New Developments in Spin Labels for Pulsed Dipolar EPR
Abstract
:1. Introduction
2. Nitroxides
2.1. Nitroxides in the Literature
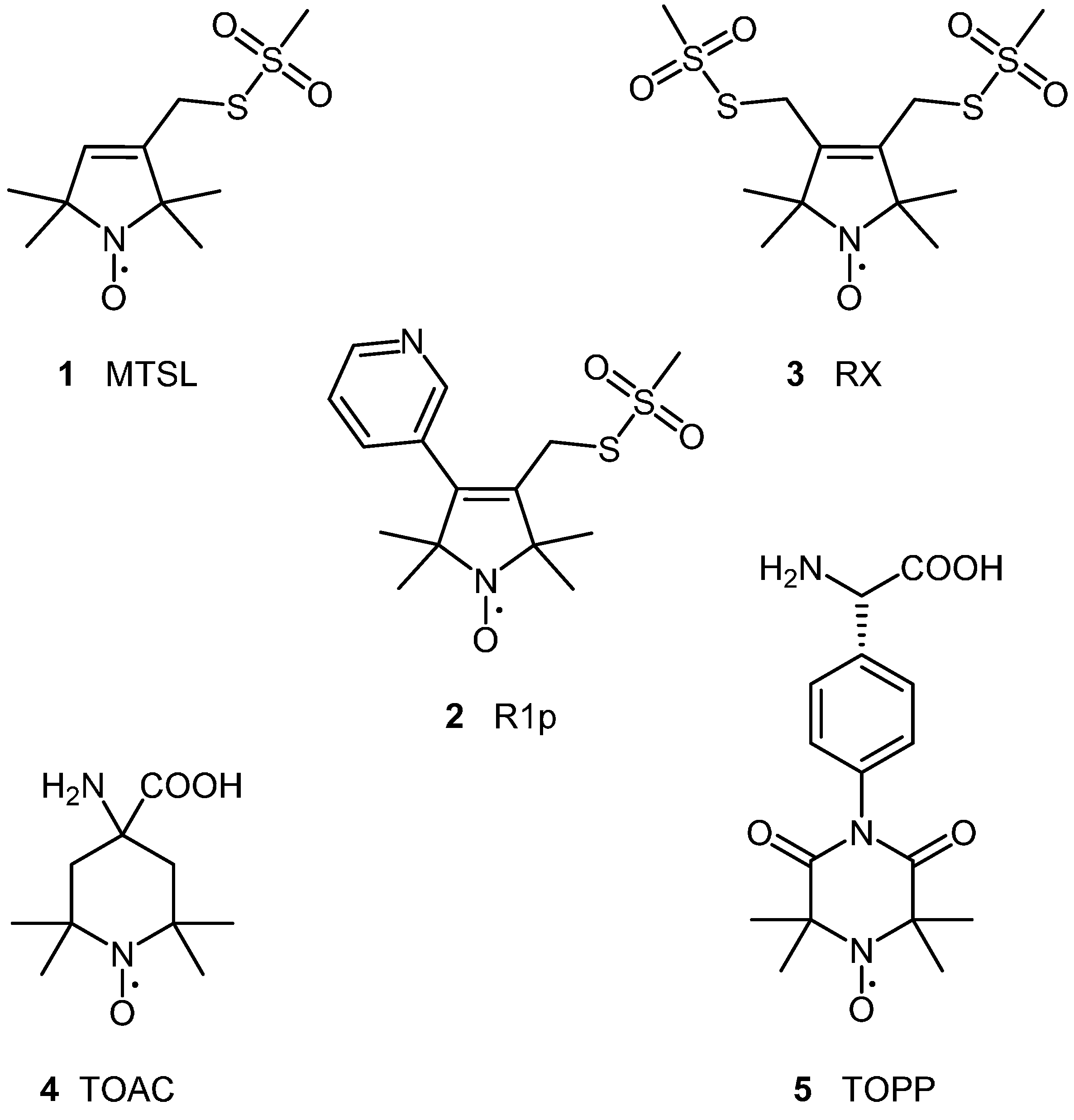
2.1.1. Spin Labelling of Amino Acids
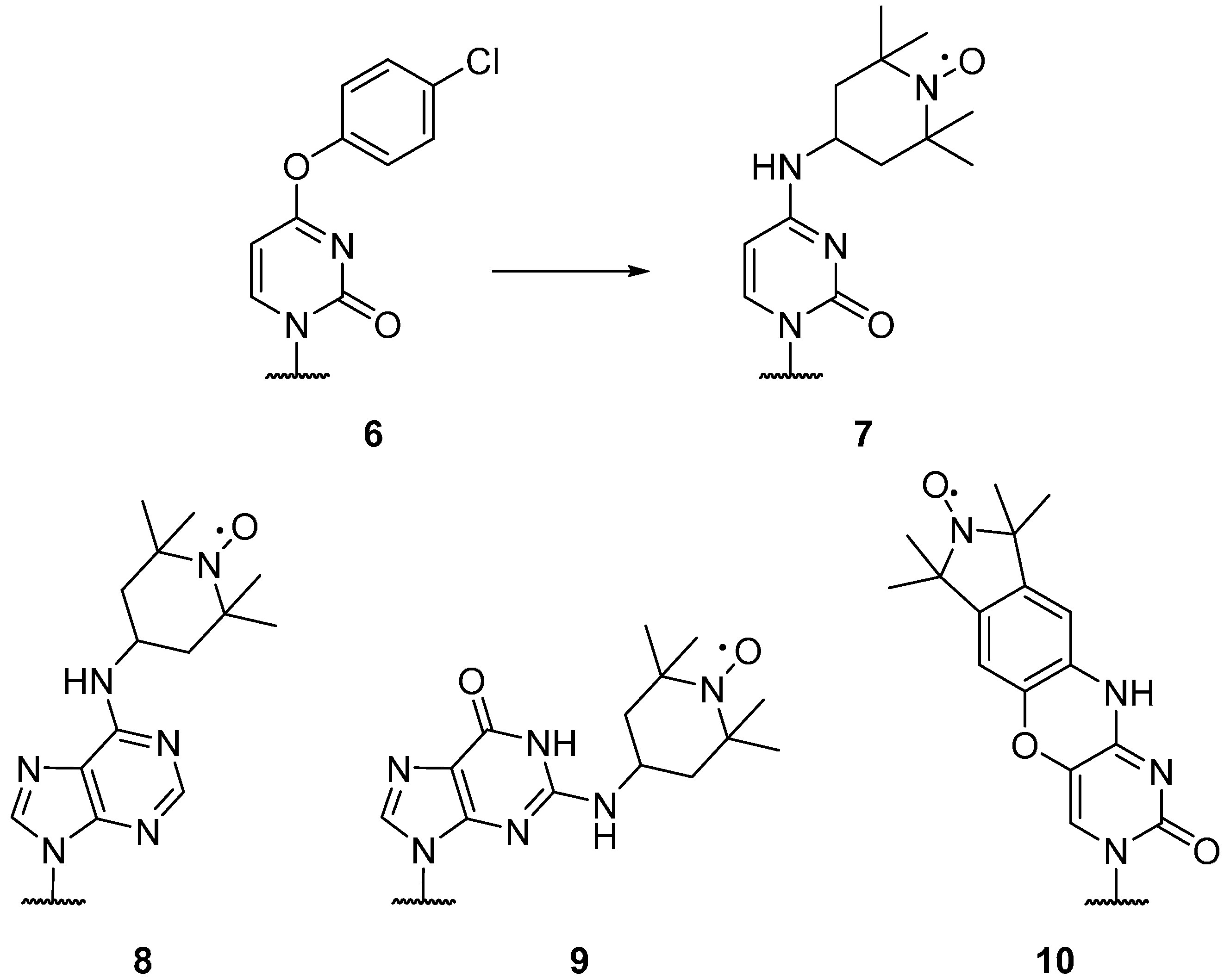
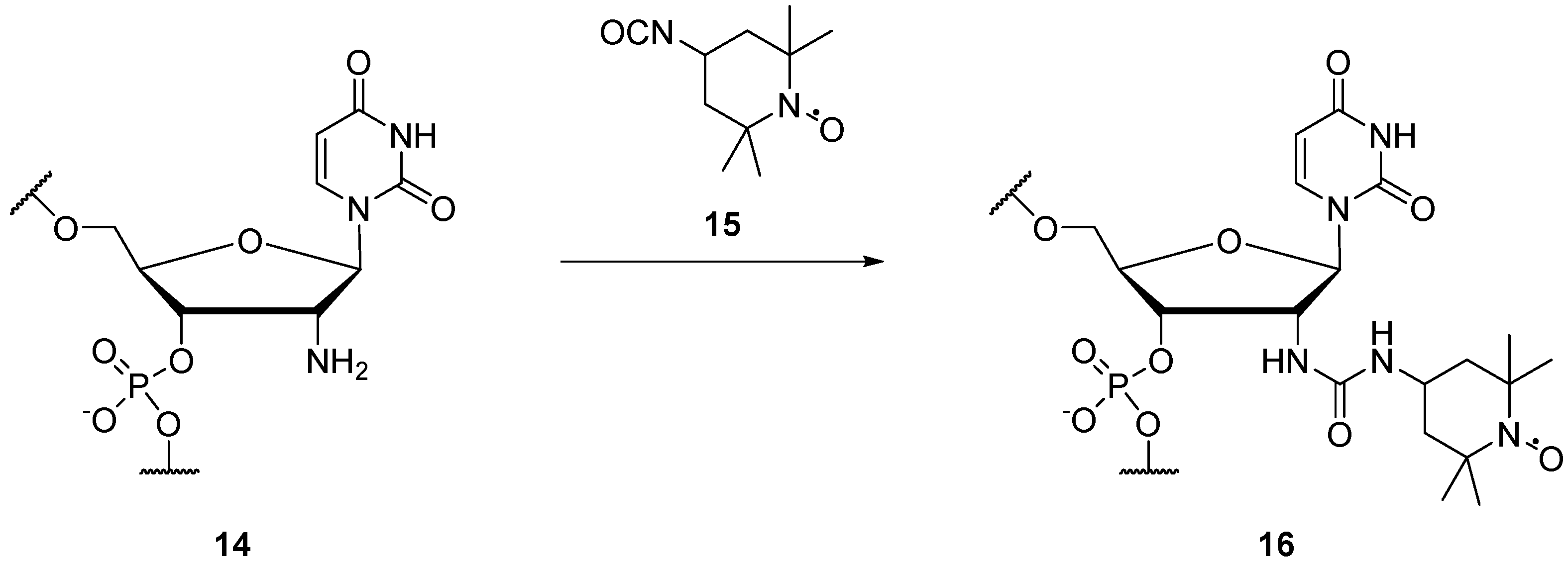
2.1.2. Spin Labels for DNA and RNA


2.2. Design Philosophy
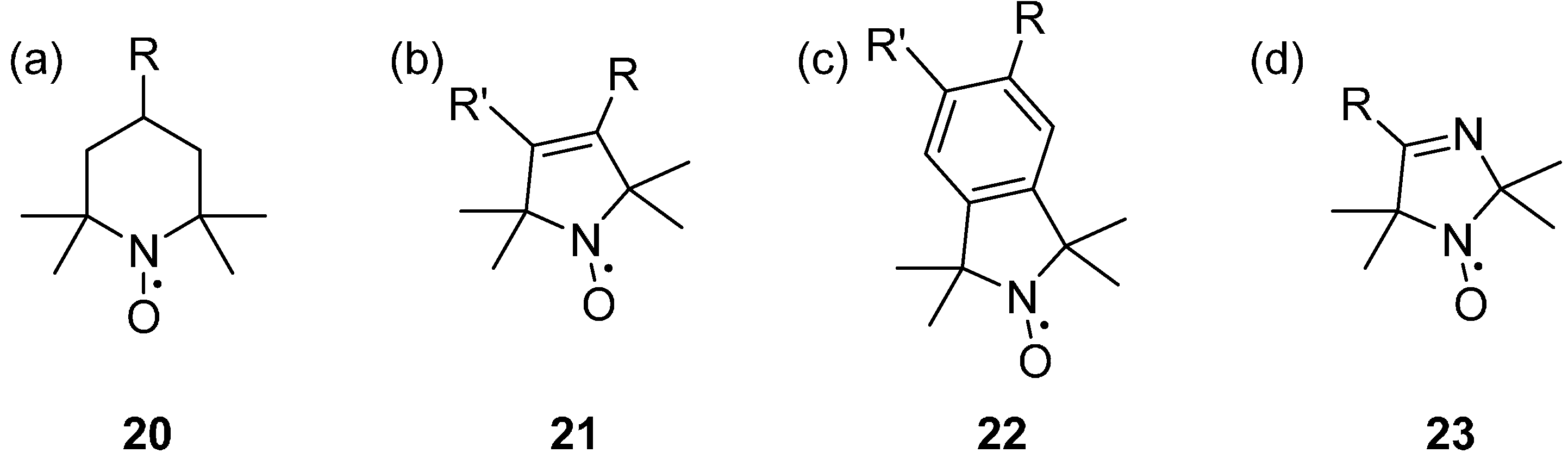
2.2.1. Parent Ring Structures
2.2.2. Alternatives to Methanethiosulfonate Linkages
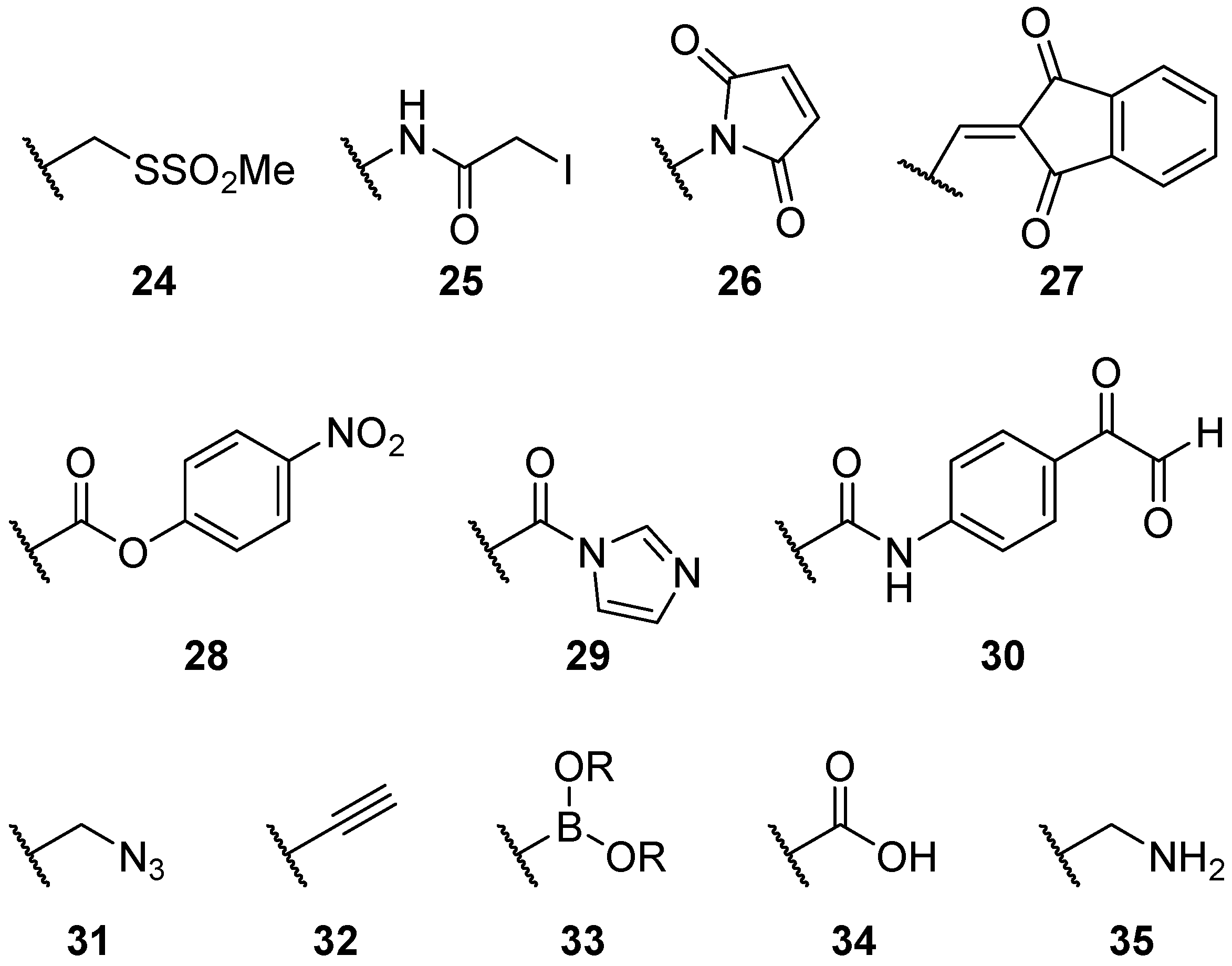
2.2.3. Steric Groups
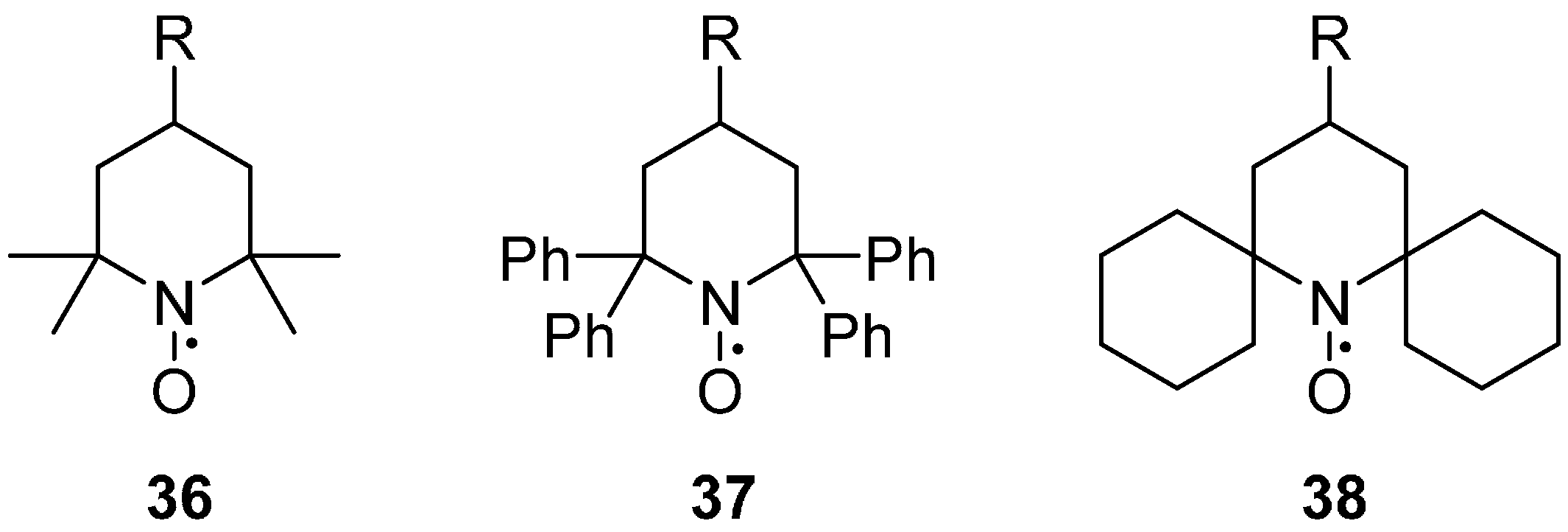
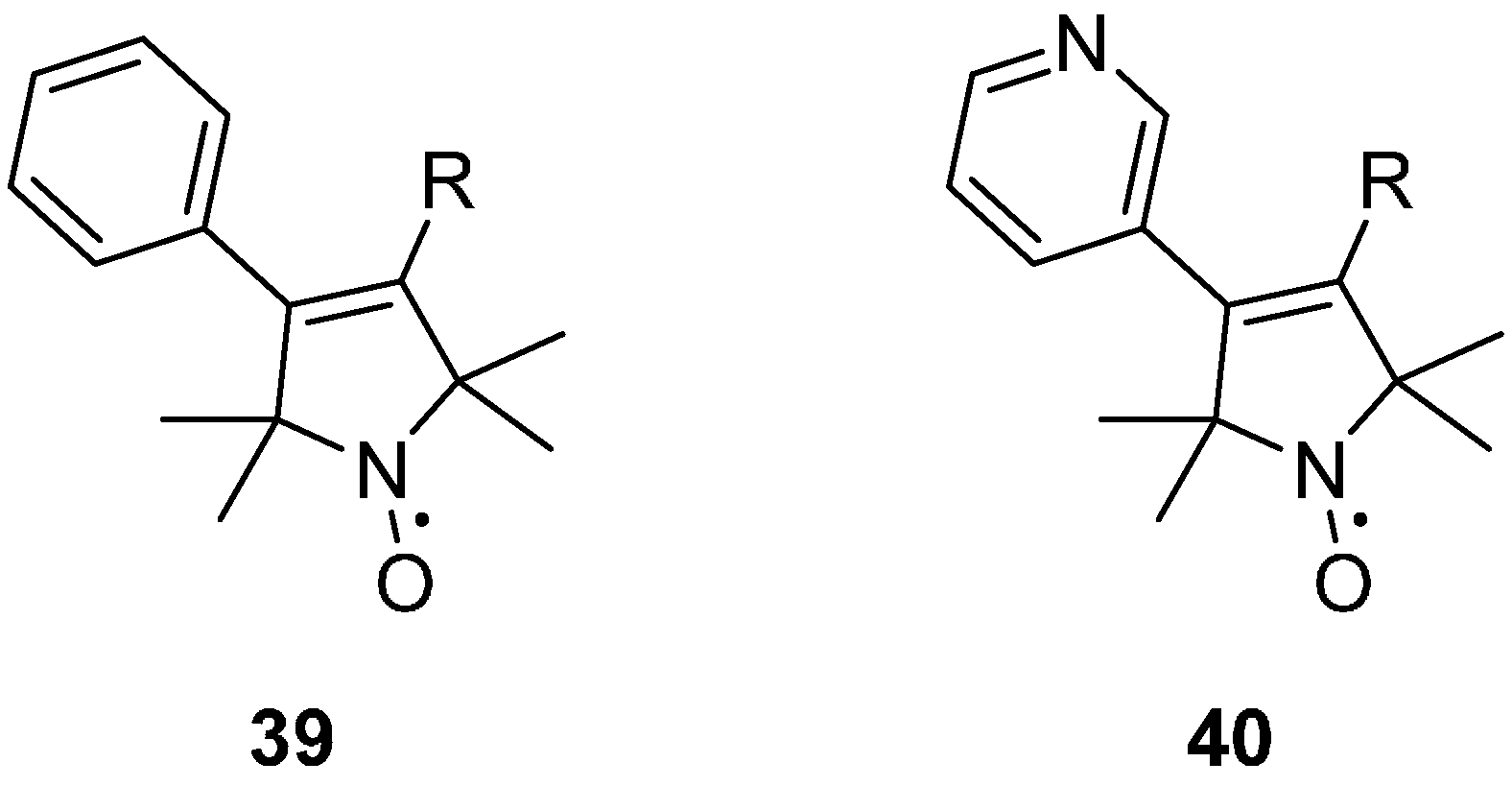
