Synthesis and Biological Evaluation of 2-Phenoxyacetamide Analogues, a Novel Class of Potent and Selective Monoamine Oxidase Inhibitors
Abstract
:1. Introduction
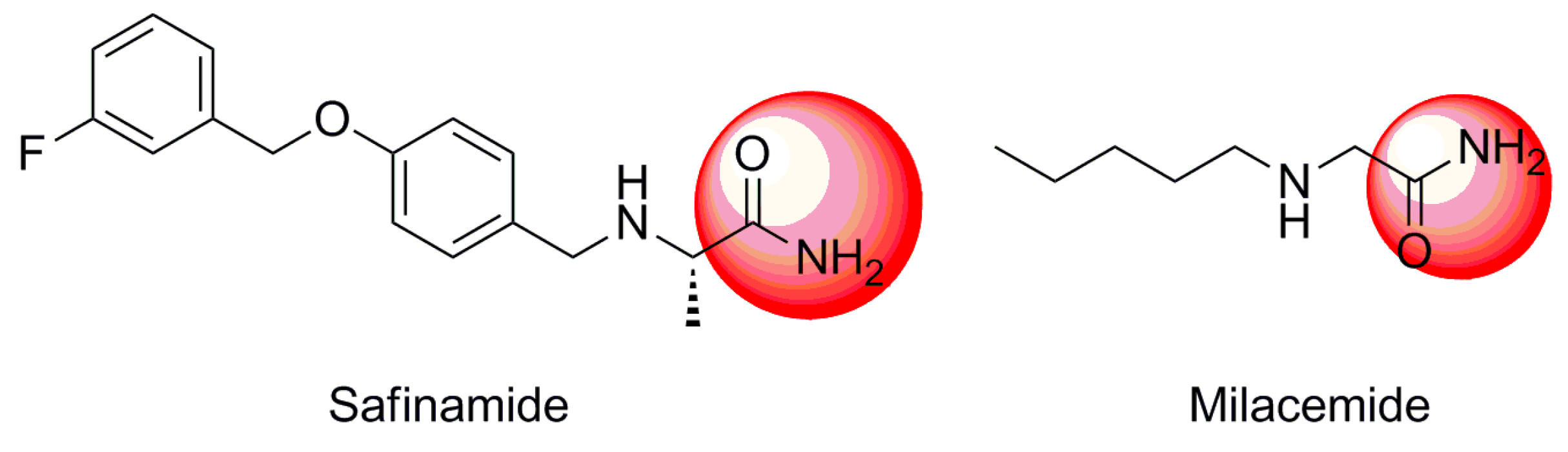
2. Results and Discussion
2.1. Chemistry


2.2. Enzyme Inhibition Studies
| Item | R | X | IC50 (μM) | SI b | |
|---|---|---|---|---|---|
| MAO-A | MAO-B | ||||
| 1 | Phenyl | O | 69 | 778 | 11.27 |
| 2 | 2-Naphthalenyl | O | 149 | 542 | 3.64 |
| 3 | 4-Fluoro-phenyl | O | 92 | 255 | 2.77 |
| 4 | 4-Chloro-phenyl | O | 490 | 202 | 0.41 |
| 5 | 2-Chloro-phenyl | O | 98 | 694 | 7.08 |
| 6 | 4-Formyl-phenyl | O | 89 | 457 | 5.13 |
| 7 | 2-Formyl-phenyl | O | 142 | 559 | 3.94 |
| 8 | 3,4-Dimethyl-phenyl | O | 113 | 534 | 4.73 |
| 9 | 2-Methyl-phenyl | O | 26 | 663 | 25.5 |
| 10 | 4-Methyl-phenyl | O | 3 | 541 | 180 |
| 11 | 2-Methoxy-phenyl | O | 96 | 775 | 8.07 |
| 12 | 4-Methoxy-phenyl | O | 4 | 980 | 245 |
| 13 | o-Carboxyphenyl | O | 217 | 177 | 0.82 |
| 14 | o-Acid ester | O | 108 | 98 | 0.91 |
| 15 | 4-( N-tert-Butyl O-acyl)amine-phenyl | O | 196 | 296 | 1.51 |
| 16 | 4-( N-acetyl)amine- phenyl | O | 61 | 553 | 9.07 |
| 17 | Phenyl | S | 166 | 642 | 3.87 |
| 18 | 3,4-Dimethyl-phenyl | S | 292 | 366 | 1.58 |
| 19 | 4-(4,5-dihydro-1H-imidazol-2-yl)-phenyl | O | 61 | 506 | 8.30 |
| 20 | 2-(4,5-dihydro-1H-imidazol-2-yl)-phenyl | O | 186 | 714 | 3.84 |
| 21 | 4-((Prop-2-ynylimino)methyl)-phenyl | O | 0.018 | 0.076 | 4.22 |
| 22 | 4-((Prop-2-ynylamino)methyl)-phenyl | O | 0.094 | 0.164 | 1.74 |
| 23 | 4-((benzylimino)methyl)-phenyl | O | 96 | 575 | 5.99 |
| 24 | 4-((benzylamino)methyl)-phenyl | O | 37 | 534 | 14.43 |
| 25 | 2-((Prop-2-ynylimino)methyl)-phenyl | O | 0.068 | 0.176 | 2.59 |
| 26 | 2-((Prop-2-ynylamino)methyl)-phenyl | O | 0.168 | 0.188 | 1.19 |
| 27 | 2-((benzylimino)methyl)-phenyl | O | 147 | 562 | 3.82 |
| 28 | 2-((benzylamino)methyl)-phenyl | O | 107 | 497 | 4.64 |
| Clorgyline | 0.0011 (0.0014) | ||||
| Pargyline | 0.0035 (0.0038) | ||||
| Enzymes/Cell Lysates | 12 | 21 | 22 | 25 |
|---|---|---|---|---|
| SH-SY5Y | 5.4 | 0.18 | 0.26 | 0.88 |
| MAO-A | 4.0 | 0.018 | 0.094 | 0.068 |
| HepG2 | N.D. b | 0.41 | 0.53 | 1.81 |
| MAO-B | 980 | 0.076 | 0.164 | 0.176 |
2.3. Reversibility of Inhibition
| Compound | MAO-A Inhibiton (%) | MAO-B Inhibiton (%) | ||
|---|---|---|---|---|
| Before Washing | After Washing | Before Washing | After Washing | |
| 12 (100 nM) | 88.7% | 12.5% | 96.9% | 8.6% |
