Isolation and Bioactivities of the Flavonoids Morin and Morin-3-O-β-D-glucopyranoside from Acridocarpus orientalis—A Wild Arabian Medicinal Plant
Abstract
:1. Introduction
2. Results and Discussion
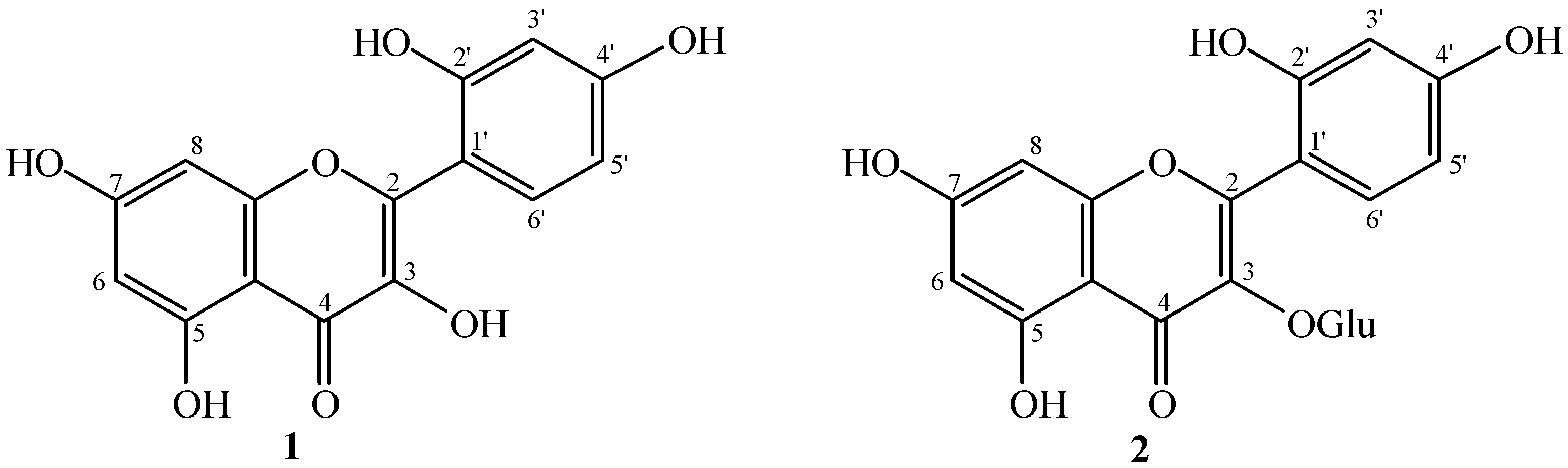
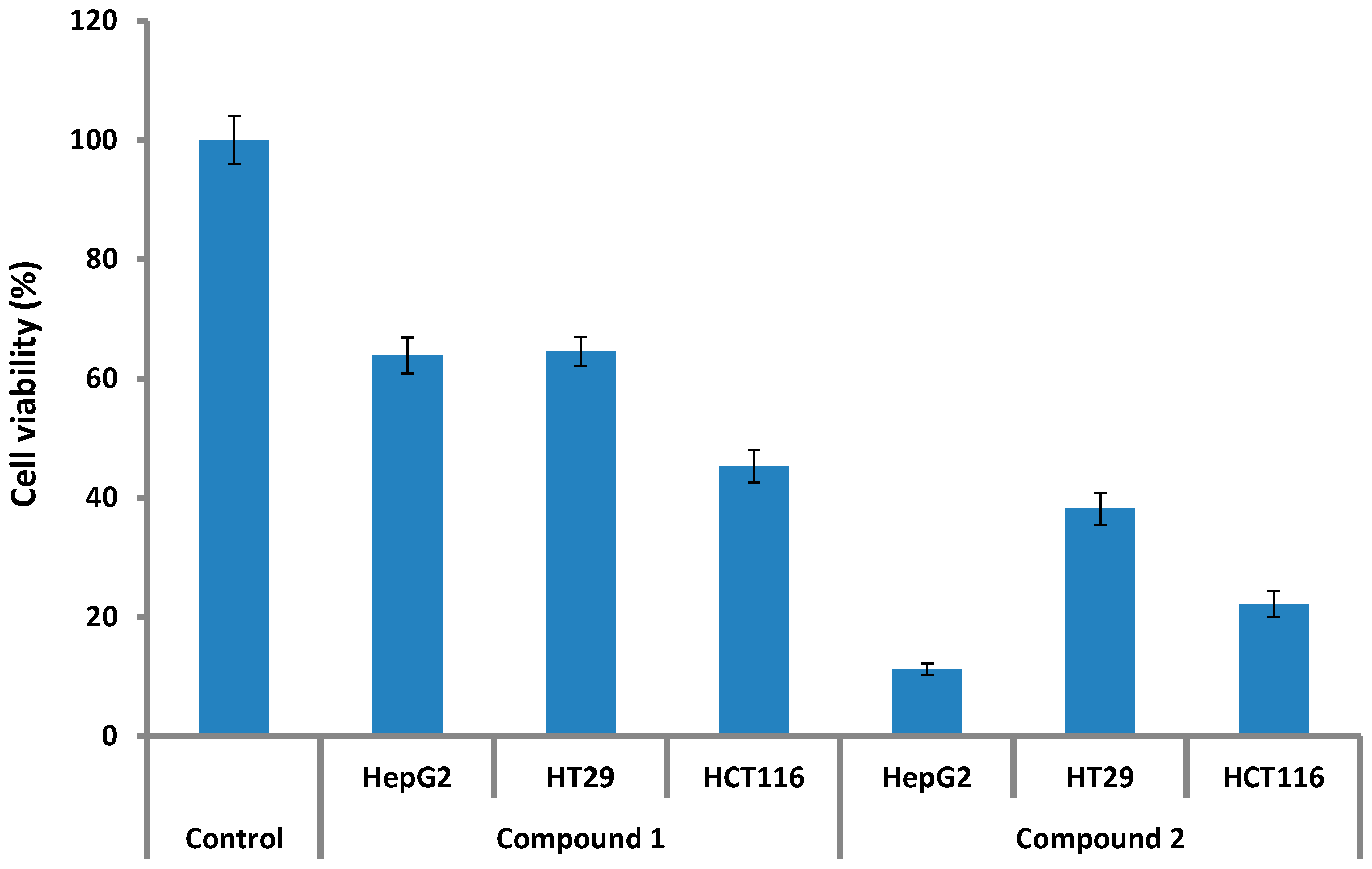
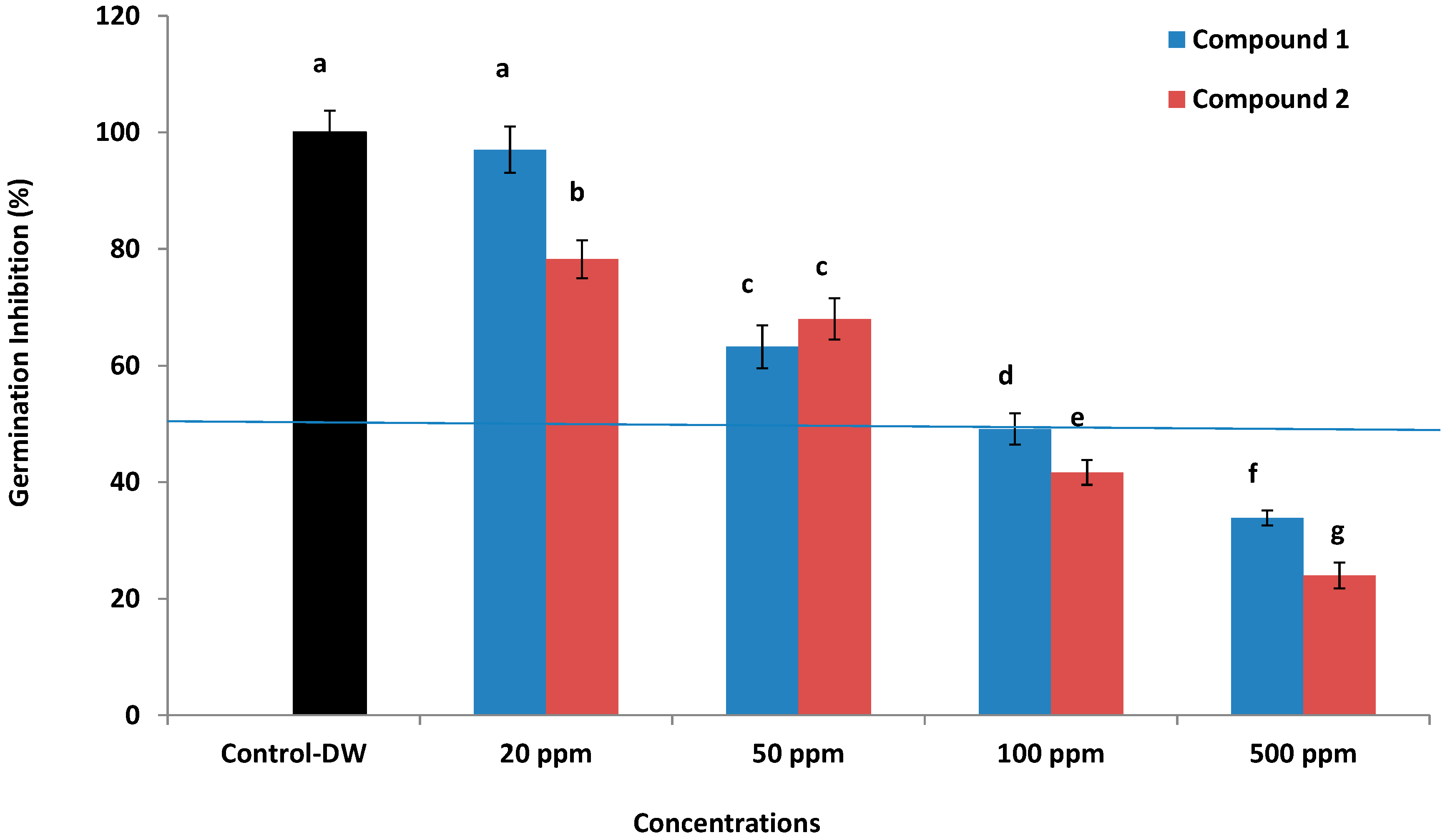
Anticancer, Allelopathic, Antifungal and Antioxidant Activities

| Compounds | Anti-Lipid Peroxidation (%) | Superoxide Anion (%) | DPPH (%) |
|---|---|---|---|
| Control | 92.34 ± 0.023a | 64.19 ± 0.73a | 98.88 ± 0.92a |
| Compound 1 | 75.22 ± 0.023c | 43.76 ± 0.0042b | 97.96 ± 1.04a |
| Compound 2 | 81.58 ± 0.12b | 37.12 ± 0.18c | 76.83 ± 0.78b |
3. Experimental Section
3.1. Plant Material
3.2. Extraction and Isolation
3.3. Anticancer Activities
3.4. Allelopathic and Antifungal Activities
3.5. Antioxidant Activities
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bako, S.P.; Bakfur, M.J.; John, I.; Bala, E.I. Ethno-medicinal and phytochemical profile of some savanna plant species in Nigeria. Int. J. Bot. 2005, 1, 147–150. [Google Scholar] [CrossRef]
- Williams, R.J.; Spencer, J.P.E.; Rice-Evans, C. Flavonoids: Antioxidants or signalling molecules? Free Radic. Biol. Med. 2004, 36, 838–849. [Google Scholar]
- Soobrattee, M.A.; Neergheen, V.S.; Luximon-Ramma, A.; Aruoma, O.; Bahorun, T. Phenolics as potential antioxidant theraputic agents: Mechanism and actions. Mutat. Res. 2005, 579, 200–213. [Google Scholar] [CrossRef] [PubMed]
- Kisksi, T.; Guenaoui, C.; Fawzi, N. Early growth stages of the rare Acridocarpus orientalis in the UAE-A First step towards conservation. Nat. Resour. 2012, 3, 1–5. [Google Scholar]
