2-(1H-Benzimidazol-2-yl)-4,5,6,7-tetrahydro-2H-indazol-3-ol, a Benzimidazole Derivative, Inhibits T Cell Proliferation Involving H+/K+-ATPase Inhibition
Abstract
:1. Introduction
2. Results and Discussion
2.1. H+/K+-ATPases Inhibition Associated with the Acidification of Cytosolic pH in BMT-1 Treated Cells
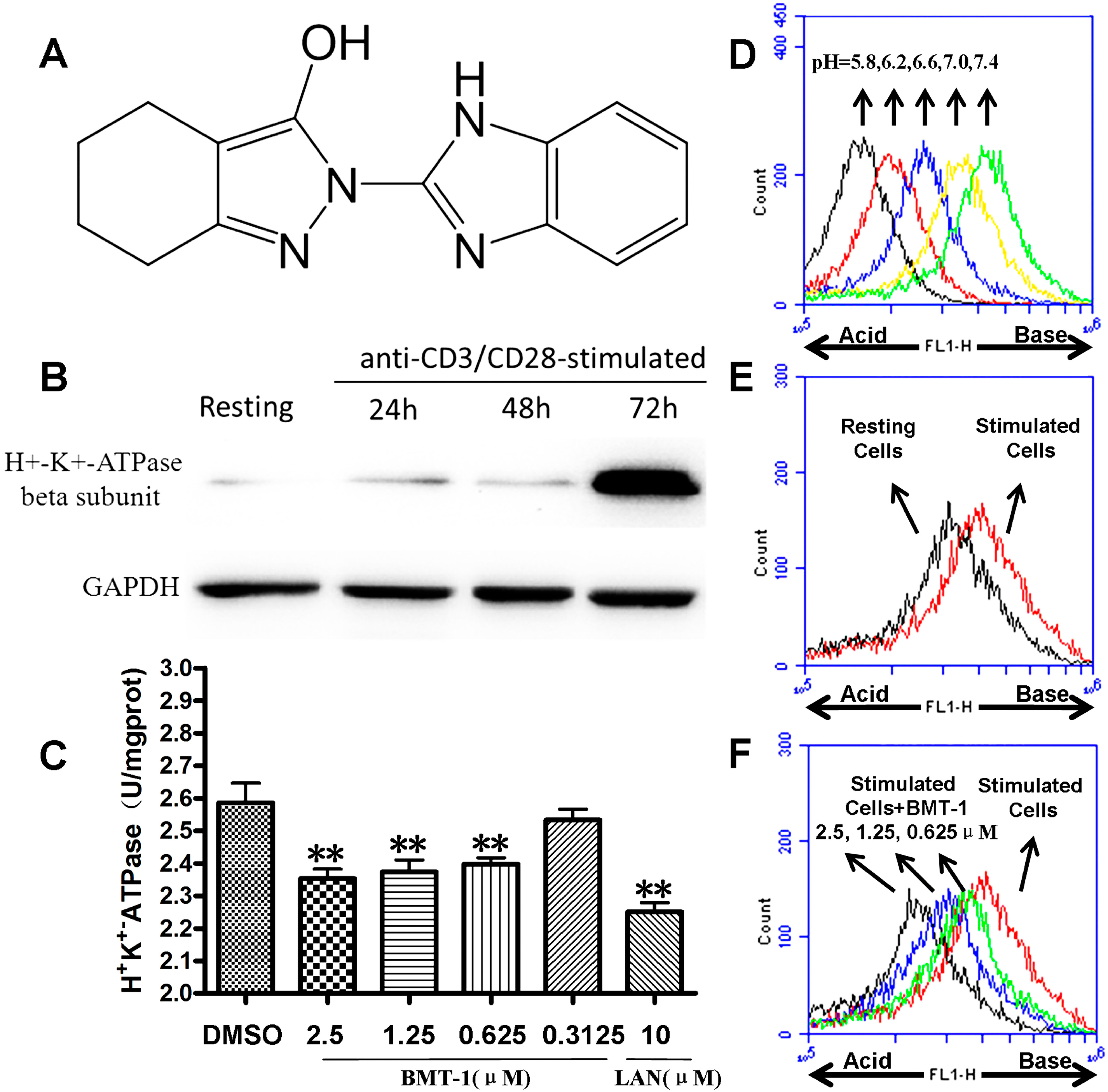
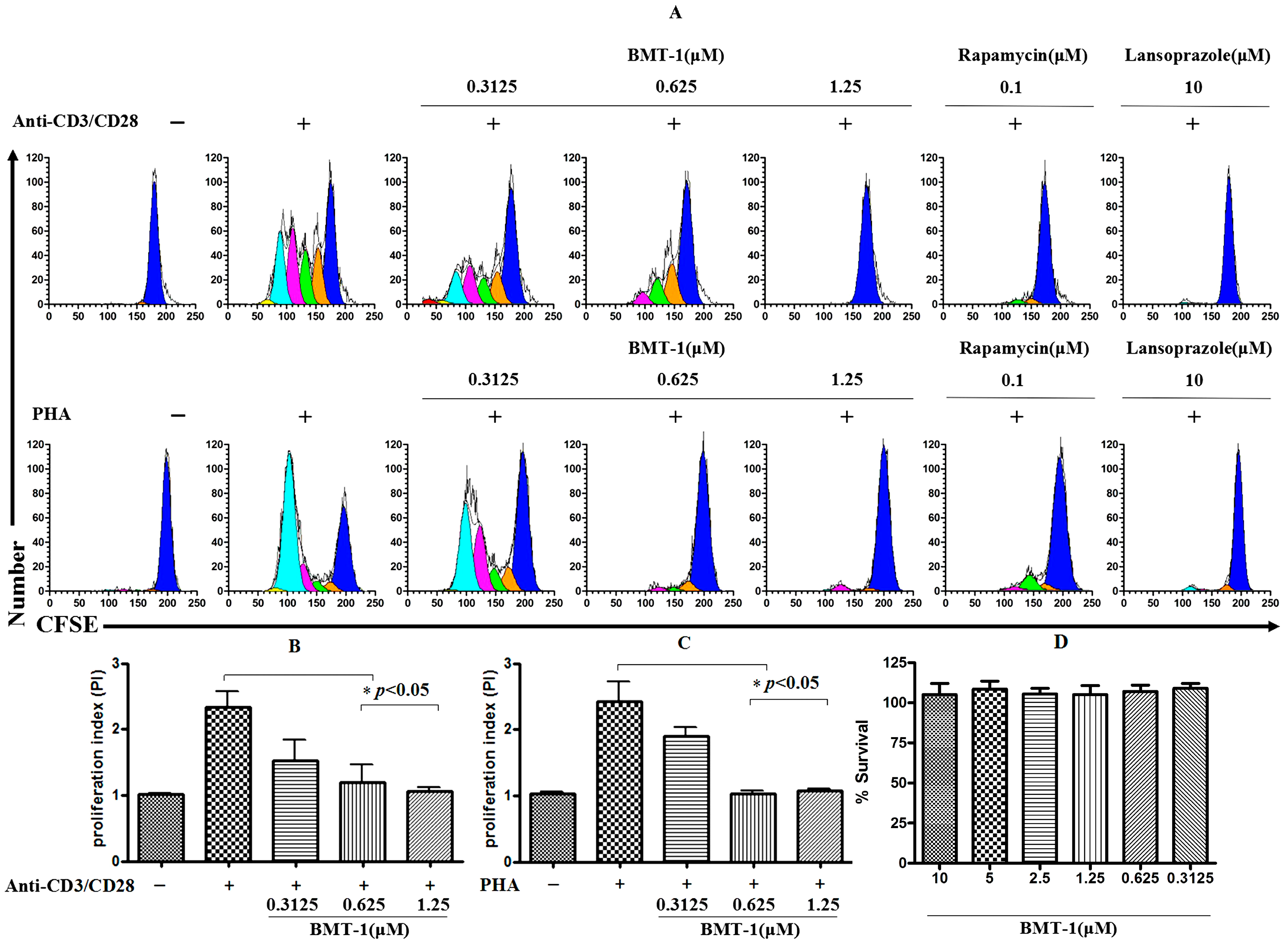
2.2. BMT-1 Inhibits T Cell Proliferation
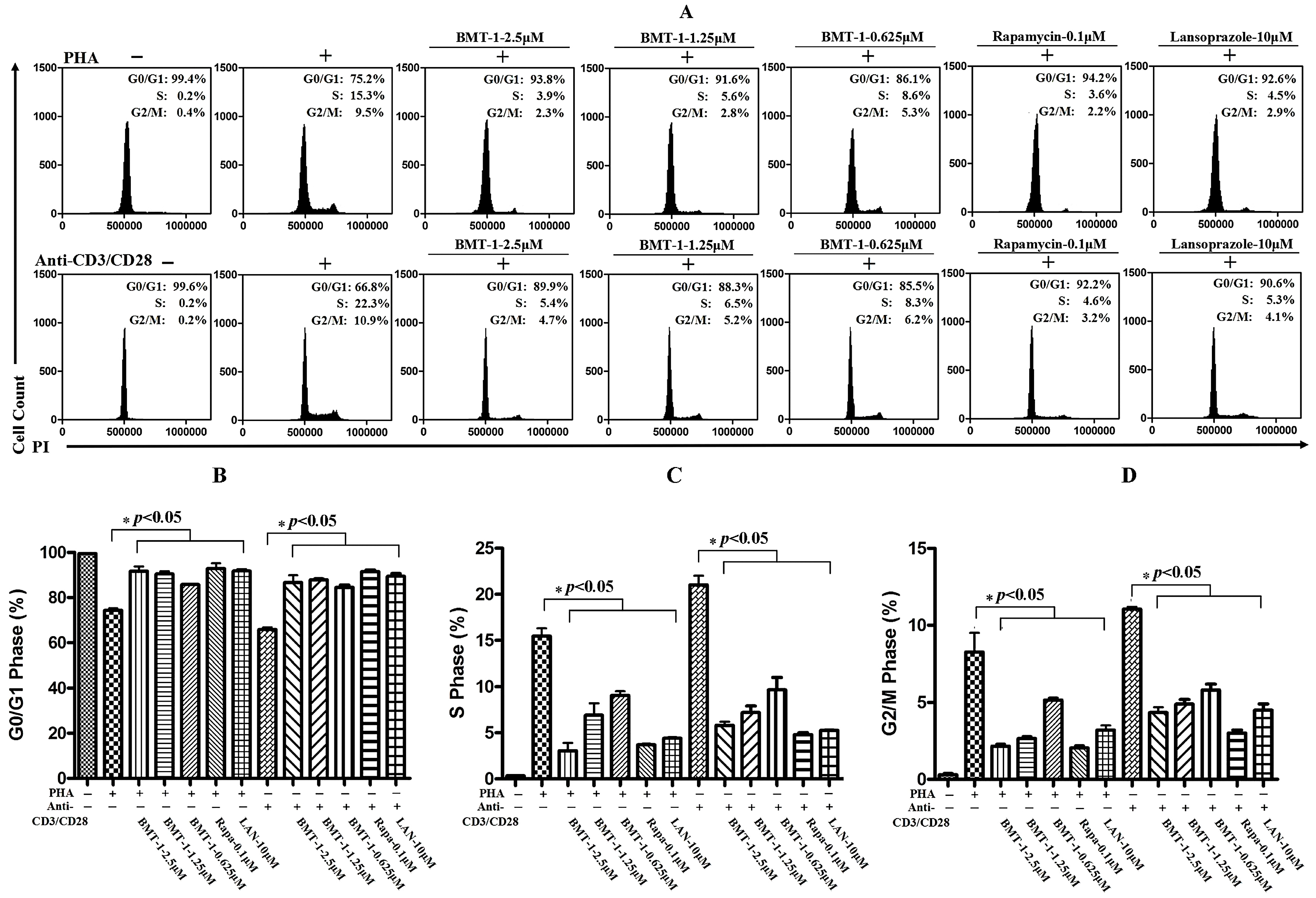
2.3. BMT-1 Decreases Anti-CD3/CD28 or PHA-Activated T Cell Proliferation by Arresting the Cell Cycle at the G0/G1 Phase
2.4. BMT-1 has no Effect on IL-2 and CD25 Expression

2.5. BMT-1 Inhibits IL-2-Dependent PBMCs Proliferation
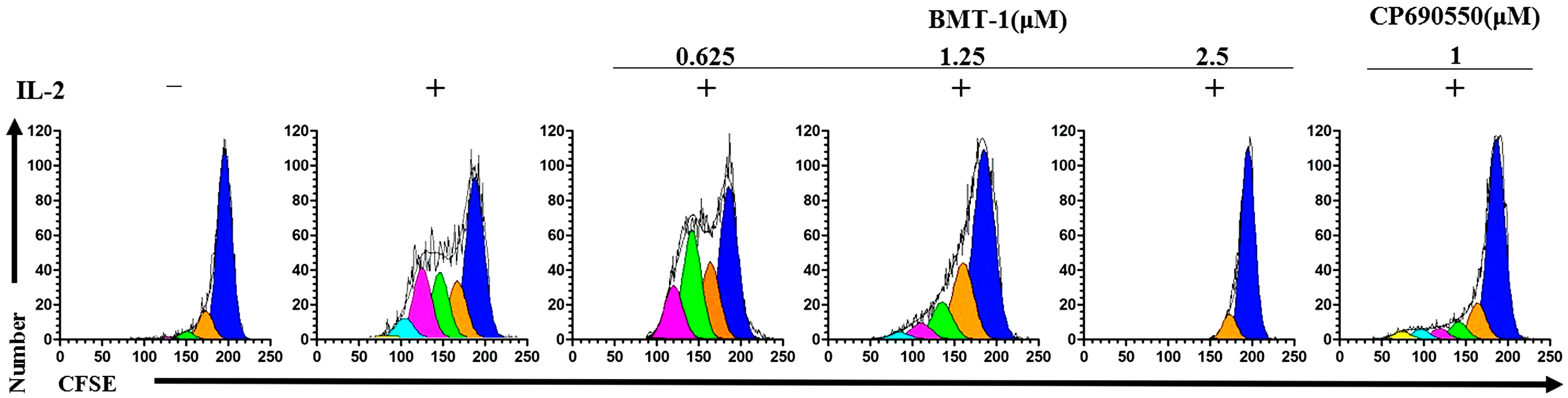
2.6. Discussion
3. Experimental Section
3.1. Chemicals
3.2. Antibodies and Reagents
3.3. Cell Isolation and Culture
3.4. Western Blot Analysis
3.5. Measurement of ATPases Activity in T Cells
3.6. Evaluation of Cytosolic pH
3.7. Proliferation Testing
3.8. Viability of Lymphocytes and BMT-1 Exposure
3.9. Cell Cycle Analysis
3.10. The Effect of BMT-1 on the Expression of CD25 (IL-2 Receptor Chain) and IL-2 on Human T Cells Stimulated with Anti-CD3/CD28
3.11. Inhibition of Human IL-2 Dependent PBMCs Blast Proliferation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jauregui, I.; Garcia-Lirio, E.; Soriano, A.M.; Gamboa, P.M.; Antepara, I. An overview of the novel HL-antihistamine bilastine in allergic rhinitis and urticaria. Expert Rev. Clin. Immunol. 2012, 8, 33–41. [Google Scholar] [CrossRef]
