Melting points both the ligands and copper(II) complexes were determined on a Boetius apparatus and are uncorrected. FT-IR spectra were measured by Nicolet-380 spectrophotometer and 1H-NMR and 13C-NMR spectra were recorded on a Varian Gemini instrument operating at 200 MHz and 50 MHz, respectively, in CDCl3 or DMSO-d6 as a solvent. Chemical shifts are shown in parts per million (ppm) on the δ scale. Coupling constants are shown in hertz (Hz).
Crystallographic data for compounds have been deposited with the Cambridge Crystallographic Data Centre, with the deposition Nos CCDC 986094, 986095, 986193–986202. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.
Elemental analyses of C, H and N were within ±0.4% of the theoretical values.
All cell culture reagents were purchased from Sigma (Deisenhofen, FRG). Cancer cell lines: human large cell lung carcinoma LCLC-103H, human urinary bladder carcinoma 5637, human lung carcinoma A-427, human uterine cervical adenocarcinoma SISO, esophageal squamous cell carcinoma KYSE-520, human bladder cell carcinoma RT-4 and human pancreas cell adenocarcinoma DAN-G were obtained from the German Collection of Microorganisms and Cell Cultures (DSMZ, Brauschweig, FRG). The culture medium for cell lines was RPMI-1640 medium containing 2 g/L HCO3, and 10% FCS. Cells were grown in 75 cm2 plastic culture flasks (Sarstedt, Nümbrecht, FRG) in a humid atmosphere of 5% CO2 at 37 °C and were passaged shortly before becoming confluent.
3.1. Synthesis of 1-Acyl-3-(2-pyridyl)imidazolidin-2-ones 3a–j (General Procedure)
Imidazolidin-2-one (0.001 mol) was refluxed in 5 mL of acetic anhydride or butyric anhydride for 6 h. The reaction mixture was concentrated under reduced pressure and basified with 20% solution of K2CO3. Precipitated was collected by suction, washed with water and dried. In case when oily residue was formed after addition of K2CO3 the product was extracted with chloroform (3 × 15 mL), dried with anhydrous MgSO4, filtrated and concentrated under reduced pressure. Product was purified by use of chromatotron, flash column chromatography or crystallization. According to described general procedure were obtained following compounds:
1-Acetyl-3-(5-methyl-2-pyridyl)imidazolidin-2-one (3a). Compound 3a was purified by use of chromatotron (eluent: dichloromethane/ethyl acetate, 7:3, v/v); yield 60%; mp. 179–181 °C; IR (KBr) ν [cm−1]: 2999, 2953, 2922, 2853, 1731, 1680, 1484, 1403, 1375, 1291, 1246, 1023; 1H-NMR (500 MHz, CDCl3): δ 2.30 (s, 3H, CH3), 2.57 (s, 3H, CH3), 3.93 (t, 2H, CH2), 4.10 (t, 2H, CH2), 7.54 (d, J = 8.3 Hz, 1H, Ar-H), 8.13 (d, J = 8.3 Hz, 1H, Ar-H), 8.16 (s, 1H, Ar-H); Anal. Calcd. for C11H13N3O2: C, 60.26; H, 5.98; N, 19.17; Found: C, 60.11; H, 5.78; N, 19.08.
1-Acetyl-3-(4-methyl-2-pyridyl)imidazolidin-2-one (3b). Compound 3b was purified by use of chromatotron (eluent: dichloromethane/acetone, 95:5, v/v); yield 70%; mp. 152–154 °C; IR (KBr) ν [cm−1]: 3017, 2920, 1732, 1679, 1605, 1485, 1426, 1373, 1296, 1249, 1195; 1H-NMR (200 MHz, (CD3)2SO): δ 2.33 (s, 3H, CH3), 2.43 (s, 3H, OCH3), 3.76 (t, 2H, CH2), 3.96 (t, 2H, CH2), 6.97 (d, J = 4.5 Hz, 1H, Ar-H), 8.02 (s, 1H, Ar-H), 8.22 (d, J = 4.5 Hz, 1H, Ar-H); Anal. Calcd. for C11H13N3O2: C, 60.26; H, 5.98; N, 19.17; Found: C, 60.08; H, 5.81; N, 18.96.
1-Butyryl-3-(4-methyl-2-pyridyl)imidazolidin-2-one (3c). Compound 3c was purified by use of chromatotron (eluent: chloroform); yield 54%; mp. 82–83 °C; IR (KBr) ν [cm−1]: 3058, 2967, 2915, 2878, 1734, 1677, 1603, 1560, 1374, 1323, 1246, 1190; 1H-NMR (500 MHz, CDCl3): δ 1.00 (t, 3H, CH3), 1.73 (sextet, 2H, CH2), 2.38 (s, 3H, CH3), 2.97 (t, 2H, CH2), 3.93 (t, 2H, CH2), 4.10 (t, 2H, CH2), 6.87 (d, J = 5.4 Hz, 1H, Ar-H), 8.08 (s, 1H, Ar-H), 8.19 (d, J = 5.4 Hz, 1H, Ar-H); Anal. Calcd. for C13H17N3O2: C, 63.14; H, 6.93; N, 16.99; Found: C, 62.99; H, 6.81; N, 16.89.
1-Acetyl-3-(4-tert-butyl-2-pyridyl)imidazolidin-2-one (3d). Compound 3d was purified by use of chromatotron (eluent: dichloromethane/ethyl acetate, 4:1, v/v); yield 59%; mp. 163–166 °C; IR (KBr) ν [cm−1]: 2970, 2919, 2870, 1726, 1685, 1598, 1547, 1484, 1420, 1374, 1293, 1252, 1120; 1H-NMR (200 MHz, CDCl3): δ 1.35 (s, 9H, C(CH3)3), 2.60 (s, 3H, CH3), 3.94–3.98 (m, 2H, CH2), 4.10–4.14 (m, 2H, CH2), 7.08 (dd, J1 = 1.3 Hz, J2 = 5.4 Hz, 1H, Ar-H), 8.26 (d, J = 5.4 Hz, 1H, Ar-H), 8.30 (s, 1H, Ar-H); Anal. Calcd. for C14H19N3O2: C, 64.35; H, 7.33; N, 16.08; Found: C, 64.17; H, 7.19; N, 15.86.
1-Acetyl-3-(4-phenyl-2-pyridyl)imidazolidin-2-one (3e). Compound 3e was purified by use of flash column chromatography (eluent: chloroform/ethyl acetate:methanol, 5:2:1, v/v/v); yield 76%; mp. 156–157 °C; IR (KBr) ν [cm−1]: 3067, 3021, 2960, 2918, 1747, 1685, 1594, 1545, 1474, 1420, 1377, 1368, 1308, 1251; 1H-NMR (200 MHz, (CD3)2SO): δ 2.45 (s, 3H, CH3), 3.77–3.85 (m, 2H, CH2), 3.99–4.07 (m, 2H, CH2), 7.45–7.58 (m, 4H, Ar-H), 7.72–7.77 (m, 2H, Ar-H), 8.43–8.48 (m, 2H, Ar-H), 13C-NMR (50 MHz, (CD3)2SO): δ 23.98, 38.55, 41.05, 110.29, 117.28, 127.07 (two overlapping signals), 129.58 (two overlapping signals), 129.66, 137.72, 148.68, 149.18, 152.32, 153.02, 170.09; Anal. Calcd. for C16H15N3O2: C, 68.31; H, 5.37; N, 14.94; Found: C, 68.22; H, 5.23; N, 14.78.
1-Butyryl-3-(4-phenyl-2-pyridyl)imidazolidin-2-one (3f). Compound 3f was purified by use of chromatotron (eluent: chloroform); yield 70%; mp. 155–156 °C; IR (KBr) ν [cm−1]: 3110, 3069, 2962, 2918, 2872, 1735, 1682, 1593, 1474, 1374, 1248, 1224; 1H-NMR (500 MHz, CDCl3): δ 1.02 (t, 3H, CH3), 1.75 (sextet, 2H, CH2), 3.00 (t, 2H, CH2), 3.97 (t, 2H, CH2), 4.16 (t, 2H, CH2), 7.28 (d, J = 5.4 Hz, 1H, Ar-H), 7.43–7.50 (m, 3H, Ar-H), 7.70 (d, J = 7.8 Hz, 2H, Ar-H), 8.39 (d, J = 5.4 Hz, 1H, Ar-H), 8.53 (s, 1H, Ar-H); Anal. Calcd. for C18H19N3O2: C, 69.88; H, 6.19; N, 13.58; Found: C, 69.79; H, 6.02; N, 13.50.