- Ksiksi, T.; Hamza, A.A. Antioxidant, lipoxygenase and histone Deacetylase inhibitory activities of Acridocarpus orientalis from Al Ain and Oman. Molecules 2012, 17, 12521–12532. [Google Scholar] [CrossRef] [PubMed]
- Monthana, R.A.; Lindequist, U.; Gruenert, R.; Bednarski, P.J. Studies of the in vitro anticancer, antimicrobial and antioxidant potentials of selected Yemeni medicinal plants from the island Soqotra. BMC Complement. Altern. Med. 2009, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Hammiche, V.; Maiza, K. Traditional medicine in central sahara: Pharmacopoeia of Tassili N’ajjer. J. Ethnopharmacol. 2006, 105, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Malebo, H.M.; Tanja, W.; Cal, M.; Swaleh, S.A.M.; Omolo, M.O.; Hassanali, A.; Séquin, U.; Hamburger, M.; Brun, R.; Ndiege, I.O. Antiplasmodial, anti-trypanosomal, anti-leishmanial and cytotoxicity activity of selected Tanzanian medicinal plants. Tanzan. J. Health Res. 2009, 11, 226–234. [Google Scholar] [PubMed]
- Ghazanfar, S.A. Herbal medicines and practices in northern Oman. In Proceedings of the III International Congress on Traditional Asian Medicine, Bombay, India, 4–7 January 1990; p. 509.
- Toker, G.; Memisoglu, M.; Yesilida, E.; Aslan, M. Flavonoids of Tilia argentea Desf. ex DC. Leaves. Turk. J. Chem. 2004, 28, 745–750. [Google Scholar]
- Branco, A.; Pinto, A.C.; Ifa, D.R.; Branz-Filho, R. Two 8C-methylated flavonols from the leaves of Vellozia candida Mikan (Velloziaceae). J. Braz. Chem. Soc. 2002, 13, 318–323. [Google Scholar] [CrossRef]
- Markham, K.R.; Chari, V.M.; Mabry, T.J. The Flavonoids: Advances in Research; Harbone, J.B., Mabry, T.J., Eds.; Chapman and Hall: London, UK, 1982. [Google Scholar]
