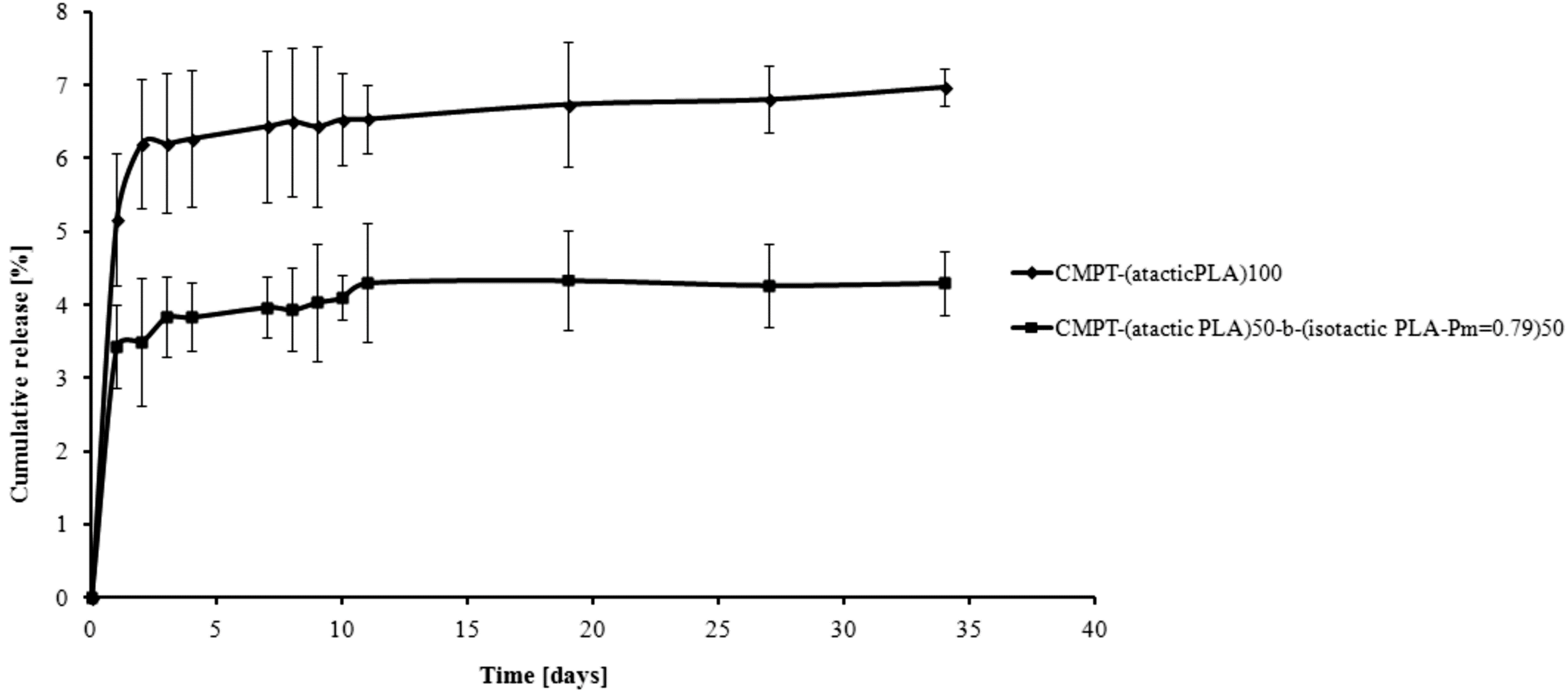
3. Experimental Section
3.1. General Information
3.2. Synthesis of PLA Matrices
3.3. Toxicity Assays
3.3.1. Microtox®
3.3.2. Protoxkit FTM
3.3.3. Spirotox
3.4. Synthesis of Camptothecin Conjugates
3.5. Camptothecin Release Study from the Macromolecular Conjugates
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wu, S.-F.; Hsieh, P.-W.; Wu, C.-C.; Lee, C.-L.; Chen, S.-L.; Lu, C.-Y.; Wu, T.-S.; Chang, F.-R.; Wu, Y.-C. Camptothecinoids from the seeds of Taiwanese Nothapodytes Foetida. Molecules 2008, 13, 1361–1671. [Google Scholar] [CrossRef]
- Fan, H.; Huang, J.; Li, Y.; Yu, J.; Chen, J. Fabrication of reduction-degradable micelle based on disulfide-linked graft copolymer-camptothecin conjugate for enhancing solubility and stability of camptothecin. Polymer 2010, 51, 5107–5114. [Google Scholar] [CrossRef]
- Sobczak, M.; Oledzka, E.; Kwietniewska, M.; Nalecz-Jawecki, G.; Kołodziejski, W. Promising macromolecular conjugates of camptothecin—The synthesis, characterization and in vitro studies. J. Macromol. Sci. Pure Appl. Chem. 2014, 51, 254–262. [Google Scholar] [CrossRef]
- Warnecke, A.; Kratz, F. Maleimide-oligo(ethylene glycol) derivatives of camptothecin as albumin-binding prodrugs: Synthesis and antitumor efficacy. Bioconjugate Chem. 2003, 14, 377–387. [Google Scholar] [CrossRef]
- Greenwald, R.B.; Zhao, H.; Xia, J. Tripartate poly(ethylene glycol) prodrugs of the open lactone form of camptothecin. Bioorg. Med. Chem. 2003, 11, 2635–2639. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, R.; de Vries, P.; Tulinsky, J.; Bellamy, G.; Baker, B.; Singer, W.; Klein, P. Synthesis and in vivo antitumor activity of poly(l-glutamic acid) conjugates of 20(S)-camptothecin. J. Med. Chem. 2003, 46, 190–193. [Google Scholar] [CrossRef] [PubMed]
- Singer, J.W.; Bhatt, R.; Tulinsky, J.; Buhler, K.R.; Heasley, E.; Klein, P.; de Vries, P. Water-soluble poly-(l-glutamic acid)-Gly-camptothecin conjugates enhance camptothecin stability and efficacy in vivo. J. Control. Release 2001, 74, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.J.; Khin, K.T.; Jensen, G.S.; Liu, A.; Davis, M.E. Synthesis of linear, β-cyclodextrin-based polymers and their camptothecin conjugates. Bioconjugate Chem. 2003, 14, 1007–1017. [Google Scholar] [CrossRef]