- Sachs, G.; Shin, J.M.; Vagin, O.; Lambrecht, N.; Yakubov, I.; Munson, K. The gastric H,K ATPase as a drug target: Past, present, and future. J. Clin. Gastroenterol. 2007, 41 (Suppl. 2), S226–S242. [Google Scholar] [CrossRef]
- Wexler, R.R.; Greenlee, W.J.; Irvin, J.D.; Goldberg, M.R.; Prendergast, K.; Smith, R.D.; Timmermans, P.B. Nonpeptide angiotensin ii receptor antagonists: The next generation in antihypertensive therapy. J. Med. Chem. 1996, 39, 625–656. [Google Scholar] [CrossRef]
- Navarrete-Vazquez, G.; Cedillo, R.; Hernandez-Campos, A.; Yepez, L.; Hernadez-Luis, F.; Valdez, J.; Morales, R.; Cortes, R.; Hernandez, M.; Castillo, R. Synthesis and antiparasitic activity of 2-(trifluoromethyl)-benzimidazole derivatives. Bioorg. Med. Chem. Lett. 2001, 11, 187–190. [Google Scholar] [CrossRef]
- Achar, K.C.; Hosamani, K.M.; Seetharamareddy, H.R. In-vivo analgesic and anti-inflammatory activities of newly synthesized benzimidazole derivatives. Eur. J. Med. Chem. 2010, 45, 2048–2054. [Google Scholar] [CrossRef]
- Lazer, E.S.; Farina, P.R.; Oliver, J.T.; Possanza, G.J.; Matteo, M.R. Antiinflammatory benzimidazole derivative with inhibitory effects on neutrophil function. Agents Actions 1987, 21, 257–259. [Google Scholar] [CrossRef]
- Kedika, R.R.; Souza, R.F.; Spechler, S.J. Potential anti-inflammatory effects of proton pump inhibitors: A review and discussion of the clinical implications. Dig. Dis. Sci. 2009, 54, 2312–2317. [Google Scholar] [CrossRef]
- Ritter, M.; Schratzberger, P.; Rossmann, H.; Woll, E.; Seiler, K.; Seidler, U.; Reinisch, N.; Kahler, C.M.; Zwierzina, H.; Lang, H.J.; et al. Effect of inhibitors of Na+/H+-exchange and gastric H+/K+ atpase on cell volume, intracellular pH and migration of human polymorphonuclear leucocytes. Br. J. Pharmacol. 1998, 124, 627–638. [Google Scholar] [CrossRef]