- Mabry, T.J.; Markham, K.R.; Thomas, M.B. The Systemic Identification of Flavonoids; Springer Verlag: New York, NY, USA; Heidelberg/Berlin, Germany, 1970. [Google Scholar]
- Tachakittirungrod, S.; Ikegami, F.; Okonogi, S. Antioxidant active principles isolated from Psidium guajava grown in Thailand. Sci. Pharm. 2007, 75, 179–193. [Google Scholar] [CrossRef]
- Agarwall, P.K. 13C-NMR of Flavonoids; Elsevier: Amsterdam, The Netherlands, 1989. [Google Scholar]
- Alford, É.R.; Vivanco, J.M.; Paschke, M.W. The Effects of flavonoid allelochemicals from Knapweeds on legume-rhizobia candidates for restoration. Restor. Ecol. 2009, 17, 506–514. [Google Scholar] [CrossRef]
- Weston, L.A.; Mathesius, U. Flavonoids: Their Structure, biosynthesis and role in the Rhizosphere, including allelopathy. J. Chem. Ecol. 2013, 39, 283–297. [Google Scholar]
- Alam, S. Synthesis, antibacterial and antifungal activity of some derivatives of 2-phenyl-chromen-4-one. J. Chem. Sci. 2004, 116, 325–331. [Google Scholar] [CrossRef]
- Edziri, H.; Mastouri, M.; Mahjoub, M.A.; Mighri, Z.; Mahjoub, A.; Verschaeve, L. Antibacterial, antifungal and cytotoxic activities of two flavonoids from Retama raetam flowers. Molecules 2012, 17, 7284–7293. [Google Scholar] [CrossRef] [PubMed]
- Cotelle, N. Role of Flavonoids in Oxidative Stress. Curr. Top. Med. Chem. 2001, 1, 569–590. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Yang, X.; Wang, K.; Yang, X. The efflux of flavonoids Morin, isorhamnetin-3-O-rutinoside and diosmetin-7-O-β-d-xylopyranosyl-(1–6)-β-d-glucopyranoside in the human intestinal cell line Caco-2. Pharm. Res. 2006, 23, 1721–1728. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Lee, W.S.; Eun, S.Y.; Jung, J.H.; Park, H.S.; Kim, G.; Choi, Y.H.; Ryu, C.H.; Jung, J.M.; Hong, S.C.; et al. Morin, a flavonoid from Moraceae, suppresses growth and invasion of the highly metastatic breast cancer cell line MDA-MB‑231 partly through suppression of the Akt pathway. Int. J. Oncol. 2014, 45, 1629–1637. [Google Scholar] [PubMed]
- Munesada, K.; Siddiqui, H.L.; Suga, T. Biologically active labdane-type diterpene glycosides from the root-stalks of Gleichenia japonica. Phytochemistry 1992, 31, 1533–1536. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growthand survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.C.; Park, S.J.; Lee, J.R.; Seo, J.C.; Yang, C.H.; Byun, S.H. Cytoprotective activity of Glycyrrhizae radix extract against arsenite-induced cytotoxicity. Evid. Based Complement. Altern. Med. 2008, 5, 165–171. [Google Scholar] [CrossRef]
- Khan, A.L.; Hussain, J.; Hamayun, M.; Kang, S.M.; Watanabe, K.N.; Lee, I.J. Allelochemical, Eudesmane-Type Sesquiterpenoids from Inula falconeri. Molecules 2010, 15, 1554–1561. [Google Scholar] [CrossRef] [PubMed]
- Hiradate, S.; Morita, S.; Sugie, H.; Fujii, Y.; Harada, J. Phytotoxic cis-cinnamoyl glucosides from Spiraea thunbergii. Phytochemistry 2004, 65, 731–739. [Google Scholar] [CrossRef]
- NCCLS. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi. Approved Standard M38-A; National Committee for Clinical Laboratory Standards: Wayne, PA, USA, 2002. [Google Scholar]
