Synthesis and Anti-Tumor Activity of Novel Aminomethylated Derivatives of Isoliquiritigenin
Abstract
:1. Introduction
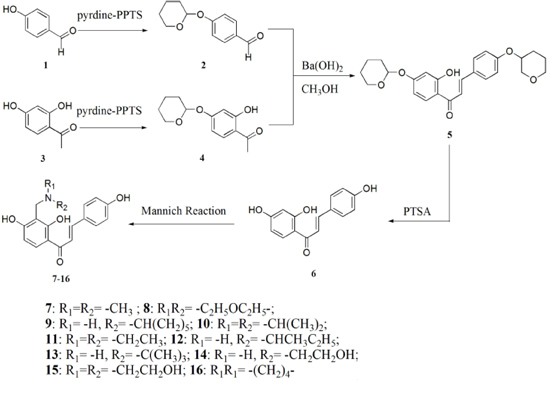
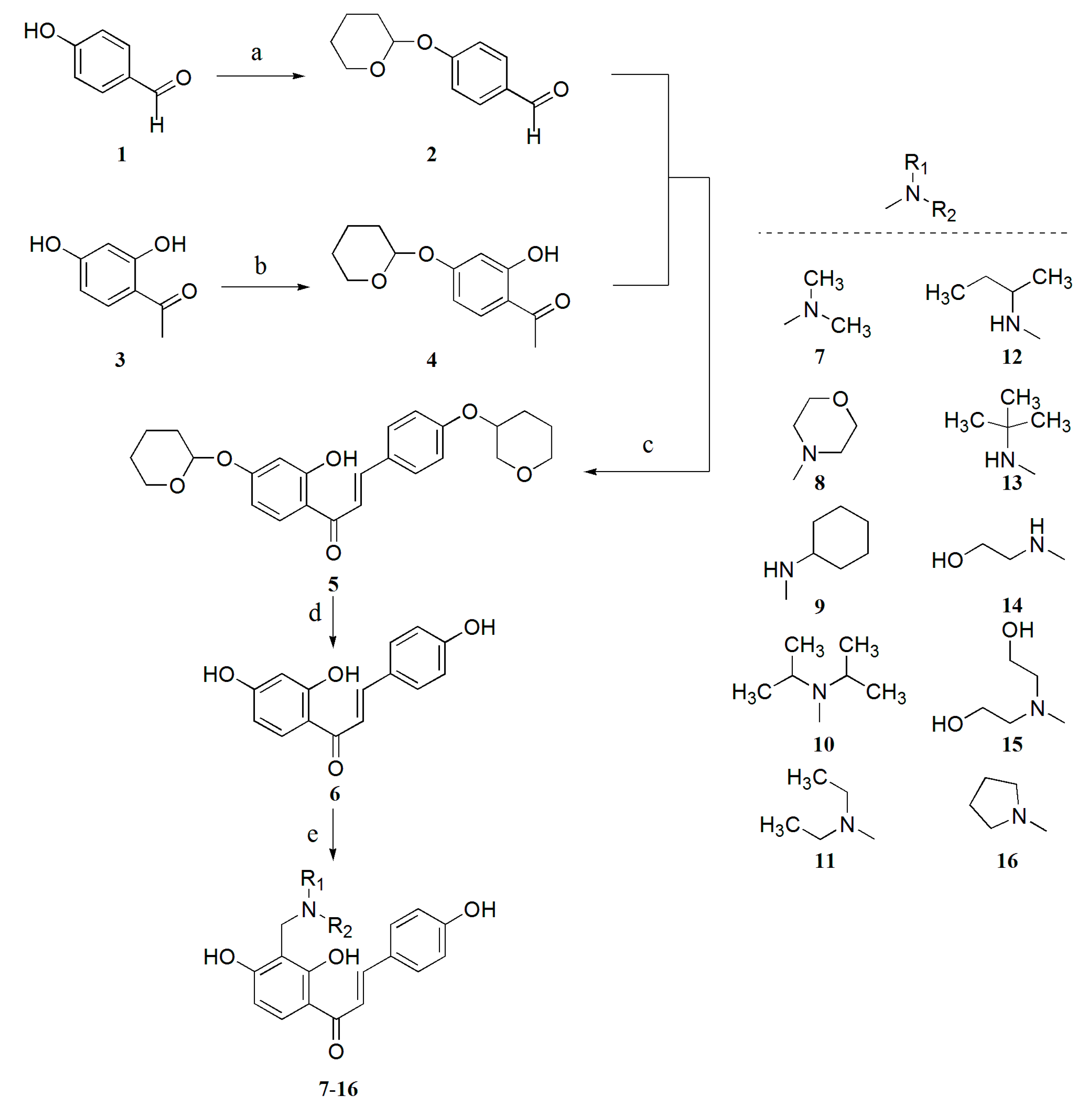
2. Results and Discussion
2.1. Chemistry
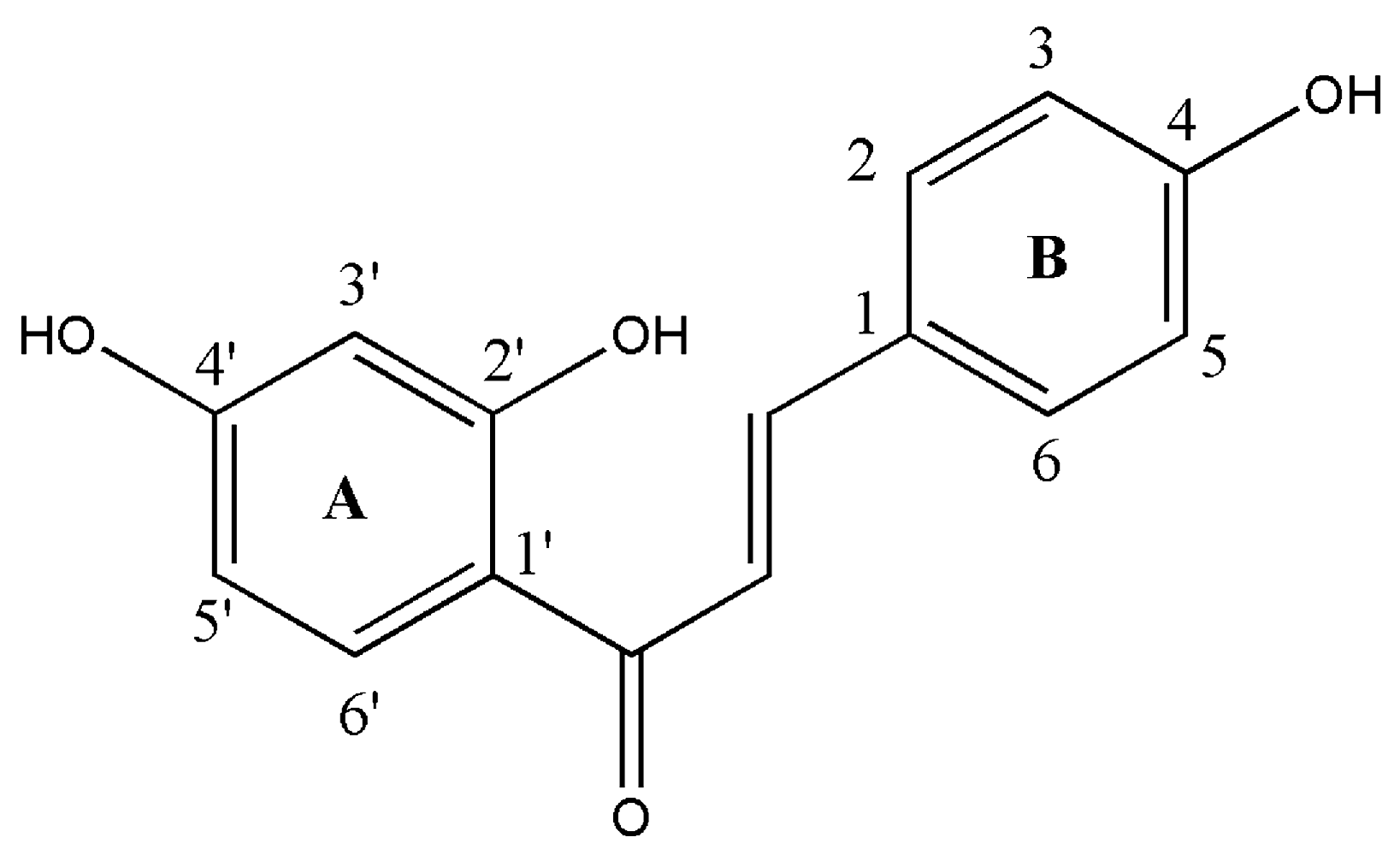
2.2. Evaluation of Biological Activity
2.2.1. Cytotoxicity Assay
| Compound | R1 | R2 | Cytotoxicity (IC50, μM) | mp, °C | |||
|---|---|---|---|---|---|---|---|
| PC-3 a | MCF-7 | HO-4980 | THP-1 | ||||
| 7 | Me | Me | >100 | >100 | >100 | >100 | >176 |
| 8 | CH2CH2OCH2CH2 | 48.64 | >100 | 96.43 | >100 | >180 | |
| 9 | H | C6H11 | 28.32 | 73.58 | >100 | >100 | >168 |
| 10 | i-Pr | i-Pr | >100 | 84.56 | 98.14 | >100 | >180 |
| 11 | Et | Et | 66.02 | >100 | 97.32 | >100 | >176 |
| 12 | H | CHCH3C2H5 | 57.05 | 84.78 | >100 | >100 | >175 |
| 13 | H | t-Bu | >100 | >100 | >100 | >100 | >170 |
| 14 | H | C2H4OH | 87.72 | 66.01 | 89.01 | >100 | >180 |
| 15 | C2H4OH | C2H4OH | 35.14 | 42.94 | 37.85 | >100 | 106.1–8 |
| 16 | CH2CH2CH2CH2 | >100 | 81.84 | 74.87 | >100 | 116.5–7.4 | |
| ISL | - | - | 36.90 | 44.11 | 58.43 | >100 | |
| Group | Treatment | Dose | Mice Number | TW (g) x ± SD | BWC a (%) | TIR b (%) | |
|---|---|---|---|---|---|---|---|
| (mg/kg) | Begin | End | |||||
| I | normal saline | - | 10 | 10 | 1.23 ± 0.534 ** | +4.4 | - |
| II | ISL | 40 | 10 | 10 | 0.85 ± 0.369 ** | −3.0 | 32.44 |
| III | ISL derivative 15 | 40 | 10 | 10 | 0.74 ± 0.618 * | +12.8 | 39.72 |
| IV | ISL derivative 15 | 80 | 10 | 10 | 0.35 ± 0.442 ** | +7.8 | 71.68 |

2.2.2. In Vivo Toxicity and Antitumor Studies
3. Experimental Section
3.1. Chemistry
3.2. Biological Assays
3.2.1. Cytotoxicity Assay
3.2.2. Animal Treatment
4. Conclusions
Supplementary Materials
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pan, D.; Li, W.; Miao, H.C.; Yao, J.; Li, Z.Y.; Wei, L.B.; Zhao, L.; Guo, Q.L. LW-214, a newly synthesized flavonoid, induces intrinsic apoptosis pathway by down-regulating Trx-1 in MCF-7 human breast cells. Biochem. Pharmacol. 2014, 87, 598–610. [Google Scholar] [PubMed]