| Safinamide (100 nM) | 36.1% | 9.8% | 91.3% | 7.1% |
3. Experimental Section
3.1. Materials and Methods
3.2. General Procedure for the Synthesis of Derivatives of Acetamides [15]
3.2.1. General Procedure for the Preparation of Compounds 1–18
3.2.2. Preparation of Compounds 19 and 20
3.2.3. Preparation of Compounds 21, 23 or 25
3.2.4. Preparation of Compound 22, 24 or 26
3.3. Biochemistry
3.3.1. MAOs Inhibitory Assay
3.3.2. Cell Lysates Preparation
3.3.3. Reversibility Experiments
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Barnham, K.J.; Bush, A.I. Biological metals and metal-targeting compounds in major neurodegenerative diseases. Chem. Soc. Rev. 2014, 43, 6727–6749. [Google Scholar]
- Youdim, M.B.H.; Edmondson, D.; Tipton, K.F. The therapeutic potential of monoamine oxidase inhibitors. Nat. Rev. Neurosci. 2006, 7, 295–309. [Google Scholar]
- Carrieri, A.; Carotti, A.; Barreca, M.L.; Altomare, C. Binding models of reversible inhibitors to type-B monoamine oxidase. J. Comput. Aid. Mol. Des. 2002, 16, 769–778. [Google Scholar]
- Gentili, F.; Pizzinat, N.; Ordener, C.; Marchal-Victorion, S.; Maurel, A.; Hofmann, R.; Renard, P.; Delagrange, P.; Pigini, M.; Parini, A.; et al. 3-[5-(4,5-Dihydro-1H-imidazol-2-yl)-furan-2-yl]phenylamine (Amifuraline), a promising reversible and selective peripheral MAO-A inhibitor. J. Med. Chem. 2006, 49, 5578–5586. [Google Scholar]
- Sant’ Anna, G.S.; Machado, P.; Sauzem, P.D.; Rosa, F.A.; Rubin, M.A.; Ferreira, J.; Bonacorso, H.G.; Zanatta, N.; Martins, M.A. Ultrasound promoted synthesis of 2-imidazolines in water: A greener approach toward monoamine oxidase inhibitors. Bioorg. Med. Chem. Lett. 2009, 19, 546–549. [Google Scholar]
- Ramsay, R.R. Monoamine Oxidases: The biochemistry of the proteins as targets in medicinal chemistry and drug discovery. Curr. Top. Med. Chem. 2012, 12, 2189–2209. [Google Scholar]
- Secci, D.; Bolasco, A.; Chimenti, P.; Carradori, S. The state of the art of pyrazole derivatives as monoamine oxidase inhibitors and antidepressant/anticonvulsant agents. Curr. Med. Chem. 2011, 18, 5114–5144. [Google Scholar]
- Christophe, J.; Kutzner, R.; Hguyen-Bui, N.D.; Damien, C.; Chatelain, P.; Gillet, L. Conversion of orally administered 2-n.pentylaminoacetamide into glycinamide and glycine in the rat brain. Life Sci. 1983, 33, 533–541. [Google Scholar]
- Chapman, A.G.; Hart, G.P. Anticonvulsant drug action and regional neurotransmitter amino acid changes. J. Neural Transm. 1988, 72, 201–212. [Google Scholar]