- Gulati, V.; Harding, I.H.; Palombo, E.A. Enzyme inhibitory and antioxidant activities of traditional medicinal plants: Potential application in the management of hyperglycemia. BMC Complement. Altern. Med. 2012, 12, 77. [Google Scholar] [CrossRef] [PubMed]
- González-Montelongo, R.; Lobo, M.G.; González, M. Antioxidant activity in banana peel extracts: Testing extraction conditions and related bioactive compounds. Food Chem. 2010, 119, 1030–1039. [Google Scholar] [CrossRef]
- Hazra, B.; Biswas, S.; Mandal, N. Antioxidant and free radical scavenging activity of Spondias pinnata. BMC Complement. Altern. Med. 2008, 8, 63. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds 1 and 2 are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, J.; Ali, L.; Khan, A.L.; Rehman, N.U.; Jabeen, F.; Kim, J.-S.; Al-Harrasi, A. Isolation and Bioactivities of the Flavonoids Morin and Morin-3-O-β-D-glucopyranoside from Acridocarpus orientalis—A Wild Arabian Medicinal Plant. Molecules 2014, 19, 17763-17772. https://doi.org/10.3390/molecules191117763
Hussain J, Ali L, Khan AL, Rehman NU, Jabeen F, Kim J-S, Al-Harrasi A. Isolation and Bioactivities of the Flavonoids Morin and Morin-3-O-β-D-glucopyranoside from Acridocarpus orientalis—A Wild Arabian Medicinal Plant. Molecules. 2014; 19(11):17763-17772. https://doi.org/10.3390/molecules191117763
Chicago/Turabian StyleHussain, Javid, Liaqat Ali, Abdul Latif Khan, Najeeb Ur Rehman, Farah Jabeen, Jong-Sang Kim, and Ahmed Al-Harrasi. 2014. "Isolation and Bioactivities of the Flavonoids Morin and Morin-3-O-β-D-glucopyranoside from Acridocarpus orientalis—A Wild Arabian Medicinal Plant" Molecules 19, no. 11: 17763-17772. https://doi.org/10.3390/molecules191117763