- Shin, I.S.; Seo, C.S.; Ha, H.K.; Lee, M.Y.; Huang, D.S.; Huh, J.I.; Shin, H.K. Genotoxicity assessment of Pyungwi-san (PWS), a traditional herbal prescription. J. Ethnopharmacol. 2011, 133, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Balunas, M.J.; Kinghorn, A.D. Drug discovery from medicinal plants. Life Sci. 2005, 78, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N.; van der Hooft, J.J.; Crozier, A. Human studies on the absorption, distribution, metabolism, and excretion of tea polyphenols. Am. J. Clin. Nutr. 2013, 98, 1619–1630. [Google Scholar] [CrossRef]
- Ko, W.C.; Shih, C.M.; Lai, Y.H.; Chen, J.H.; Huang, H.L. Inhibitory effects of flavonoids on phosphodiesterase isozymes from guinea pig and their structure–activity relationships. Biochem. Pharmacol. 2004, 68, 2087–2094. [Google Scholar] [CrossRef] [PubMed]
- Zhan, C.; Yang, J. Protective effects of isoliquiritigenin in transient middle cerebral artery occlusion-induced focal cerebral ischemia in rats. Pharmacol Res. 2006, 53, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Deodhar, M.; Black, D.S.; Kumar, N. Acid catalyzed stereoselective rearrangement and dimerization of flavenes: Synthesis of dependensin. Tetrahedron 2007, 63, 5227–5235. [Google Scholar] [CrossRef]
- Sano, S.; Okubo, Y.; Handa, A.; Nakao, M.; Kitaike, S.; Nagao, Y.; Kakegawa, H. Reinvestigation of the synthesis of isoliquiritigenin: Application of Horner–Wadsworth–Emmons Reaction and Claisen–Schmidt Condensation. Chem. Pharm. Bull. 2011, 59, 885–888. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.P.; Zhang, Z.Y.; Li, Y.P.; Li, D.; Huang, S.L.; Gu, L.Q.; Xu, J.; Huang, Z.S. Syntheses and evaluation of novel isoliquiritigen derivatives as potenial dual inhibitors for amyloid-beta aggregation and 5-lipoxygenase. Eur. J. Med. Chem. 2013, 66, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Gho, W.M.; Chan, F.L.; Chen, S.; Leung, L.K. Dietary administration of the licorice flavonoid isoliquiritigenin deters the growth of MCF-7 cells overexpressing aromatase. Int. J. Cancer 2009, 124, 1028–1036. [Google Scholar] [CrossRef] [PubMed]