1-Acetyl-3-[4-(3-phenylpropyl)-2-pyridyl]imidazolidin-2-one (3g). Compound 3g was purified by use of chromatotron (eluent: dichloromethane/ethyl acetate, 4:1, v/v); yield 64%; mp. 89–90 °C; IR (KBr) ν [cm−1]: 3060, 3024, 2943, 2925, 2858, 1722, 1683, 1601, 1560, 1483, 1438, 1402, 1379, 1305, 1278, 1254; 1H-NMR (500 MHz, (CD3)2SO): δ 1.90 (q, 2H, CH2), 2.45 (s, 3H, CH3), 2.60–2.64 (m, 4H, 2×CH2), 3.79 (t, 2H, CH2), 3.98 (t, 2H, CH2), 7.02 (d, J = 4.9 Hz, 1H, Ar-H), 7.16–7.29 (m, 5H, Ar-H), 8.07 (s, 1H, Ar-H), 8.27 (d, J = 4.9 Hz, 1H, Ar-H); Anal. Calcd. for C19H21N3O2: C, 70.57; H, 6.55; N, 12.99; Found: C, 70.43; H, 6.51; N, 13.20.
1-Acetyl-3-(4-methoxy-2-pyridyl)imidazolidin-2-one (3h). Compound 3h was purified by use of chromatotron (eluent: dichloromethane/ethyl acetate, 4:1, v/v); yield 87%; mp. 139–140 °C; IR (KBr) ν [cm−1]: 3023, 2983, 2920, 1731, 1687, 1594, 1566, 1455, 1406, 1382, 1309, 1257, 1223, 1179; 1H-NMR (500 MHz, (CD3)2SO): δ 2.44 (s, 3H, CH3), 3.78 (t, 2H, CH2), 3.84 (s, 3H, OCH3), 3.97 (t, 2H, CH2), 6.77 (dd, J1 = 1.9 Hz, J2 = 5.9 Hz, 1H, Ar-H), 7.77 (d, J = 1.9 Hz, 1H, Ar-H), 8.19 (d, J = 5.9 Hz, 1H, Ar-H); Anal. Calcd. for C11H13N3O3: C, 56.16; H, 5.57; N, 17.86; Found: C, 55.99; H, 5.51; N, 17.80.
1-Butyryl-3-(4-methoxy-2-pyridyl)imidazolidin-2-one (3i). Compound 3i was purified by use of chromatotron (eluent: chloroform); yield 66%; mp. 112–113 °C; IR (KBr) ν [cm−1]: 3112, 3016, 2964, 2921, 2875, 1736, 1691, 1595, 1564, 1482, 1398, 1375, 1255, 1216, 1178; 1H-NMR (200 MHz, CDCl3): δ 1.01 (t, 3H, CH3), 1.72 (sextet, 2H, CH2), 2.98 (t, 2H, CH2), 3.89 (s, 3H, OCH3), 3.92–4.00 (m, 2H, CH2), 4.07–4.16 (m, 2H, CH2), 6.61 (dd, J1 = 2.2 Hz, J2 = 5.9 Hz, 1H, Ar-H), 7.86 (d, J = 2.2 Hz, 1H, Ar-H), 8.14 (d, J = 5.9 Hz, 1H, Ar-H); Anal. Calcd. for C13H17N3O3: C, 59.30; H, 6.51; N, 15.96; Found: C, 59.21; H, 6.46; N, 16.00.
1-Acetyl-3-(4-benzyloxy-2-pyridyl)imidazolidin-2-one (3j). Compound 3j was purified by use of chromatotron (eluent: chloroform/ethyl acetate, 4:1, v/v); yield 70%; mp. 151–153 °C; IR (KBr) ν [cm−1]: 3101, 3069, 3027, 2916, 1743, 1737, 1673, 1596, 1563, 1481, 1452, 1386, 1318, 1255, 1215, 1011, 868; 1H-NMR (500 MHz, (CD3)2SO): δ 2.45 (s, 3H, OCH3), 3.78 (t, 2H, CH2), 3.98 (t, 2H, CH2), 5.21 (s, 2H, OCH2), 6.86 (dd, J1 = 1.9 Hz, J2 = 5.9 Hz, 1H, Ar-H), 7.36–7.49 (m, 5H, Ar-H), 7.87 (d, J = 1.9 Hz, 1H, Ar-H), 8.21 (d, J = 5.9 Hz, 1H, Ar-H); Anal. Calcd. for C17H17N3O3: C, 65.58; H, 5.50; N, 13.50; Found: C, 65.42; H, 5.28; N, 13.71.
3.2. Synthesis of N-(2-Pyridyl)imidazolidine-2-thiones 4a–c, e–g (General Procedure)
Appropriate N-(2-pyridyl)imidazolidin-2-one (0.001 mol) was refluxed with Lawesson’s reagent (0.00075 mol) in anhydrous toluene (8 mL) for 12 h and concentrated under reduced pressure. The residue was extracted with chloroform (2 × 20 mL), dried with anhydrous MgSO4, filtrated and concentrated under reduced pressure. Product was separated from oily residue by use of chromatotron. According above given procedure were obtained following compounds:
1-(2-Pyridyl)imidazolidine-2-thione (4a). Compound 4a was purified by use of chromatotron (eluent: chloroform/ethyl acetate/acetone, 8:1:1, v/v/v); yield 50%; mp. 99–102 °C; IR (KBr) ν [cm−1]: 3196, 3036, 2993, 1591, 1567, 1533, 1466, 1438, 1413, 1347, 1228; 1H-NMR (200 MHz, CDCl3): δ 3.68 (t, 2H, CH2); 4.44 (t, 2H, CH2); 6.96 (bs, 1H, NH); 7.01–7.07 (m, 1H, Ar-H); 7.64–7.73 (m, 1H, Ar-H); 8.33–8.36 (m, 1H, Ar-H); 8.93 (d, J = 8.0 Hz, 1H, Ar-H); 13C-NMR (50 MHz, CDCl3): δ 40.93, 49.43, 116.33, 119.62, 136.69, 147.32, 152.32, 181.27; Anal. Calcd. for C8H9N3S: C, 53.61; H, 5.06; N, 23.44; Found: C, 53.54; H, 4.92; N, 23.37.
1-(6-Methyl-2-pyridyl)imidazolidine-2-thione (4b). Compound 4b was purified by use of chromatotron (eluent: dichloromethane/ethyl acetate, 1:1, v/v); yield 31%; mp. 133–135 °C; IR (KBr) ν [cm−1]: 3222, 3101, 3029, 2974, 1588, 1519, 1456, 1428, 1397, 1346, 1254, 1231; 1H-NMR (200 MHz, (CD3)2SO): δ 2.41 (s, 3H, CH3), 3.53 (t, 2H, CH2), 4.26 (t, 2H, CH2), 6.97 (d, J = 7.3 Hz, 1H, Ar-H), 7.64 (t, 1H, Ar-H), 8.60 (d, J = 8.4 Hz, 1H, Ar-H), 9.00 (s, 1H, NH); 13C-NMR (50 MHz, (CD3)2SO): δ 24.25, 41.05, 49.09, 113.18, 118.54, 137.04, 152.14, 156.09, 180.19; Anal. Calcd. for C9H11N3S: C, 55.93; H, 5.74; N, 21.74; Found: C, 55.86; H, 5.64; N, 21.48.
1-(5-Methyl-2-pyridyl)imidazolidine-2-thione (4c). Compound 4c was purified by use of chromatotron (eluent: dichloromethane/ethyl acetate, 7:3, v/v); yield 51%; mp. 200–203 °C; IR (KBr) ν [cm−1]: 3271, 2969, 2904, 1608, 1570, 1514, 1479, 1388, 1341, 1239, 1217; 1H-NMR (200 MHz, (CD3)2SO): δ 2.26 (s, 3H, CH3); 3.53 (t, 2H, CH2); 4.24 (t, 2H, CH2); 7.59 (dd, J1 = 2.1 Hz, J2 = 8.5 Hz, 1H, Ar-H), 8.18 (s, 1H, Ar-H), 8.67 (d, J = 8.5 Hz, 1H, Ar-H), 8.96 (s, 1H, NH); 13C-NMR (50 MHz, (CD3)2SO): δ 17.52, 40.65, 49.12, 115.84, 128.49, 137.28, 147.28, 150.70, 180.15; Anal. Calcd. for C9H11N3S: C, 55.93; H, 5.74; N, 21.74; Found: C, 55.81; H, 5.63; N, 21.68.
1-(4-Tert-butyl-2-pyridyl)imidazolidine-2-thione (4e). Compound 4e was purified by use of chromatotron (eluent: dichloromethane/ethyl acetate, 7:3, v/v); yield 50%; mp. 160–163 °C; IR (KBr) ν [cm−1]: 3197, 3018, 2962, 2927, 2859, 1602, 1548, 1521, 1482, 1412, 1311, 1236, 1119, 830, 553; 1H-NMR (200 MHz, CDCl3): δ 1.34 (s, 9H, 3×CH3), 3.68 (t, 2H, CH2), 4.46 (t, 2H, CH2), 6.76 (br.s, 1H, NH), 7.06 (dd, J1 = 1.6 Hz, J2 = 5.5 Hz, 1H, Ar-H), 8.25 (d, J = 5.5 Hz, 1H, Ar-H), 9.01 (d, J = 1.6 Hz, 1H, Ar-H); 13C-NMR (50 MHz, CDCl3): δ 31.02 (three overlapping signals), 35.73, 41.52, 50.06, 114.33, 117.67, 147.21, 152.85, 161.89, 181.86; Anal. Calcd. for C12H17N3S: C, 61.24; H, 7.28; N, 17.85; Found C, 61.02; H, 6.18; N, 17.78.