- Yang, T.; Li, M.H.; Liu, J.; Huang, N.; Li, N.; Liu, S.N.; Liu, Y.; Zhang, T.; Zou, Q.; Li, H. Benzimidazole derivative, BMT-1, induces apoptosis in multiple myeloma cells via a mitochondrial-mediated pathway involving H+/K+-ATPase inhibition. Oncol. Rep. 2014, 31, 2743–2750. [Google Scholar]
- Babcock, D.F.; Rufo, G.A., Jr.; Lardy, H.A. Potassium-dependent increases in cytosolic pH stimulate metabolism and motility of mammalian sperm. Proc. Natl. Acad. Sci. USA 1983, 80, 1327–1331. [Google Scholar] [CrossRef]
- Gerson, D.F.; Kiefer, H. Intracellular pH and the cell cycle of mitogen-stimulated murine lymphocytes. J. Cell. Physiol. 1983, 114, 132–136. [Google Scholar] [CrossRef]
- De Milito, A.; Iessi, E.; Logozzi, M.; Lozupone, F.; Spada, M.; Marino, M.L.; Federici, C.; Perdicchio, M.; Matarrese, P.; Lugini, L.; et al. Proton pump inhibitors induce apoptosis of human B-Cell tumors through a caspase-independent mechanism involving reactive oxygen species. Cancer Res 2007, 67, 5408–5417. [Google Scholar]
- Yeo, M.; Kim, D.K.; Park, H.J.; Cho, S.W.; Cheong, J.Y.; Lee, K.J. Blockage of intracellular proton extrusion with proton extrusions with proton pump inhibitor induces apoptosis in gastric cancer. Cancer Sci. 2008, 99, 185. [Google Scholar]
- Bonnefoy-Berard, N.; Verrier, B.; Vincent, C.; Revillard, J.P. Inhibition of CD25 (IL-2Rα) expression and T-cell proliferation by polyclonal anti-thymocyte globulins. Immunology 1992, 77, 61–67. [Google Scholar]