2.2.4. Isotope Effects
3. Carbon Centred Spin Labels
Trityl Radicals
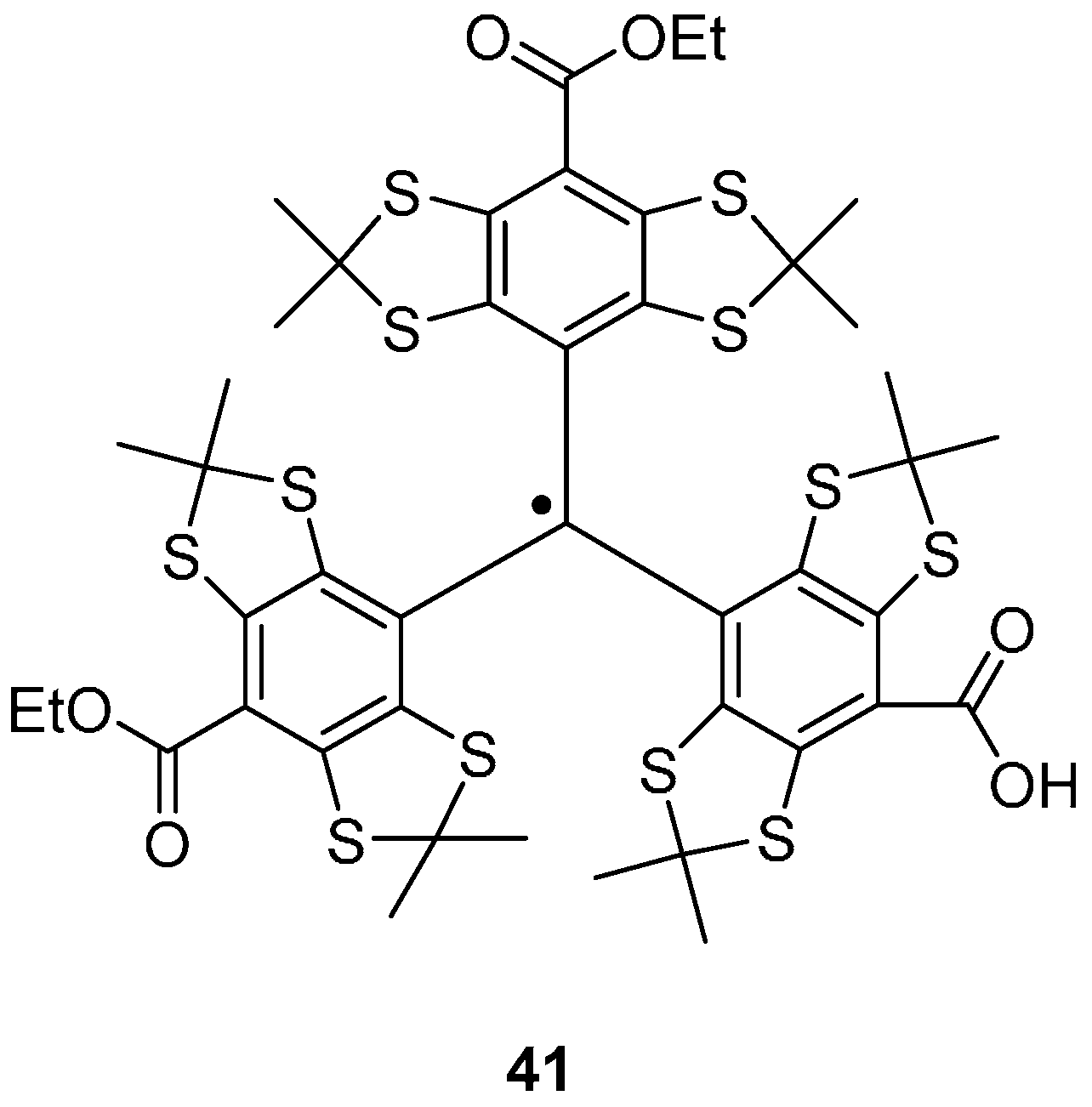

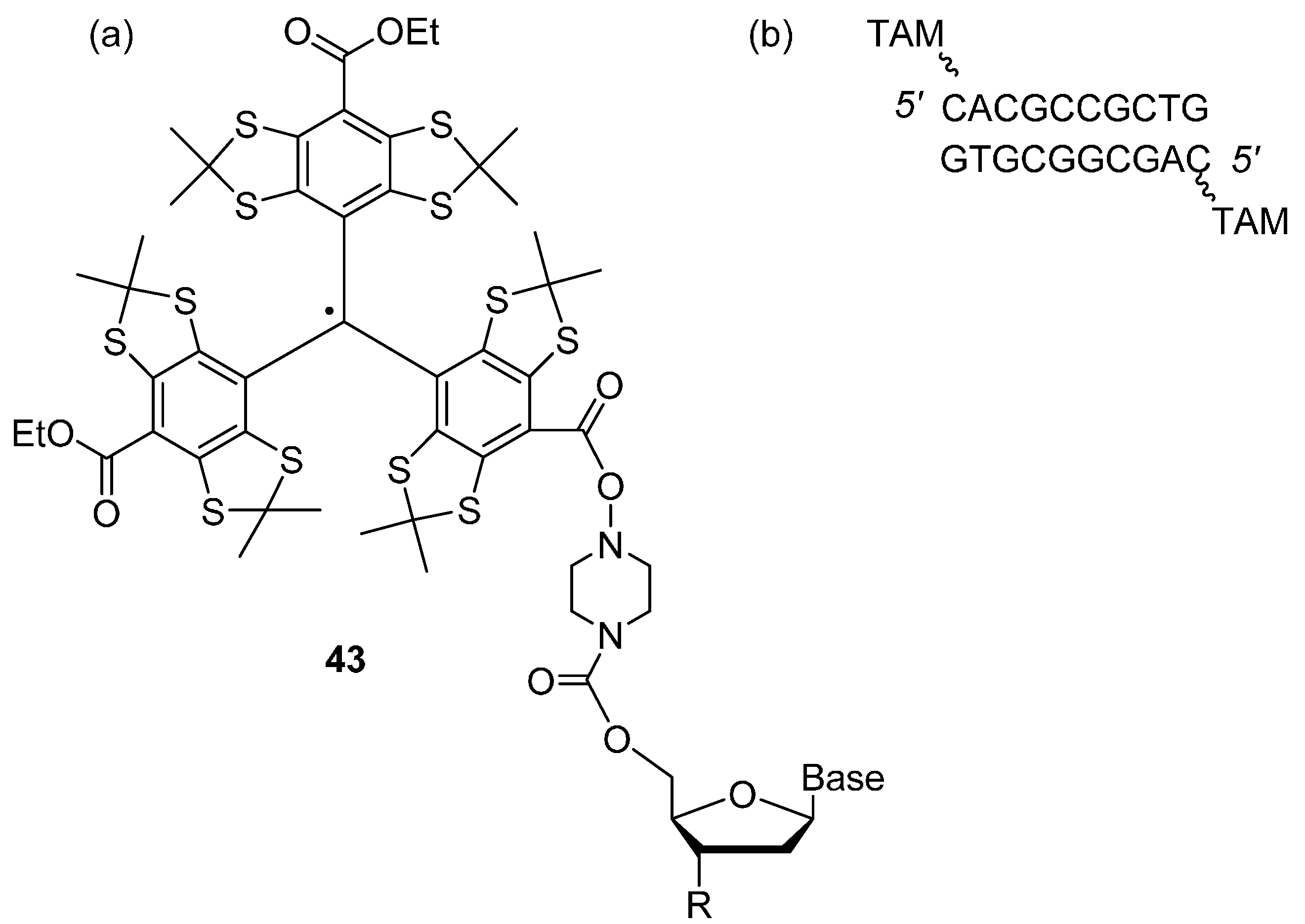
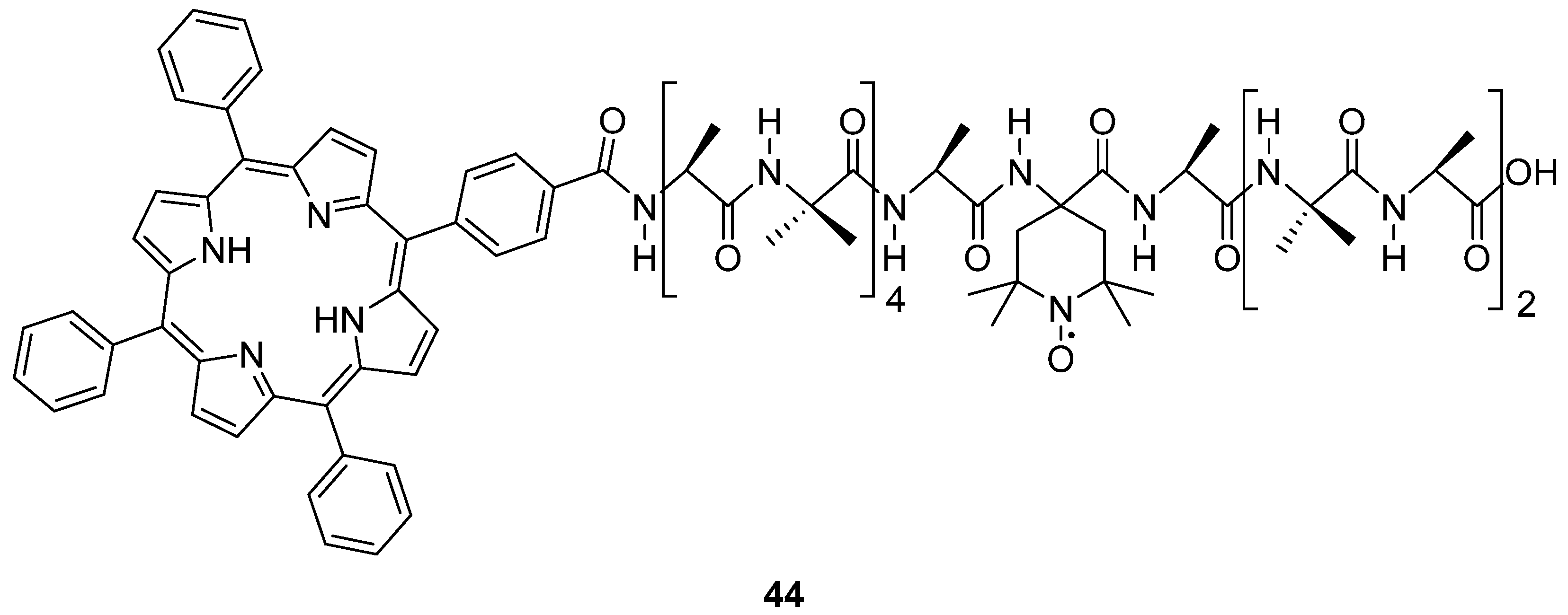
4. Photo-Excited Triplet States
5. Transition Metals
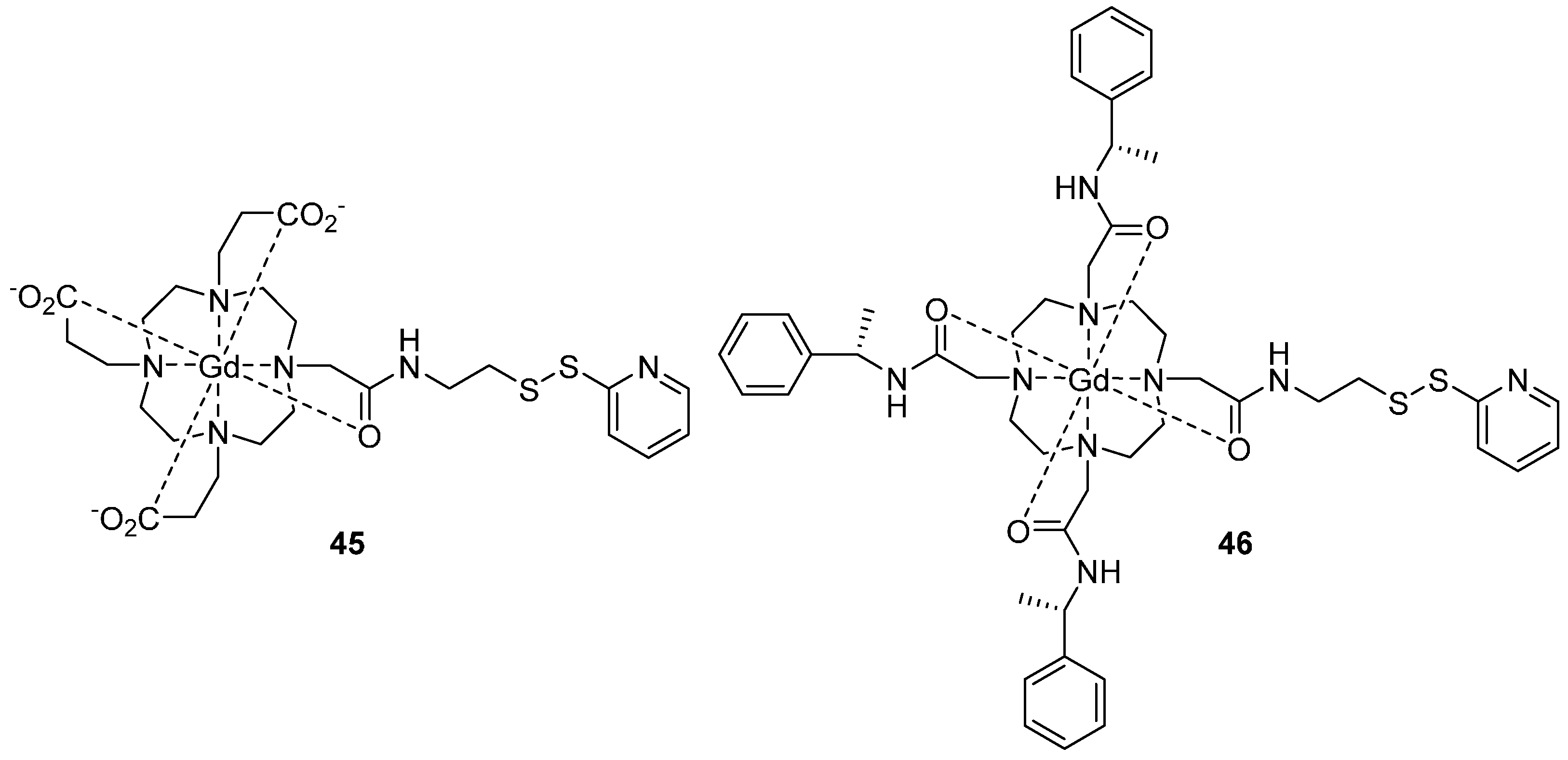
5.1. Gadolinium (III)

5.2. Copper (II)/Nickel (II) Porphyrin/Nitroxide Systems
6. Expression in Cells
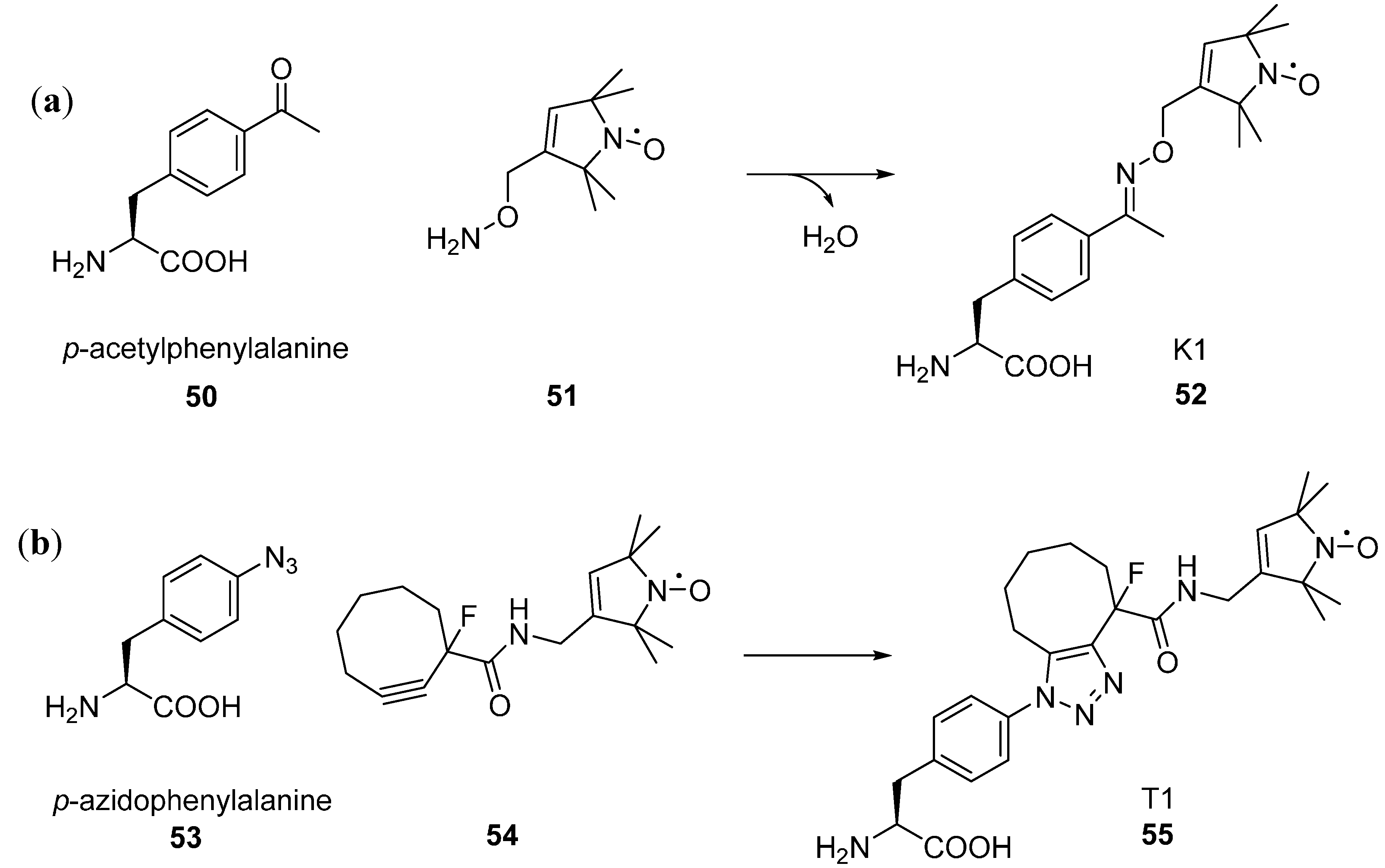
6.1. Introducing Spin Labels via Unnatural Amino Acids
6.1.1. Unnatural Amino Acids with Paramagnetic Centres

6.1.2. Unnatural Amino Acids with Orthogonal Chemical Reactivity