- Honda, H.; Nagai, Y.; Matsunaga, T.; Saitoh, S.; Akashi-Takamura, S.; Hayashi, H.; Fujii, I.; Miyake, K.; Muraguchi, A.; Takatsu, K. Glycyrrhizin and isoliquiritigenin suppress the LPS sensor Toll-like receptor 4/MD-2 complex signaling in a different manner. J. Leukoc. Biol. 2012, 91, 967–976. [Google Scholar] [CrossRef]
- Lee, Y.M.; Lim, D.Y.; Choi, H.J.; Jung, J.I.; Chung, W.Y.; Park, J.H. Induction of cell cycle arrest in prostate cancer cells by the dietary compound isoliquiritigenin. J. Med. Food 2009, 12, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, M.; Satomi, Y.; Mizutani, Y.; Ukimura, O.; Kawauchi, A.; Sakai, T.; Baba, M.; Okuyama, T.; Nishino, H.; Miki, T. Isoliquiritigenin inhibits the growth of prostate cancer. Eur. Urol. 2003, 43, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, S.; Morita, T.; Endo, H.; Hamamoto, T.; Baba, M.; Joichi, Y.; Kaneko, S.; Okada, Y.; Okuyama, T.; Nishino, H.; Tokue, A. Isoliquiritigenin suppresses pulmonary metastasis of mouse renal cell carcinoma. Cancer Lett. 2002, 183, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.L.; Kuo, P.L.; Lin, C.C. Isoliquiritigenin induces apoptosis and cell cycle arrest through p53-dependent pathway in Hep G2 cells. Life Sci. 2005, 77, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Baba, M.; Nishino, H.; Okuyama, T. Cyclooxygenase-2 plays a suppressive role for induction of apoptosis in isoliquiritigenin-treated mouse colon cancer cells. Cancer Lett. 2006, 231, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, W.; Wang, Y.; Asada, Y.; Koike, K. Prenylflavonoids from Glycyrrhiza uralensis and their protein tyrosine phosphatase-1B inhibitory activities. Bioorg. Med. Chem. Lett. 2010, 20, 5398–5401. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Jeong, G.S.; Lim, H.D.; An, R.B.; Kim, Y.C.; Kim, E.C. Isoliquiritigenin 2′-methyl ether induces growth inhibition and apoptosis in oral cancer cells via heme oxygenase-1. Toxicol. In Vitro 2010, 24, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.F.; Gang, Y.Z.; Luo, H.; Ma, Y.T.; Kong, Y.; Lee, S.M.; Luo, M. New Isoliquiritigenin Derivatives with Anti-Tumor Activity and the Synthetic Method. CN 101648883 A, 17 February 2010. [Google Scholar]