- Kudlacz, E.; Perry, B.; Sawyer, P.; Conklyn, M.; McCurdy, S.; Brissette, W.; Flanagan, M.; Changelian, P. The novel JAK-3 inhibitor CP-690550 is a potent immunosuppressive agent in various murine models. Am. J. Transplant. 2004, 4, 51–57. [Google Scholar] [CrossRef]
- Becknell, B.; Caligiuri, M.A. Interleukin-2, interleukin-15, and their roles in human natural killer cells. Adv. Immunol. 2005, 86, 209–239. [Google Scholar]
- Theze, J.; Alzari, P.M.; Bertoglio, J. Interleukin 2 and its receptors: Recent advances and new immunological functions. Immunol. Today 1996, 17, 481–486. [Google Scholar] [CrossRef]
- Shin, J.M.; Kim, N. Pharmacokinetics and pharmacodynamics of the proton pump inhibitors. J. Neurogastroenterol. Motil. 2013, 19, 25–35. [Google Scholar] [CrossRef]
- Chen, M.; Huang, S.L.; Zhang, X.Q.; Zhang, B.; Zhu, H.; Yang, V.W.; Zou, X.P. Reversal effects of pantoprazole on multidrug resistance in human gastric adenocarcinoma cells by down-regulating the V-ATPases/mTOR/HIF-1alpha/P-gp and MRP1 signaling pathway in vitro and in vivo. J. Cell. Biochem. 2012, 113, 2474–2487. [Google Scholar] [CrossRef]
- Daniel, C.; Bell, C.; Burton, C.; Harguindey, S.; Reshkin, S.J.; Rauch, C. The role of proton dynamics in the development and maintenance of multidrug resistance in cancer. Biochim. Biophys. Acta 2013, 1832, 606–617. [Google Scholar] [CrossRef]
- Putney, L.K.; Barber, D.L. Na-h exchange-dependent increase in intracellular pH times G2/M entry and transition. J. Biol. Chem. 2003, 278, 44645–44649. [Google Scholar] [CrossRef]