1-(4-Phenyl-2-pyridyl)imidazolidine-2-thione (4f). Compound 4f was purified by use of chromatotron (eluent: dichloromethane/ethyl acetate, 4:1, v/v); yield 51%; mp. 201–202 °C; IR (KBr) ν [cm−1]: 3204, 3026, 2969, 2924, 1597, 1532, 1466, 1412, 1230; 1H-NMR (200 MHz, (CD3)2SO): δ 3.58 (t, 2H, CH2), 4.33 (t, 2H, CH2), 7.44–7.60 (m, 4H, Ar-H), 7.73–7.77 (m, 2H, Ar-H), 8.43 (d, J = 5.2 Hz, 1H, Ar-H), 9.16 (s, 1H, NH), 9.25 (s, 1H, Ar-H); 13C-NMR (50 MHz, (CD3)2SO): δ 41.06, 49.12, 113.36, 117.10, 127.01 (two overlapping signals), 129,58 (three overlapping signals), 137.82, 147.69, 148.29, 153.54, 180.19; Anal. Calcd. for C14H13N3S: C, 65.85; H, 5.13; N, 16.46; Found: C, 65.78; H, 5.04; N, 16.24.
1-(6-Methoxy-2-pyridyl)imidazolidine-2-thione (4g). Compound 4g was purified by use of chromatotron (eluent: chloroform); yield 43%; mp. 186–190 °C; IR (KBr) ν [cm−1]: 3365, 3008, 2947, 1594, 1584, 1431, 1397, 1361, 1247; 1H-NMR (500 MHz, (CD3)2SO): δ 3.56 (t, 2H, CH2), 3.84 (s, 3H, OCH3), 4.32 (t, 2H, CH2), 6.53 (d, J = 7.8 Hz, 1H, Ar-H), 7.68 (t, 1H, Ar-H), 8.47 (d, J = 7.8 Hz, 1H, Ar-H), 9.05 (s, 1H, NH); 13C-NMR [50 MHz, (CD3)2SO]: δ 39.40, 48.88, 53.20, 104.42, 107.59, 139.81, 150.72, 162.09, 179.98; Anal. Calcd. for C9H11N3OS: C, 51.65; H, 5.30; N, 20.08; Found: C, 51.52; H, 5.24; N, 19.79.
3.3. Synthesis of Copper(II) Complexes 5a–8k (General Procedure)
To a solution of appropriate ligand in 5 mL of ethanol or methanol was added dropwise at ambient temperature, copper(II) chloride dissolved in 1 mL of ethanol or methanol (in 1:1 molar ratio). The solution was left at room temperature and then the solvent was slowly evaporated. The resulting precipitate (a few minutes to 48 h) was filtered and washed with ethanol or methanol and dried in a desiccator. The following complexes were prepared according to above given procedure:
Dichloro[1-(2-pyridyl)imidazolidin-2-one]copper(II) (5a). Solvent: ethanol, dark green crystals, yield 55%; mp. 241–245 °C; IR (KBr) ν [cm−1]: 3251, 3126, 2923, 1675, 1606, 1474, 1451, 1436, 1317, 1284, 1170, 769; Anal. Calcd. for C8H9Cl2CuN3O (297.63): C, 32.28; H, 3.05; N, 14.12; Found: C, 32.14; H, 2.91; N, 13.79.
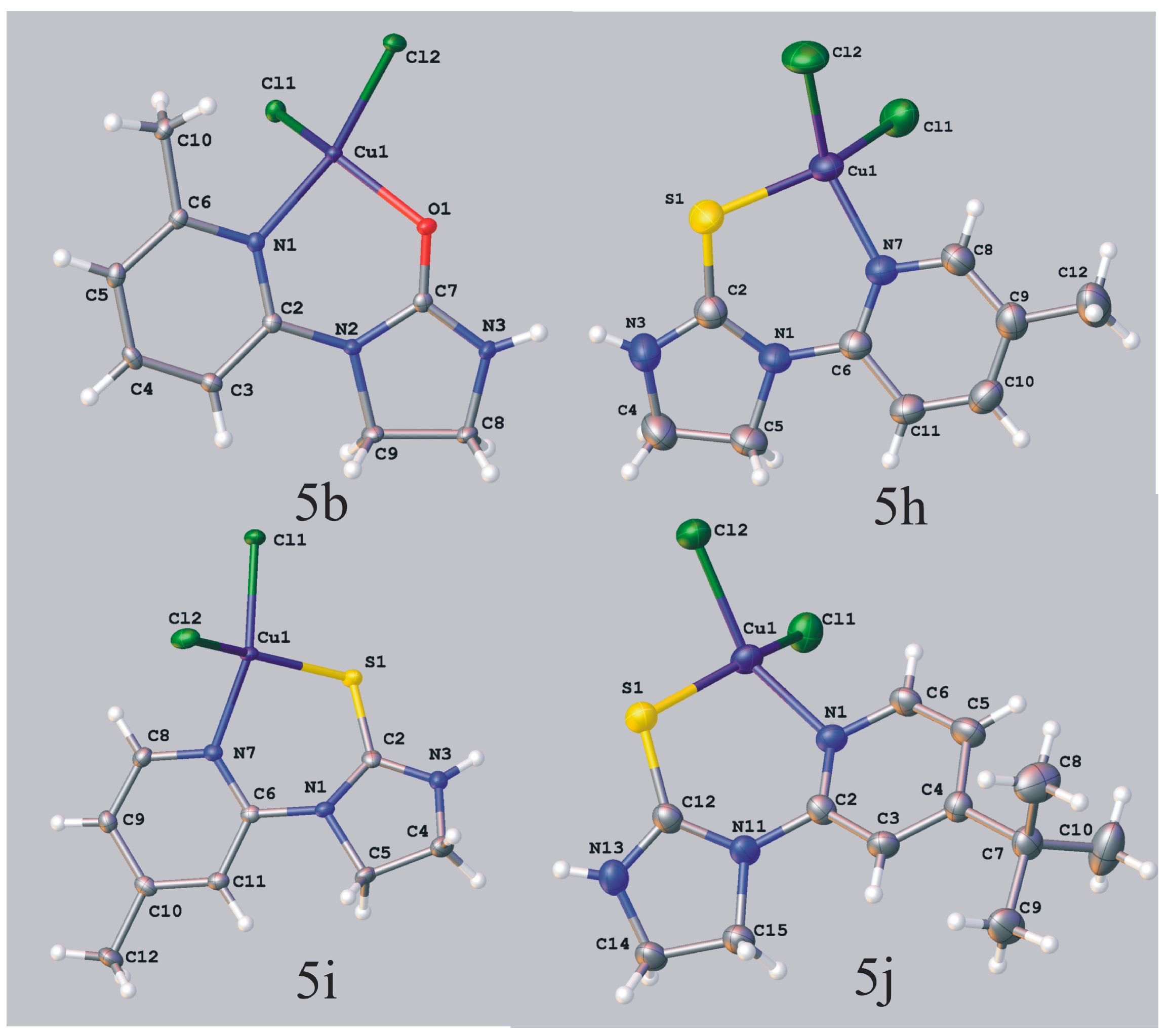
Dichloro[1-(6-methyl-2-pyridyl)imidazolidin-2-one]copper(II) (5b). Solvent: methanol, dark brown crystals; mp. 211–215 °C; IR (KBr) ν [cm−1]: 3316, 3069, 2920, 1662, 1604, 1494, 1443, 1351, 1285, 1087; Anal. Calcd. for C9H11Cl2CuN3O (311.65): C, 34.68; H, 3.56; N, 13.48; Found: C, 34.42; H, 3.50; N, 13.28.
Crystal data for 5b CCDC no. 986196: C9H11Cl2CuN3O, M = 311.65, monoclinic, space group P21/n (no. 14), Z = 4, a = 6.7379(2) Å, b = 16.9634(3) Å, c = 9.9351(2) Å, β = 105.290(2), V = 1095.36(4) Å3, T = 100 K, μ(MoKα) = 2.460 mm−1, 12880 reflections measured, 2816 unique (Rint = 0.0200) which were used in all calculations. The final wR2 was 0.0630 (all data) and R1 was 0.0217 [I > 2σ (I)].
Dichloro[1-(4-methyl-2-pyridyl)imidazolidin-2-one]copper(II) (5c). Solvent: methanol, light green crystals, yield 65%; mp. 237–238 °C; IR (KBr) ν [cm−1]: 3202, 1658, 1625, 1508, 1480, 1461, 1317, 1290, 1249, 1024, 828, 818, 742; Anal. Calcd. for C9H11Cl2CuN3O (311.66): C, 34.68; H, 3.56; N, 13.48; Found: C, 34.42; H, 3.46; N, 13.17.