- Silverman, R.B.; Nishimura, K.; Lu, X. Mechanism of inactivation of monoamine oxidase-B by the anticonvulsant agent milacemide (2-(n-pentylamino)acetamide). J. Am. Chem. Soc. 1993, 115, 4949–4954. [Google Scholar]
- Lu, Y.Y.; Zhu, Q. A novel fluorogenic probe for monoamine oxidase assays. Chin. Chem. Lett. 2008, 19, 947–950. [Google Scholar]
- Jia, Z.; Zhu, Q. Monoamine oxidase inhibitors: Benzylidene-prop-2-ynyl-amines analogues. Biol. Pharm. Bull. 2010, 33, 725–728. [Google Scholar]
- Naoi, M.; Maruyama, W.; Akao, Y.; Yi, H.; Yamaoka, Y. Involvement of type a monoamine oxidase in neurodegeneration: Regulation of mitochondrial signaling leading to cell death or neuroprotection. J. Neural Transm. Suppl. 2006, 71, 67–77. [Google Scholar]
- Shih, J.C.; Chen, K. Regulation of MAO-A and MAO-B gene expression. Curr. Med. Chem. 2004, 11, 1995–2005. [Google Scholar]
- Valhondo, M.; Marco, I.; Martin-Fontecha, M.; Vázquez-Villa, H.; Ramos, J.A.; Berkels, R.; Lauterbach, T.; Benhamú, B.; López-Rodríguez, M.L. New serotonin 5-HT1A receptor agonists endowed with antinociceptive activity in vivo. J. Med. Chem. 2013, 56, 7851–7861. [Google Scholar]
- Chimenti, F.; Carradori, S.; Secci, D.; Bolasco, A.; Bizzarri, B.; Chimenti, P.; Granese, A.; Yáñez, M.; Orallo, F. Synthesis and inhibitory activity against human monoamine oxidase of N1-thiocarbamoyl-3,5-di(hetero)aryl-4,5-dihydro-(1H)-pyrazole derivatives. Eur. J. Med. Chem. 2010, 45, 800–804. [Google Scholar]
- Sample Availability: Samples of the compounds are not available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, W.; Yu, S.; Zhang, J.; Jia, W.; Zhu, Q. Synthesis and Biological Evaluation of 2-Phenoxyacetamide Analogues, a Novel Class of Potent and Selective Monoamine Oxidase Inhibitors. Molecules 2014, 19, 18620-18631. https://doi.org/10.3390/molecules191118620
Shen W, Yu S, Zhang J, Jia W, Zhu Q. Synthesis and Biological Evaluation of 2-Phenoxyacetamide Analogues, a Novel Class of Potent and Selective Monoamine Oxidase Inhibitors. Molecules. 2014; 19(11):18620-18631. https://doi.org/10.3390/molecules191118620
Chicago/Turabian StyleShen, Wei, Shian Yu, Jiaming Zhang, Weizheng Jia, and Qing Zhu. 2014. "Synthesis and Biological Evaluation of 2-Phenoxyacetamide Analogues, a Novel Class of Potent and Selective Monoamine Oxidase Inhibitors" Molecules 19, no. 11: 18620-18631. https://doi.org/10.3390/molecules191118620