- Nguyen, T.B.; Wang, Q.; Guéritte, F. An Efficient One-Step Synthesis of Piperidin-2-yl and Pyrrolidin-2-yl Flavonoid Alkaloids through Phenolic Mannich Reactions. Eur. J. Org. Chem. 2011, 35, 7076–7079. [Google Scholar] [CrossRef]
- Zhang, S.X.; Ma, J.G.; Bao, Y.M.; Yang, P.W.; Zou, L.; Li, K.J.; Sun, X.D. Nitrogen-containing flavonoid analogues as CDK1/cyclin B inhibitors: Synthesis, SAR analysis, and biological activity. Bioorg. Med. Chem. 2008, 16, 7127–7132. [Google Scholar] [CrossRef]
- Karthikeyan, M.S.; Prasad, D.J.; Poojary, B.; Bhat, K.S.; Holla, B.S.; Kumari, N.S. Synthesis and biological activity of Schiff and Mannich bases bearing 2,4-dichloro-5-fluorophenyl moiety. Bioorg. Med. Chem. 2006, 14, 7482–7489. [Google Scholar] [CrossRef] [PubMed]
- Pandeya, S.N.; Sriram, D.; Nath, G.; DeClercq, E. Synthesis, antibacterial, antifungal and anti-HIV activities of Schiff and Mannich bases derived from isatin derivatives and N-[4-(49-chlorophenyl)thiazol-2-yl] thiosemicarbazide. Eur. J. Pharm. Sci. 1999, 9, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Pandeya, S.N.; Sriram, D.; Nath, G.; Clercq, E.D. Synthesis and antimicrobial activity of Schiff and Mannich bases of isatin and its derivatives with pyrimidine. Farmaco 1999, 54, 624–628. [Google Scholar] [CrossRef] [PubMed]
- Pandeya, S.N.; Sriram, D.; Nath, G.; Clercq, E.D. Synthesis, antibacterial, antifungal and anti-HIV evaluation of Schiff and Mannich bases of isatin derivatives with 3-amino-2-methylmercapto quinazolin-4(3H)-one. Pharm. Acta Helv. 1999, 74, 11–17. [Google Scholar] [CrossRef] [PubMed]
- List, B. The direct catalytic asymmetric three-component Mannich Reaction. J. Am. Chem. Soc. 2000, 122, 9336–9337. [Google Scholar] [CrossRef]