Dichloro[1-(4-methoxy-2-pyridyl)imidazolidin-2-one]copper(II) (5d). Solvent: ethanol, green crystals, yield 47%; mp. 223–227 °C; IR (KBr) ν [cm−1]: 3185, 2975, 1678, 1619, 1561, 1485, 1474, 1455, 1294, 1062, 1029, 833, 751, 737; Anal. Calcd. for C9H11Cl2CuN3O2 (327.65): C, 32.99; H, 3.38; N, 12.82; Found: C, 32.86; H, 3.32; N, 12.48.
Dichloro[1-(4-ethoxy-6-methyl-2-pyridyl)imidazolidin-2-one]copper(II) (5e). Solvent: ethanol, brown crystals, yield 87%; mp. 189–193 °C; IR (KBr) ν [cm−1]: 3336, 2985, 1673, 1612, 1459, 1430, 1300, 1205, 1154, 1047, 851, 837, 744, 715, 636; Anal. Calcd. for C11H15Cl2CuN3O2 (355.71): C, 37.14; H, 4.25; N, 11.81; Found: C, 37.02; H, 4.17; N, 11.68.
Dichloro[1-(2-pyridyl)imidazolidine-2-thione]copper(II) (5f). Solvent: ethanol, dark green crystals, yield 74%; mp. 195–198 °C; IR (KBr) ν [cm−1]: 3198, 1601, 1575, 1540, 1466, 1440, 1419, 1353, 1323, 1238, 777, 670, 543; Anal. Calcd. for C8H9Cl2CuN3S (313.69): C, 30.63; H, 2.89; N, 13.40; Found: C, 30.52; H, 2.86; N, 13.76.
Dichloro[1-(6-methyl-2-pyridyl)imidazolidine-2-thione]copper(II) (5g). Solvent: ethanol, dark green crystals, yield 65%; mp. 230–233 °C; IR (KBr) ν [cm−1]: 3322, 3064, 1662, 1604, 1462, 1444, 1351, 1286, 1087, 801, 747, 734; Anal. Calcd. for C9H11Cl2CuN3S (327.72): C, 32.98; H, 3.38; N, 12.82; Found: C, 32.88; H, 3.30; N, 12.58.
Dichloro[1-(5-methyl-2-pyridyl)imidazolidine-2-thione]copper(II) (5h). Solvent: ethanol, dark green crystals, yield 59%; mp. 186–190 °C; IR (KBr) ν [cm−1]: 3202, 3058, 2962, 2912, 1613, 1539, 1504, 1429, 1385, 1321, 1232, 1053, 821; Anal. Calcd. for C9H11Cl2CuN3S (327.71): C, 32.98; H, 3.38; N, 12.82; Found: C, 32.84; H, 3.27; N, 12.61.
Crystal data for 5h CCDC no. 986201: C9H11Cl2CuN3S, M = 327.71, triclinic, space group P-1 (no. 2), Z = 2, a = 8.1363(3) Å, b = 9.0505(4) Å, c = 9.9441(3) Å, α = 63.452(4), β = 77.213(3), γ = 69.989(4), V = 613.46(4) Å3, T = 296 K, μ(CuKα) = 7.908 mm−1, 12399 reflections measured, 2531 unique (Rint = 0.0378) which were used in all calculations. The final wR2 was 0.0942 (all data) and R1 was 0.0318 [I > 2σ (I)].
Dichloro[1-(4-methyl-2-pyridyl)imidazolidine-2-thione]copper(II) (5i). Solvent: methanol, dark green crystals; mp. 179–181 °C; IR (KBr) ν [cm−1]: 3174, 3070, 1618, 1566, 1547, 1440, 1347, 1239; Anal. Calcd. for C9H11Cl2CuN3S (327.71): C, 32.98; H, 3.38; N, 12.82; Found: C, 32.84; H, 3.32; N, 12.78.
Crystal data for 5i CCDC no. 986193: C9H11Cl2CuN3S, M = 327.71, monoclinic, space group P21/c (no. 14), Z = 4, a = 8.4124(4) Å, b = 13.8054(6) Å, c = 11.4288(5) Å, β = 110.782(4), V = 1240.94(1) Å3, T = 140 K, μ(MoKα) = 2.333 mm−1, 10224 reflections measured, 2538 unique (Rint = 0.0189) which were used in all calculations. The final wR2 was 0.0545 (all data) and R1 was 0.0201 [I > 2σ (I)].
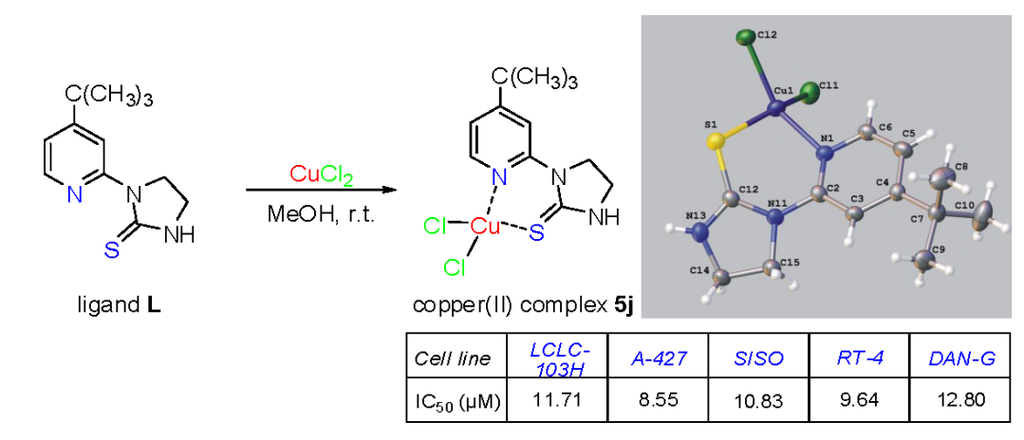
Dichloro[1-(4-tert-butyl-2-pyridyl)imidazolidine-2-thione]copper(II) (5j). Solvent: methanol, dark green crystals, yield 76%; mp. 165–168 °C; IR (KBr) ν [cm−1]: 3163, 3054, 2957, 2923, 1615, 1554, 1536, 1442, 1294, 1247, 1020, 863, 843; Anal. Calcd. for C12H17Cl2CuN3S (369.79): C, 38.97; H, 4.63; N, 11.36; Found: C, 38.92; H, 4.58; N, 11.21.
Crystal data for 5j CCDC no. 986094: C12H17Cl2CuN3S, M = 369.79, orthorhombic, space group Pbca (no. 61), Z = 8, a = 14.6475(9) Å, b = 11.3493(8) Å, c = 18.1891(13) Å, V = 3023.7(4) Å3, T = 130 K, μ(CuKα) = 6.490 mm−1, 16551 reflections measured, 3117 unique (Rint = 0.0944) which were used in all calculations. The final wR2 was 0.1678 (all data) and R1 was 0.0583 (I > 2σ (I)). In the diffraction pattern reflections with l = 2n + 1 were weak. The structure is strongly disordered with the complex molecule adopting three different overlapping orientations. The main orientation has an occupancy of 0.689(4) and the remaining ones 0.153(4) and 0.158(4). The atoms forming the minor orientation of the molecule were refined with a common isotropic temperature factor, except Cu, Cl and S atoms which were refined anisotropically. The geometry of the molecules in minor orientation was restricted to be the same as for the major orientation. Some restraints were also imposed on the planar fragments of the molecules.
Dichloro[1-(4-phenyl-2-pyridyl)imidazolidine-2-thione]copper(II) (5k). Solvent: ethanol, dark green, yield 59%; mp. 165–170 °C; IR (KBr) ν [cm−1]: 3207, 3052, 3004, 2960, 1613, 1552, 1466, 1437, 1276, 1236, 763, 696, 556; Anal. Calcd. for C14H13Cl2CuN3S (389.79): C, 43.14; H, 3.36; N, 10.78; Found: C, 42.99; H, 3.26; N, 10.43.
Dichloro[1-(6-methoxy-2-pyridyl)imidazolidin-2-one]copper(II) (6a). Solvent: ethanol, brown crystals, yield 91%; mp. 207–209 °C; IR (KBr) ν [cm−1]: 3316, 3293, 3082, 3030, 2905, 1673, 1606, 1474, 1440, 1295, 1267, 1168, 1117, 1030, 796, 746, 736; Anal. Calcd. for C9H11Cl2CuN3O2 (327.65): C, 32.99; H, 3.38; N, 12.82; Found: C, 32.83; H, 3.34; N, 13.16.