- Webb, B.A.; Chimenti, M.; Jacobson, M.P.; Barber, D.L. Dysregulated pH: A perfect storm for cancer progression. Nat. Rev. Cancer 2011, 11, 671–677. [Google Scholar] [CrossRef]
- Srivastava, J.; Barber, D.L.; Jacobson, M.P. Intracellular pH sensors: Design principles and functional significance. Physiology 2007, 22, 30–39. [Google Scholar] [CrossRef]
- Chen, M.; Zou, X.; Luo, H.; Cao, J.; Zhang, X.; Zhang, B.; Liu, W. Effects and mechanisms of proton pump inhibitors as a novel chemosensitizer on human gastric adenocarcinoma (SGC7901) cells. Cell Biol. Int. 2009, 33, 1008–1019. [Google Scholar] [CrossRef]
- Schneider, D.; Gerhardt, E.; Bock, J.; Muller, M.M.; Wolburg, H.; Lang, F.; Schulz, J.B. Intracellular acidification by inhibition of the Na+/H+-exchanger leads to caspase-independent death of cerebellar granule neurons resembling paraptosis. Cell Death Differ. 2004, 11, 760–770. [Google Scholar] [CrossRef]
- Wang, Q.J.; Cai, X.B.; Liu, M.H.; Hu, H.; Tan, X.J.; Jing, X.B. Apoptosis induced by emodin is associated with alterations of intracellular acidification and reactive oxygen species in EC-109 cells. Biochem. Cell Biol. 2010, 88, 767–774. [Google Scholar] [CrossRef]
- Pinto, M.C.; Dias, D.F.; Del Puerto, H.L.; Martins, A.S.; Teixeira-Carvalho, A.; Martins-Filho, O.A.; Badet, B.; Durand, P.; Alves, R.J.; Souza-Fagundes, E.M. Discovery of cytotoxic and pro-apoptotic compounds against leukemia cells: tert-Butyl-4-[(3-nitrophenoxy)methyl]-2,2-dimethyloxazolidine-3-carboxylate. Life Sci. 2011, 89, 786–794. [Google Scholar] [CrossRef]
- Ni, H.T.; Deeths, M.J.; Mescher, M.F. LFA-1-mediated costimulation of CD8+ T-cell proliferation requires phosphatidylinositol 3-kinase activity. J. Immunol. 2001, 166, 6523–6529. [Google Scholar] [CrossRef]