- Artico, M.; DiSanto, R.; Costi, R.; Novellino, E.; Greco, G.; Massa, S.; Tramontano, E.; Marongiu, M.E.; DeMontis, A.; LaColla, P. Geometrically and conformationally restrained cinnamoyl compounds as inhibitors of HIV-1 integrase: Synthesis, biological evaluation, and molecular modeling. J. Med. Chem. 1998, 41, 3948–3960. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.W.; Zhao, D.H.; Quan, Y.C.; Sun, L.P.; Yin, X.M.; Guan, L.P. Synthesis and evaluation of antiinflammatory activity of substituted chalcone derivatives. Med. Chem. Res. 2010, 19, 403–412. [Google Scholar] [CrossRef]
- Bukhari, S.N.; Jasamai, M.; Jantan, I. Synthesis and biological evaluation of chalcone derivatives (mini review). Mini Rev. Med. Chem. 2012, 12, 1394–1403. [Google Scholar] [PubMed]
- Tramontini, M.; Angiolini, L. Further advances in the chemistry of Mannich bases. Tetrahedron 1990, 46, 1791–1837. [Google Scholar] [CrossRef]
- Scudiero, D.A.; Shoemaker, R.H.; Paul, K.D.; Monks, A.; Tierney, S.; Nofziger, T.H.; Currens, M.J.; Seniff, D.; Boyd, M.R. Evaluation of a soluble Tetrazolium/Formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 1988, 48, 4827–4833. [Google Scholar] [PubMed]
- Wu, D.D.; Gao, Y.F.; Chen, L.X.; Qi, Y.M.; Kang, Q.Z.; Wang, H.L.; Zhu, L.Y.; Ye, Y.; Zhai, M.X. Anti-tumor effects of a novel chimeric peptide on S180 and H22 xenografts bearing nude mice. Peptides 2010, 31, 850–864. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, H.; Zhang, Y.; Wang, X.; Han, Y.; Peng, X.; Efferth, T.; Fu, Y. Synthesis and Anti-Tumor Activity of Novel Aminomethylated Derivatives of Isoliquiritigenin. Molecules 2014, 19, 17715-17726. https://doi.org/10.3390/molecules191117715
Fu H, Zhang Y, Wang X, Han Y, Peng X, Efferth T, Fu Y. Synthesis and Anti-Tumor Activity of Novel Aminomethylated Derivatives of Isoliquiritigenin. Molecules. 2014; 19(11):17715-17726. https://doi.org/10.3390/molecules191117715
Chicago/Turabian StyleFu, Haoran, Yuhang Zhang, Xiqing Wang, Yingzhi Han, Xiao Peng, Thomas Efferth, and Yujie Fu. 2014. "Synthesis and Anti-Tumor Activity of Novel Aminomethylated Derivatives of Isoliquiritigenin" Molecules 19, no. 11: 17715-17726. https://doi.org/10.3390/molecules191117715