Crystal data for 6a CCDC no. 986200: C9H11Cl2CuN3O2, M = 327.65, triclinic, space group P-1 (no. 2), Z = 2, a = 7.3569(8) Å, b = 8.7648(8) Å, c = 9.7086(10) Å, α = 86.242(8), β = 86.557(9), γ = 77.925(9), V = 610.17(11) Å3, T = 293 K, μ(CuKα) = 6.521 mm−1, 7294 reflections measured, 2226 unique (Rint = 0.0366) which were used in all calculations. The final wR2 was 0.1024 (all data) and R1 was 0.0342 (I >2 σ (I)).
Dichloro[1-(6-ethoxy-2-pyridyl)imidazolidin-2-one]copper(II) (6b). Solvent: ethanol, brown crystals, yield 23%; mp. 199–202 °C; IR (KBr) ν [cm−1]: 3329, 3079, 2982, 2925, 1670, 1605, 1480, 1466, 1451, 1429, 1294, 1264, 1164, 1118, 1034, 1017, 786; Anal. Calcd. for C10H13Cl2CuN3O2 (341.68): C, 35.15; H, 3.83; N, 12.30; Found: C, 35.02; H, 3.79; N, 12.63.
Dichloro[1-(6-n-propoxy-2-pyridyl)imidazolidin-2-one]copper(II) (6c). Solvent: ethanol, brown crystals, yield 68%; mp. 185–187 °C; IR (KBr) ν [cm−1]: 3249, 3094, 2958, 2925, 2877, 1712, 1676, 1605, 1474, 1445, 1425, 1294, 1266, 1165, 1113, 1091, 993, 962, 792, 735; Anal. Calcd. for C11H15Cl2CuN3O2 (355.71): C, 37.14; H, 4.25; N, 11.81; Found: C, 37.10; H, 4.08; N, 12.08.
Dichloro[1-(6-isopropoxy-2-pyridyl)imidazolidin-2-one]copper(II) (6d). Solvent: ethanol, brown crystals, yield 56%; mp. 211–212 °C; IR (KBr) ν [cm−1]: 3303, 3112, 3056, 2983, 2931, 1672, 1603, 1469, 1441, 1425, 1372, 1293, 1266, 1166, 1115, 1092, 987, 952, 798, 734; Anal. Calcd. for C11H15Cl2CuN3O2 (355.71): C, 37.14; H, 4.25; N, 11.81; Found: C, 36.98; H, 4.18; N, 11.80.
Dichloro[1-(6-n-butoxy-2-pyridyl)imidazolidin-2-one]copper(II) (6e). Solvent: ethanol, golden crystals, yield 48%; mp. 187–189 °C; IR (KBr) ν [cm−1]: 3229, 3104, 2956, 2871, 1678, 1607, 1467, 1444, 1428, 1294, 1294, 1265, 1166, 1094, 790, 735; Anal. Calcd. for C12H17Cl2CuN3O2 (369.73): C, 38.98; H, 4.63; N, 11.36; Found: C, 38.91; H, 4.56; N, 11.62.
Dichloro[1-(6-methoxy-2-pyridyl)imidazolidine-2-thione]copper(II) (6f). Solvent: ethanol, brown crystals, yield 49%; mp. 149–154 °C; IR (KBr) ν [cm−1]: 3159, 3066, 2953, 2923, 1604, 1543, 1468, 1435, 1418, 1286, 1232, 1129, 787; Anal. Calcd. for C9H11Cl2CuN3OS (343.72): C, 31.45; H, 3.23; N, 12.23; Found: C: 31.38; H, 3.19; N, 11.88.
Dichloro[1,3-bis(4-methyl-2-pyridyl)imidazolidin-2-one]copper(II).H2O (7). Solvent: methanol, green crystals; mp. 170–172°C; IR (KBr) ν [cm−1]: 3372, 1635, 1506, 1472, 1436, 1335, 1261, 1195.
Crystal data for 7 CCDC no. 986194: C15H18Cl2CuN4O2, M = 420.77, triclinic, space group P-1 (no. 2), Z = 2, a = 8.9310(7) Å, b = 10.6693(7) Å, c = 10.9789(9) Å, α = 112.749(7), β = 95.298(7), γ = 111.370(7), V = 864.67(11) Å3, T = 293 K, μ(MoKα) = 1.587 mm−1, 7039 reflections measured, 3517 unique (Rint = 0.0181) which were used in all calculations. The final wR2 was 0.0742 (all data) and R1 was 0.0287 (I > 2σ (I)).
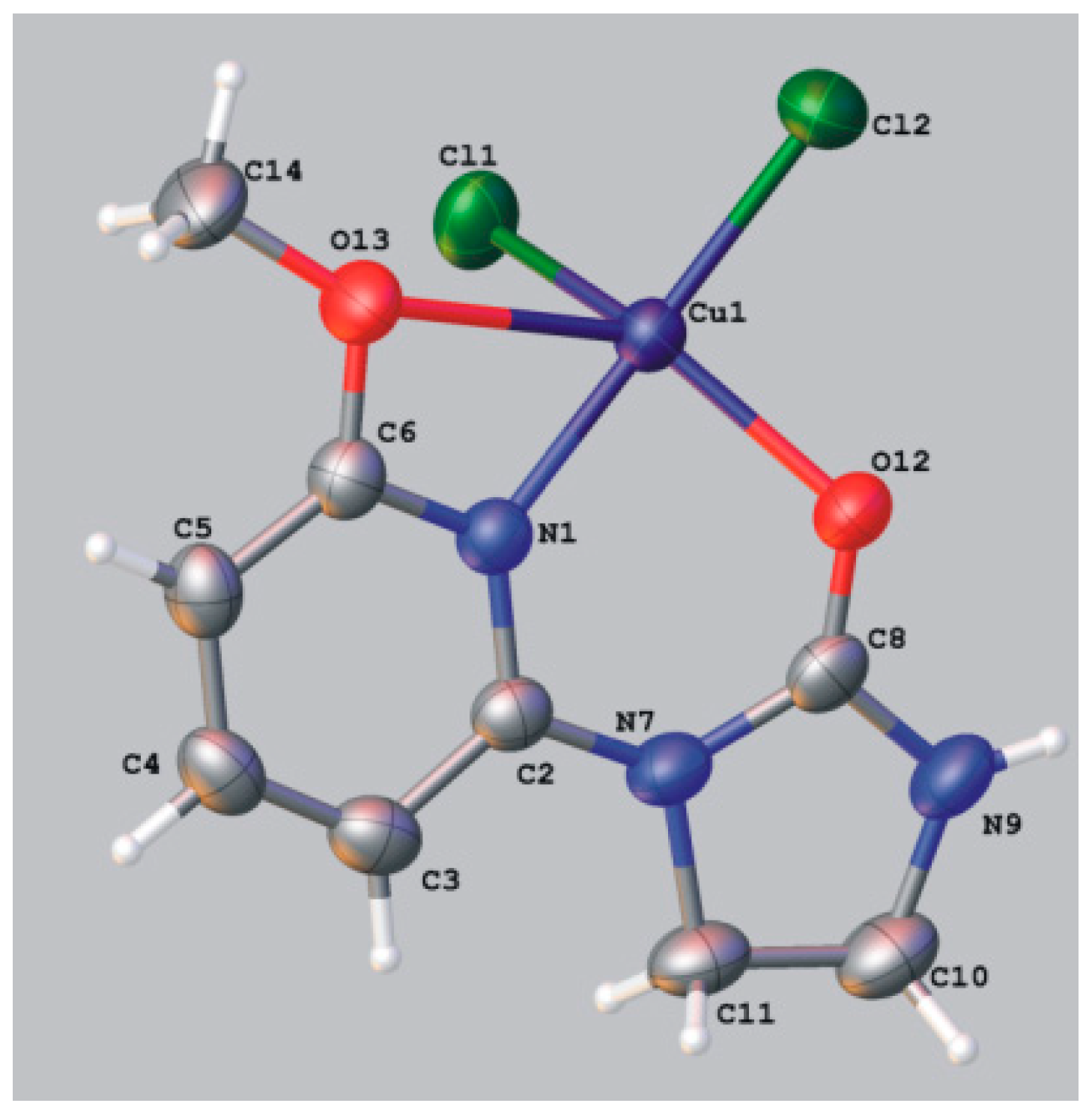
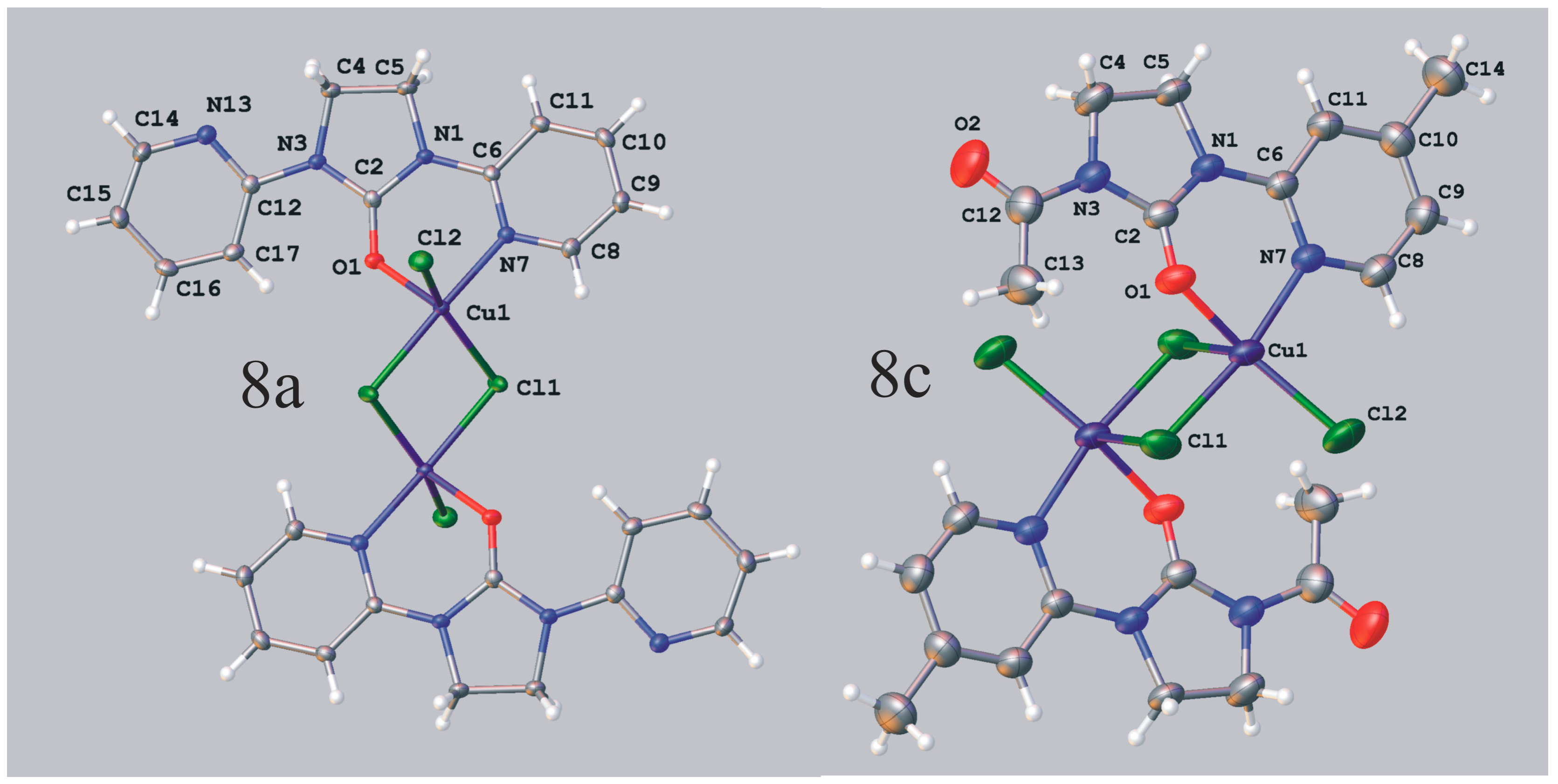
Dichloro[1,3-bis(2-pyridyl)imidazolidin-2-one]copper(II) (8a). Solvent: ethanol, yellow-green crystals, yield 77%; mp. 265–267 °C; IR (KBr) ν [cm−1]: 3074, 2901, 1679, 1604, 1587, 1463, 1438, 1412, 1351, 1312, 1245, 1138, 781, 752, 740; Anal. Calcd. for C13H12Cl2CuN4O (374.71): C, 41.67; H, 3.23; N, 14.95; Found: C, 41.64; H, 3.19; N, 14.92.