- Caiolfa, V.R.; Zamai, M.; Fiorino, A.; Frigerio, E.; Pellizzoni, C.; D’Argy, R.; Ghiglieri, A.; Castelli, M.G.; Farao, M.; Pesenti, E.; et al. Polymer-bound camptothecin: Initial biodistribution and antitumour activity studies. J. Control. Release 2000, 65, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Fan, N.; Duan, K.; Wang, C.; Liu, S.; Luo, S.; Yu, J.; Huang, J.; Li, Y.; Wang, D. Fabrication of nanomicelle with enhanced solubility and stability of camptothecin based on α,β-poly[(N-carboxybutyl)-l-aspartamide]-camptothecin conjugate. Colloids Surf. B 2010, 75, 543–549. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, J.; Fan, N.; Yu, J.; Liu, Y.; Liu, S.; Wang, D.; Li, Y. Nanomicelle with long-term circulation and enhanced stability of camptothecin based on mPEGylated α,β-poly (l-aspartic acid)-camptothecin conjugate. Colloids Surf. B 2010, 81, 297–303. [Google Scholar] [CrossRef]
- Chun, C.; Kuh, H.-J.; Song, S.-C. Injectable poly(organophosphazene)-camptothecin conjugate hydrogels: Synthesis, characterization, and antitumor activities. Eur. J. Pharm. Biopharm. 2012, 81, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Ignatius, A.A.; Claes, L.E. In vitro biocompatibility of bioresorbable polymers: Poly(l,dl-lactide) and poly(l-lactide-co-glycolide). Biomaterials 1996, 17, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Jelonek, K.; Kasperczyk, J.; Li, S.; Dobrzyński, P.; Jarząbek, B. Controlled poly(l-lactide-co-trimethylene carbonate) delivery system of cyclosporine A and rapamycine—The effect of copolymer chain microstructure on drug release rate. Int. J. Pharm. 2011, 414, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, P.J.; Du, H.; Feijen, J. Single site catalysts for stereoselective ring-opening polymerization of lactides. Polym. Chem. 2011, 2, 520–527. [Google Scholar] [CrossRef]
- Słomkowski, S.; Penczek, S.; Duda, A. Polylactides—An overview. Polym. Adv. Technol. 2014, 25, 436–447. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, Y.; Liu, X.; Chen, X.; Cui, D.; Chen, E.Y.-Z. Protic compound mediated living cross-chain-transfer polymerization of rac-lactide: Synthesis of isotactic (crystalline)-heterotactic (amorphous) stereomultiblock polylactide. Chem. Commun. 2012, 48, 6375–6377. [Google Scholar] [CrossRef]
- Horeglad, P.; Szczepaniak, G.; Dranka, M.; Zachara, J. The first facile stereoselectivity switch in the polymerization of rac-lactide-from heteroselective to isoselective dialkylgallium alkoxides with the help of N-heterocyclic carbenes. Chem. Commun. 2012, 48, 1171–1173. [Google Scholar] [CrossRef]
- Horeglad, P.; Litwińska, A.; Żukowska, G.Z.; Kubicki, D.; Szczepaniak, G.; Dranka, M.; Zachara, J. The influence of organosuperbases on the structure and activity of dialkylgallium alkoxides in the polymerization of rac-lactide: The road to stereo diblock PLA copolymers. Appl. Organomet. Chem. 2013, 27, 328–336. [Google Scholar] [CrossRef]
- Horeglad, P.; Kruk, P.; Pécaut, J. Heteroselective Polymerization of rac-lactide in the presence of dialkylgallium alkoxides: The effect of Lewis base on polymerization stereoselectivity. Organometallics 2010, 29, 3729–3734. [Google Scholar] [CrossRef]