- Flanagan, M.E.; Blumenkopf, T.A.; Brissette, W.H.; Brown, M.F.; Casavant, J.M.; Shang-Poa, C.; Doty, J.L.; Elliott, E.A.; Fisher, M.B.; Hines, M.; et al. Discovery of CP-690,550: A potent and selective Janus kinase (JAK) inhibitor for the treatment of autoimmune diseases and organ transplant rejection. J. Med. Chem. 2010, 53, 8468–8484. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 2-(1H-Benzimidazol-2-yl)-4,5,6,7-tetrahydro-2H-indazol-3-ol are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Huang, N.; Li, N.; Liu, S.-N.; Li, M.-H.; Li, H.; Luo, X.-Y.; Wang, Y.-T.; Li, L.-M.; Zou, Q.; et al. 2-(1H-Benzimidazol-2-yl)-4,5,6,7-tetrahydro-2H-indazol-3-ol, a Benzimidazole Derivative, Inhibits T Cell Proliferation Involving H+/K+-ATPase Inhibition. Molecules 2014, 19, 17173-17186. https://doi.org/10.3390/molecules191117173
Liu J, Huang N, Li N, Liu S-N, Li M-H, Li H, Luo X-Y, Wang Y-T, Li L-M, Zou Q, et al. 2-(1H-Benzimidazol-2-yl)-4,5,6,7-tetrahydro-2H-indazol-3-ol, a Benzimidazole Derivative, Inhibits T Cell Proliferation Involving H+/K+-ATPase Inhibition. Molecules. 2014; 19(11):17173-17186. https://doi.org/10.3390/molecules191117173
Chicago/Turabian StyleLiu, Jin, Ning Huang, Ning Li, Si-Nian Liu, Min-Hui Li, Hua Li, Xing-Yan Luo, Yan-Tang Wang, Li-Mei Li, Qiang Zou, and et al. 2014. "2-(1H-Benzimidazol-2-yl)-4,5,6,7-tetrahydro-2H-indazol-3-ol, a Benzimidazole Derivative, Inhibits T Cell Proliferation Involving H+/K+-ATPase Inhibition" Molecules 19, no. 11: 17173-17186. https://doi.org/10.3390/molecules191117173