Crystal data for 8a CCDC no. 986095: C26H24Cl4Cu2N8O2, M = 749.43, monoclinic, space group P21/n (no. 14), Z = 2, a = 7.83450(10) Å, b = 16.3329(2) Å, c = 10.8358(2) Å, β = 90.7160(10), V = 1386.44(4) Å3, T = 130 K, μ(MoKα) = 1.963 mm−1, 22938 reflections measured, 3444 unique (Rint = 0.0379) which were used in all calculations. The final wR2 was 0.0665 (all data) and R1 was 0.0293 [I > 2σ (I)].
Dichloro[1-acetyl-3-(5-methyl-2-pyridyl)imidazolidin-2-one]copper(II) (8b). Solvent: ethanol, green crystals, yield 50%; mp. 233–235 °C; IR (KBr) ν [cm−1]: 3091, 2984, 2927, 1698, 1655, 1468, 1434, 1401, 1323, 1291, 1256, 1042, 962, 839, 740, 617; Anal. Calcd. for C11H13Cl2CuN3O2 (353.69): C, 37.35; H, 3.70; N, 11.88; Found: C, 37.28; H, 3.64; N, 12.18.
Dichloro[1-acetyl-3-(4-methyl-2-pyridyl)imidazolidin-2-one]copper(II) (8c). Solvent: ethanol, green crystals, yield 62%; mp. 222–225 °C; IR (KBr) ν [cm−1]: 3091, 3060, 2986, 1708, 1683, 1622, 1455, 1412, 1372, 1321, 1271, 1192, 1150, 1038, 971, 841, 749, 742, 619, 456; Anal. Calcd. for C11H13Cl2CuN3O2 (353.69): C, 37.35; H, 3.70; N, 11.88; Found: C, 37.27; H, 3.63; N, 12.22.
Crystal data for 8c CCDC no. 986202: C22H26Cl4Cu2N6O4, M = 707.39, triclinic, space group P-1 (no. 2), Z = 1, a = 8.6259(2) Å, b = 9.2649(3) Å, c = 10.4228(3) Å, α = 102.178(3), β = 98.752(2), γ = 116.880(3), V = 696.37(3) Å3, T = 293 K, μ(Cu Kα) = 5.765 mm−1, 7991 reflections measured, 2869 unique (Rint = 0.0137) which were used in all calculations. The final wR2 was 0.0738 (all data) and R1 was 0.0268 (I > 2σ (I)).
Dichloro[1-butyryl-3-(4-methyl-2-pyridyl)imidazolidin-2-one]copper(II) (8d). Solvent: ethanol, green crystals, yield 93%; mp. 236–240 °C; IR (KBr) ν [cm−1]: 3123, 3089, 3054, 2960, 2936, 2877, 1705, 1681, 1622, 1455, 1413, 1384, 1322, 1272, 1227, 1211, 1180, 907, 840, 743, 709, 663, 458; Anal. Calcd. for C13H17Cl2CuN3O2 (381.74): C, 40.90; H, 4.49; N, 11.01; Found: C, 40.82; H, 4.42; N, 10.98.
Dichloro[1-acetyl-3-(4-tert-butyl-2-pyridyl)imidazolidin-2-one]copper(II) (8e). Solvent: ethanol, green crystals, yield 53%; mp. 150–155 °C; IR (KBr) ν [cm−1]: 2966, 1662, 1619, 1478, 1445, 1377, 1283, 1063, 734, 617; Anal. Calcd. for C14H19Cl2CuN3O2 (395.77): C, 42.49; H, 4.84; N, 10.62; Found: C, 42.38; H, 4.82; N, 10.31.
Dichloro[1-acetyl-3-(4-phenyl-2-pyridyl)imidazolidin-2-one]copper(II) (8f). Solvent: ethanol, green crystals, yield 95%; mp. 227–233 °C; IR (KBr) ν [cm−1]: 3055, 2962, 2926, 1718, 1673, 1615, 1473, 1436, 1410, 1372, 1278, 1237, 965, 771, 740, 630, 615; Anal. Calcd. for C16H15Cl2CuN3O2 (415.76): C, 46.22; H, 3.64; N, 10.11; Found: C, 46.01; H, 3.61; N, 10.02.
Dichloro[1-butyryl-3-(4-phenyl-2-pyridyl)imidazolidin-2-one]copper(II) (8g). Solvent: ethanol, dark green crystals, yield 77%; mp. 178–184 °C; IR (KBr) ν [cm−1]: 3051, 2963, 2873, 1671, 1616, 1474, 1437, 1409, 1372, 1284, 1222, 1178, 768, 629; Anal. Calcd. for C18H19Cl2CuN3O2 (443.81): C, 48.71; H, 4.32; N, 9.47; Found: C, 48.68; H, 4.26; N, 9.45.
Dichloro{1-acetyl-3-[4-(3-phenylpropyl)-2-pyridyl]imidazolidin-2-one}copper(II) (8h). Solvent: ethanol, dark green crystals, yield 56%; mp. 160–164 °C; IR (KBr) ν [cm−1]: 3078, 2920, 2860, 1713, 1671, 1649, 1623, 1476, 1451, 1420, 1375, 1282, 1245, 966, 735, 701, 615; Anal. Calcd. for C19H21Cl2CuN3O2 (457.84): C, 49.84; H, 4.62; N, 9.18; Found: C, 49.79; H, 4.58; N, 9.46.