- Sobczak, M.; Plichta, A.; Oledzka, E.; Jaklewicz, A.; Kuras, M.; Cwil, A.; Kołodziejski, W.L.; Florjanczyk, Z.; Szatan, K.; Udzielak, I. Some spectrometric determination of metals in aliphatic polyester and polycarbonate biomedical polymers. Polimery 2009, 54, 114–119. [Google Scholar]
- Chemberlain, B.M.; Cheng, M.; Moore, D.R.; Ovitt, T.M.; Lobkovsky, E.B.; Coates, G.W. Polymerization of lactide with zinc and magnesium β-diiminate complexes: Mtereocontrol and mechanism. J. Am. Chem. Soc. 2001, 123, 3229–3238. [Google Scholar] [CrossRef] [PubMed]
- Kasperczyk, J.E. Microstructure analysis of poly(lactic acid) obtained by lithium tert-butoxide as initiator. Macromolecules 1995, 28, 3937–3939. [Google Scholar] [CrossRef]
- Abbina, S.; Du, G. Zinc-catalyzed highly isoselective ring opening polymerization of rac-lactide. ACS Macro Lett. 2014, 3, 689–692. [Google Scholar] [CrossRef] [PubMed]
- Amna, T.; Hassan, M.S.; Nam, K.-T.; Bing, Y.Y.; Barakat, N.A.M.; Khil, M.-S.; Kim, H.Y. Preparation, characterization, and cytotoxicity of CPT/Fe2O3-embedded PLGA ultrafine composite fibers: A synergistic approach to develop promising anticancer material. Int. J. Nanomedicine 2012, 7, 1659–1670. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Lee, C.; Sai, P.; Choe, Y.H.; Boro, M.; Pendri, A.; Guan, S.Y.; Greenwald, R.B. 20-O-acylcamptothecin derivatives: Evidence for lactone stabilization. J. Org. Chem. 2000, 65, 4601–4606. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the obtained compounds are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oledzka, E.; Horeglad, P.; Gruszczyńska, Z.; Plichta, A.; Nałęcz-Jawecki, G.; Sobczak, M. Polylactide Conjugates of Camptothecin with Different Drug Release Abilities. Molecules 2014, 19, 19460-19470. https://doi.org/10.3390/molecules191219460
Oledzka E, Horeglad P, Gruszczyńska Z, Plichta A, Nałęcz-Jawecki G, Sobczak M. Polylactide Conjugates of Camptothecin with Different Drug Release Abilities. Molecules. 2014; 19(12):19460-19470. https://doi.org/10.3390/molecules191219460
Chicago/Turabian StyleOledzka, Ewa, Paweł Horeglad, Zuzanna Gruszczyńska, Andrzej Plichta, Grzegorz Nałęcz-Jawecki, and Marcin Sobczak. 2014. "Polylactide Conjugates of Camptothecin with Different Drug Release Abilities" Molecules 19, no. 12: 19460-19470. https://doi.org/10.3390/molecules191219460
APA StyleOledzka, E., Horeglad, P., Gruszczyńska, Z., Plichta, A., Nałęcz-Jawecki, G., & Sobczak, M. (2014). Polylactide Conjugates of Camptothecin with Different Drug Release Abilities. Molecules, 19(12), 19460-19470. https://doi.org/10.3390/molecules191219460