7. In-Cell EPR
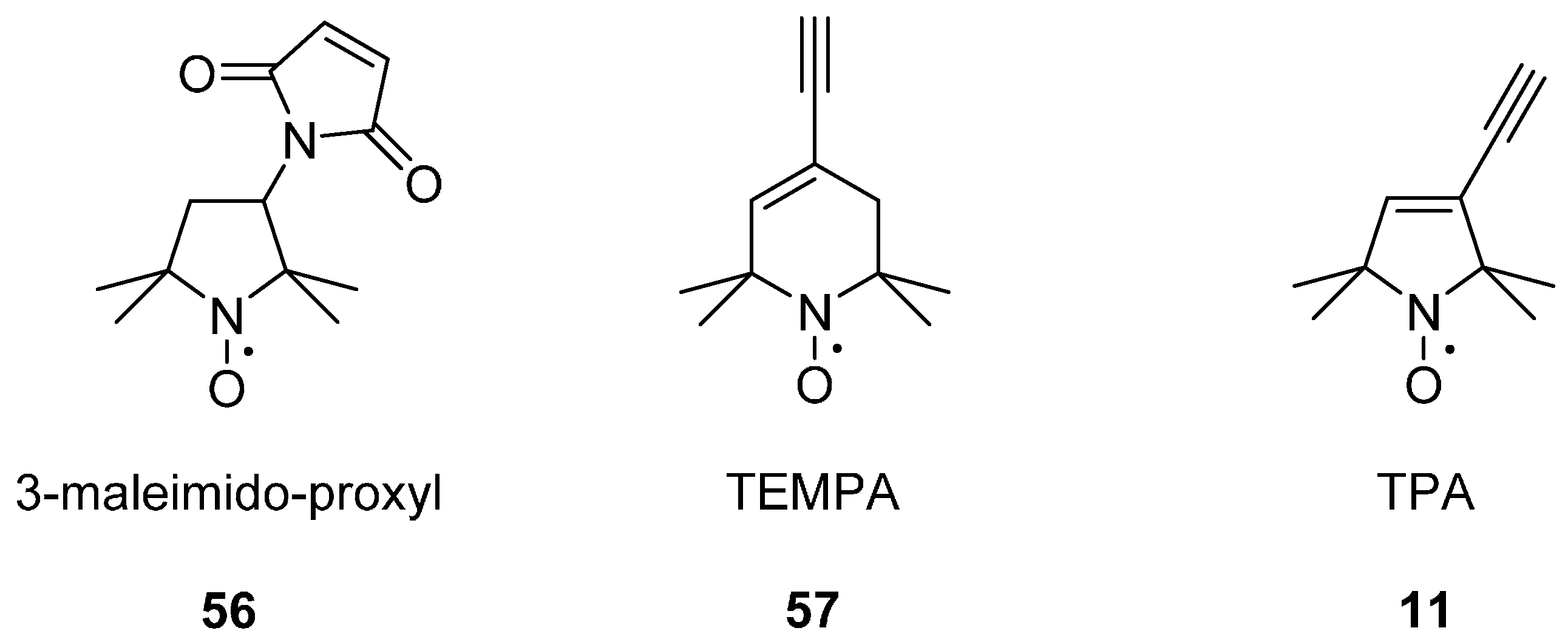
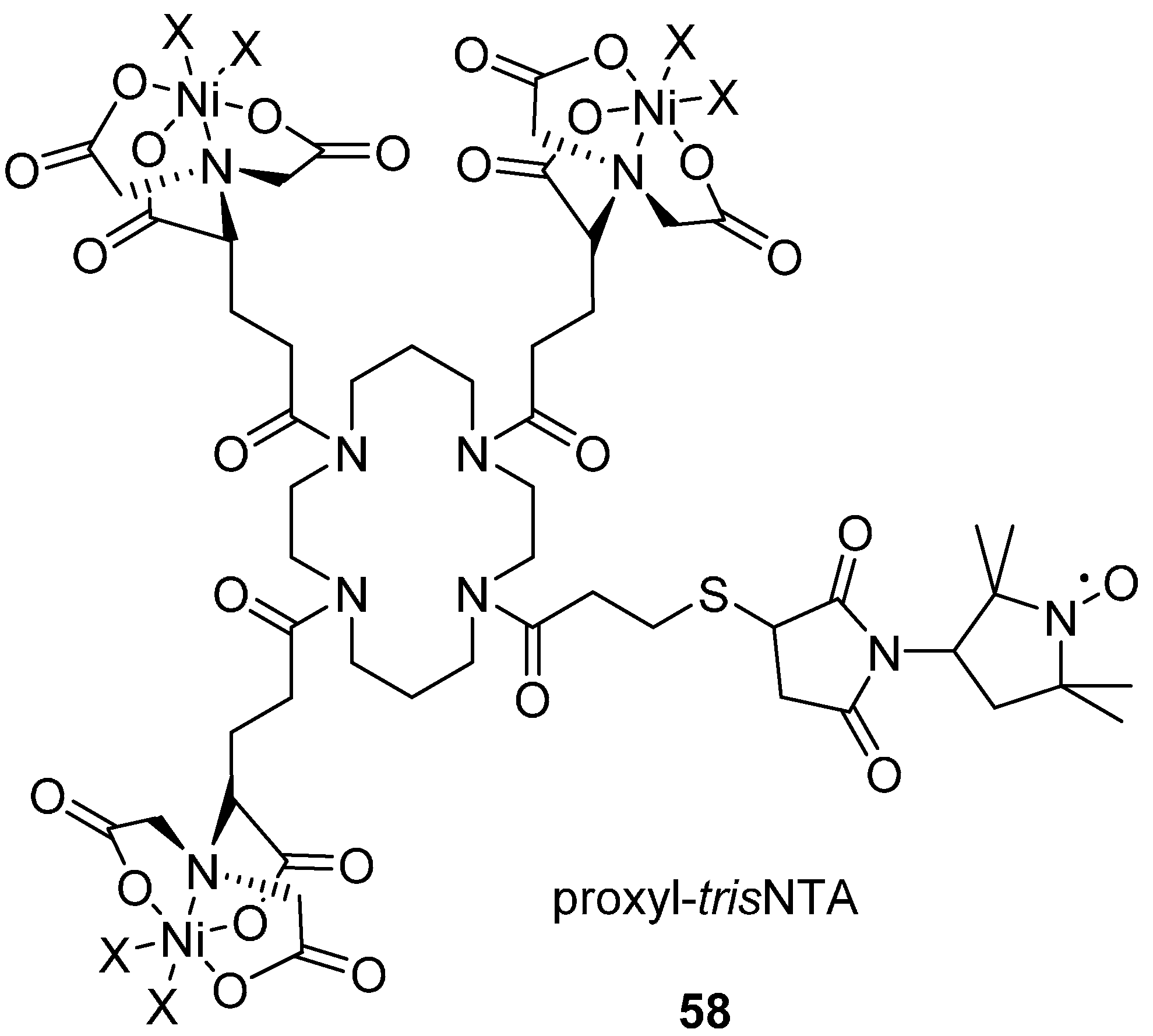
In Situ His Tag Labelling
8. Conclusions
Acknowledgments
Conflicts of Interest
References
- Schweiger, A. Principles of pulse electron paramagnetic resonance; Oxford University Press: Oxford, UK, 2001. [Google Scholar]
- Bowman, M.K. Pulsed Electron Paramagnetic Resonance. In Electron Paramagnetic Resonance: A Practitioner’s Toolkit; Brustolon, M., Giamello, E., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; pp. 159–194. [Google Scholar]