Dichloro[1-acetyl-3-(4-methoxy-2-pyridyl)imidazolidin-2-one]copper(II) (8i). Solvent: ethanol, light green crystals, yield 69%; mp. 190–194 °C; IR (KBr) ν [cm−1]: 3143, 3117, 3085, 3030, 2993, 2929, 1709, 1667, 1617, 1477, 146, 1377, 1281, 1232, 1037, 968, 845, 741, 616; Anal. Calcd. for C11H13Cl2CuN3O3 (369.69): C, 35.74; H, 3.54; N, 11.37; Found: C, 35.69; H, 3.46; N, 11.38.
Dichloro[1-butyryl-3-(4-methoxy-2-pyridyl)imidazolidin-2-one]copper(II) (8j). Solvent: ethanol, green crystals, yield 66%; mp. 210–213 °C; IR (KBr): 3104, 2967, 2930, 2873, 1671, 1618, 1468, 1419, 1382, 1285, 1220, 1176, 1048, 842, 738, 708; Anal. Calcd. for C13H17Cl2CuN3O3 (397.74): C, 39.26; H, 4.31; N, 10.56; Found: C, 39.11; H, 4.26; N, 10.35.
Dichloro[1-acetyl-3-(4-benzyloxy-2-pyridyl)imidazolidin-2-one]copper(II) (8k). Solvent: ethanol, green crystals, yield 49%; mp. 195–199 °C; IR (KBr) ν [cm−1]: 3086, 2990, 2923, 1683, 1613, 1480, 1448, 1415, 1378, 1278, 1245, 1036, 1022, 834, 738, 622; Anal. Calcd. for C17H17Cl2CuN3O3 (445.79): C, 45.80; H, 3.84; N, 9.43; Found: C, 45.71; H, 3.76; N, 9.76.
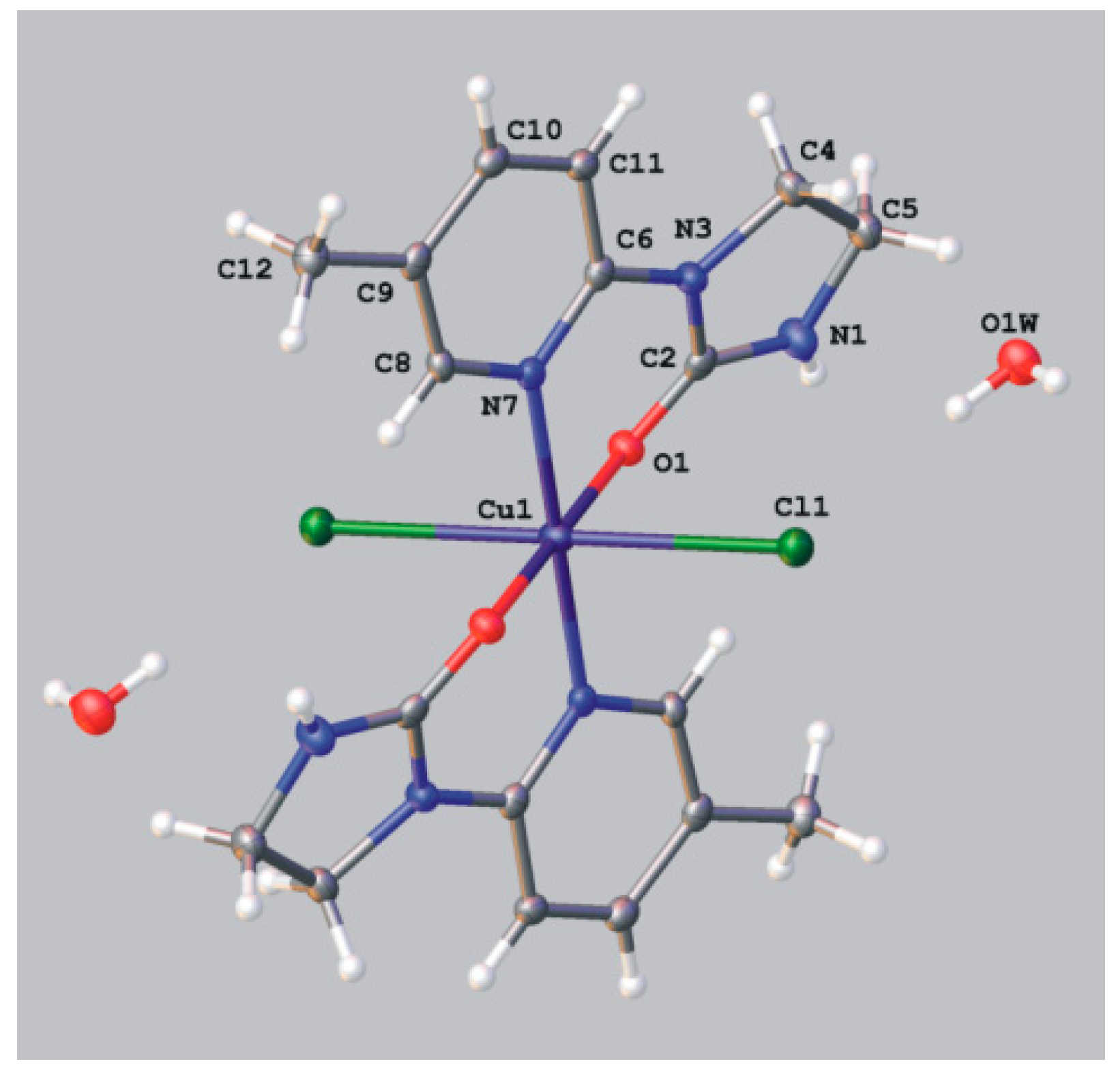
Dichloro{bis[1-(5-methyl-2-pyridyl)imidazolidin-2-one]}copper(II).2H2O (9). 1-(5-Methyl-2-pyridyl)imidazolidin-2-one (0.1 g; 0.00056 mol) was dissolved in 7 mL of N,N-dimethylformamide and copper(II) chloride (0.144 g, 0.00085 mol) was added. After week of slow evaporation at room temperature green crystals suitable for the X-ray analysis were collected, washed with small amount of solvent and dried. Obtained 0.06 g of complex compound 9, yield 43%: C18H22Cl2CuN6O2.2H2O (524.89); mp. 224–229 °C; IR (KBr) ν [cm−1]: 3169, 3068, 2915, 1657, 1618, 1576, 1483, 1453, 1319, 1287, 1170, 824, 742.
Crystal data for 9 CCDC no. 986199: (C18H22CuN6O2)Cl2·2(H2O) , M = 524.89, monoclinic, space group C2/c (no. 15), Z = 4, a = 12.3708(7) Å, b = 13.6659(5) Å, c = 13.1220(16) Å, β = 109.417(1), V = 2092.2(3) Å3, T = 130 K, μ(MoKα) = 1.340 mm−1, 7717 reflections measured, 1832 unique (Rint = 0.0450) which were used in all calculations. The final wR2 was 0.0803 (all data) and R1 was 0.0321 (I > 2σ (I)).