- Berliner, L.J.; Eaton, G.R.; Eaton, S.S. Distance Measurements in Biological Systems by EPR; Kluwer Academic: New York, NY, USA, 2002. [Google Scholar]
- Borbat, P.P.; Freed, J.H. Pulse Dipolar Electron Spin Resonance: Distance Measurements. In Structural Information from Spin-Labels and Intrinsic Paramagnetic Centres in the Biosciences; Timmel, C.R., Harmer, J.R., Eds.; Structure and Bonding; Springer: Berlin, Germany, 2013; pp. 1–82. [Google Scholar]
- Milov, A.D.; Salikhov, K.M.; Shchirov, M.D. Application of ENDOR in electron-spin echo for paramagnetic center space distribution in solids. Fiz. Tverd. Tela 1981, 23, 975–982. [Google Scholar]
- Milov, A.D.; Ponomarev, A.B.; Tsvetkov, Y.D. Electron-electron double resonance in electron spin echo: Model biradical systems and the sensitized photolysis of decalin. Chem. Phys. Lett. 1984, 110, 67–72. [Google Scholar] [CrossRef]
- Pannier, M.; Veit, S.; Godt, A.; Jeschke, G.; Spiess, H.W. Dead-time free measurement of dipole–dipole interactions between electron spins. J. Magn. Reson. 2011, 213, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Ward, R.; Bowman, A.; Sozudogru, E.; El-Mkami, H.; Owen-Hughes, T.; Norman, D.G. EPR distance measurements in deuterated proteins. J. Magn. Reson. San Diego Calif. 1997 2010, 207, 164–167. [Google Scholar] [CrossRef]