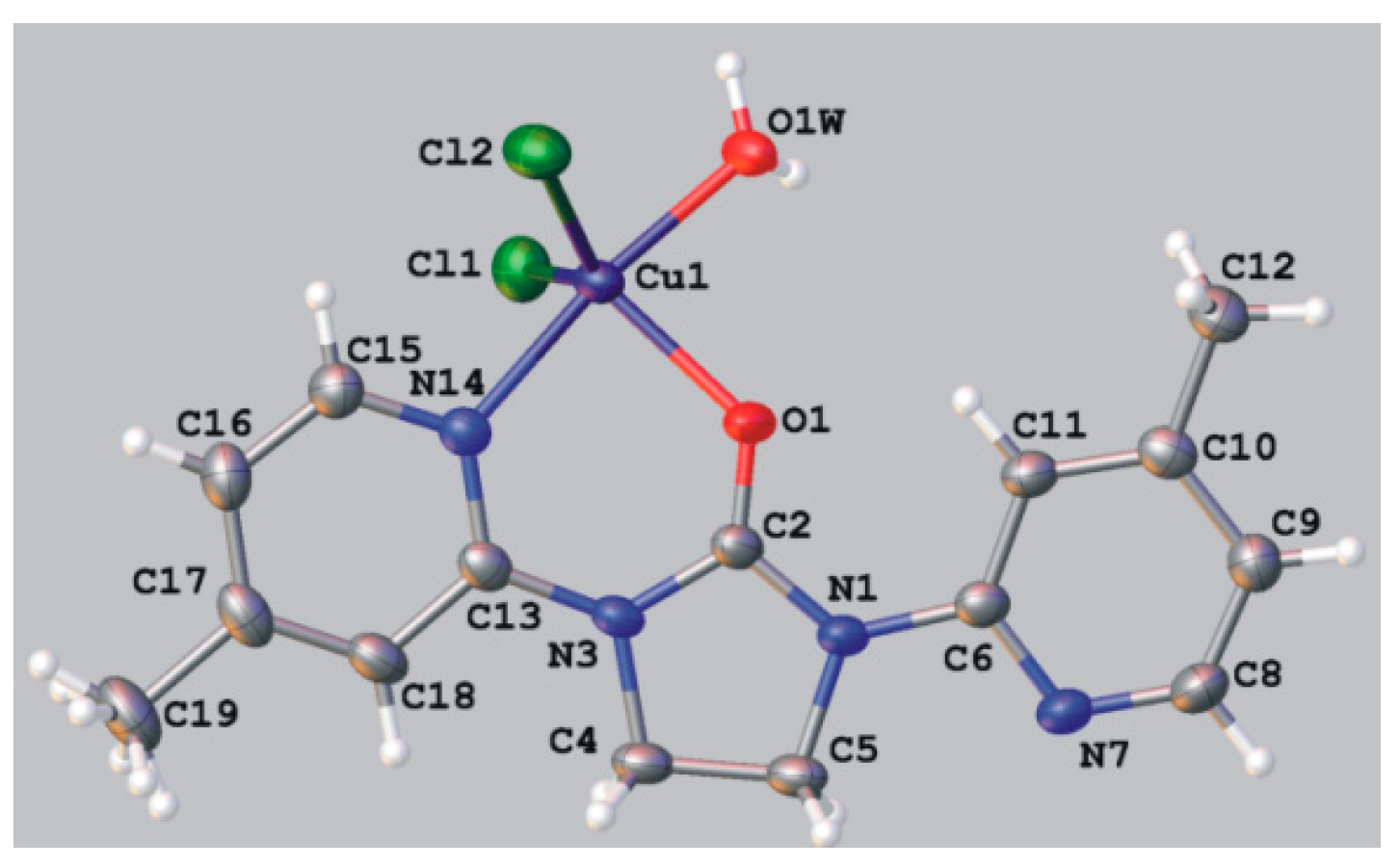
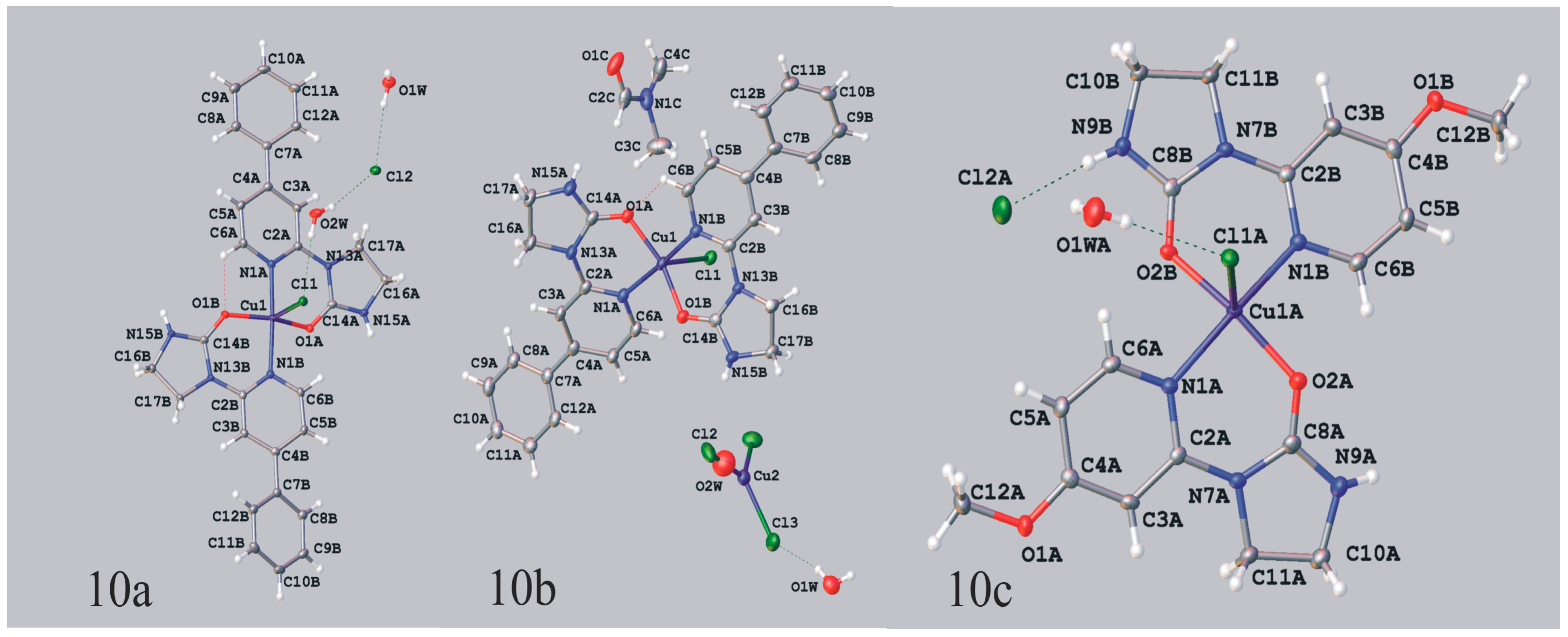
Dichloro{bis[1-(4-phenyl-2-pyridyl)imidazolidin-2-one]}copper(II) (10a, 10b). 1-(4-Phenyl-2-pyridyl)imidazolidin-2-one (0.1 g, 0.00042 mol) was dissolved in 7 mL of N,N-dimethylformamide and copper(II) chloride (0.107 g, 0.00063 mol) was added. After two weeks of slow evaporation at room temperature green crystals suitable for the X-ray analysis were collected, washed with small amount of solvent and dried. Obtained 0.06 g of a mixture of complex compounds 10a and 10b, mp. 208–211 °C; IR (KBr) ν [cm−1]: 3447, 3056, 1669, 1616, 1473, 1446, 1319, 1296, 1070, 1014, 855, 763, 740.
Crystal data for 10a CCDC no. 986198: (C28H26ClCuN6O2)Cl·2(H2O), M = 649.02, triclinic, space group P-1 (no. 2), Z = 2, a = 10.5234(4) Å, b = 11.7728(5) Å, c = 12.5141(4) Å, α = 97.231(3), β = 106.598(3), γ = 108.593(4), V = 1367.46(9) Å3, T = 100 K, μ(MoKα) = 1.042 mm−1, 14433 reflections measured, 5552 unique (Rint = 0.0169) which were used in all calculations. The final wR2 was 0.0695 (all data) and R1 was 0.0263 (I > 2σ (I)).
Crystal data for 10b CCDC no. 986197: (C28H26ClCuN6O2)2[CuCl3]0.75[Cl]0.5P· 2.15(H2O)·C3H7NO, M = 1413.85, monoclinic, space group I2/a (no. 15), Z = 4, a = 13.8269(3) Å, b = 16.8610(3) Å, c = 25.7704(5) Å, β = 93.988(2), V = 5993.4(2) Å3, T = 100 K, μ(MoKα) = 1.246 mm−1, 34078 reflections measured, 6096 unique (Rint = 0.0240) which were used in all calculations. The final wR2 was 0.0827 (all data) and R1 was 0.0297 (I > 2σ (I)).The [CuCl3]2− anion and one of the water molecules are located on a twofold axis. The CuI and the Cl atom of the anion in special position occupy their positions in 75% whereas the water molecule in 25%. The remaining Cl atoms fully occupy their positions.
Dichloro{bis[1-(4-methoxy-2-pyridyl)imidazolidin-2-one]}copper(II).H2O (10c). 1-(4-Methoxy-2-pyridyl)imidazolidin-2-one (0.1 g; 0.00052 mol) was dissolved in 7 mL of N,N-dimethylformamide and copper(II) chloride (0.132 g, 0.00078 mol) was added. After two weeks of slow evaporation at room temperature blue crystals suitable for the X-ray analysis were collected, washed with small amount of solvent and dried. Obtained 0.05 g of complex compound 10c, yield 36%: C18H22Cl2CuN6O4.H2O (538.87); mp. 214–216 °C; IR (KBr) ν [cm−1]: 3431, 3176, 2985, 1670, 1615, 1472, 1452, 1428, 1291, 1248, 1064, 1030.
Crystal data for 10c CCDC no. 986195: (C18H22ClCuN6O4)Cl.H2O, M = 538.87, monoclinic, space group P21 (no. 4), Z = 4, a = 10.9160(1) Å, b = 13.4955(2) Å, c = 15.1544(2) Å, β = 107.429(2), V = 2130.00(5) Å3, T = 120 K, μ(MoKα) = 1.322 mm−1, 36883 reflections measured, 9877 unique (Rint = 0.0225) which were used in all calculations. The final wR2 was 0.0576 (all data) and R1 was 0.0227 (I > 2σ (I)).