- Jeschke, G. DEER distance measurements on proteins. Annu. Rev. Phys. Chem. 2012, 63, 419–446. [Google Scholar] [CrossRef] [PubMed]
- Larsen, R.G.; Singel, D.J. Double electron—electron resonance spin-echo modulation—spectroscopic measurement of electron-spin pair separations in orientationally disordered solids. J. Chem. Phys. 1993, 98, 5134–5146. [Google Scholar] [CrossRef]
- Denysenkov, V.P.; Prisner, T.F.; Stubbe, J.; Bennati, M. High-field pulsed electron-electron double resonance spectroscopy to determine the orientation of the tyrosyl radicals in ribonucleotide reductase. Proc. Natl. Acad. Sci. USA 2006, 103, 13386–13390. [Google Scholar] [CrossRef] [PubMed]
- Bowen, A.M.; Tait, C.E.; Timmel, C.R.; Harmer, J.R. Orientation-Selective DEER Using Rigid Spin Labels, Cofactors, Metals, and Clusters. In Structural Information from Spin-Labels and Intrinsic Paramagnetic Centres in the Biosciences; Timmel, C.R., Harmer, J.R., Eds.; Structure and Bonding; Springer: Berlin, Germany, 2013; Volume 152, pp. 283–327. [Google Scholar]
- Saxena, S.; Freed, J.H. Double quantum two-dimensional Fourier transform electron spin resonance: Distance measurements. Chem. Phys. Lett. 1996, 251, 102–110. [Google Scholar] [CrossRef]
- Saxena, S.; Freed, J.H. Theory of double quantum two-dimensional electron spin resonance with application to distance measurements. J. Chem. Phys. 1997, 107, 1317–1340. [Google Scholar] [CrossRef]
- Borbat, P.P.; Freed, J.H. Multiple-quantum ESR and distance measurements. Chem. Phys. Lett. 1999, 313, 145–154. [Google Scholar] [CrossRef]
- Borbat, P.P.; Freed, J.H. Pros and cons of pulse dipolar ESR: DQC and DEER. EPR Newsl. 2007, 21–33. [Google Scholar]
- Kay, C.W.M.; Elsässer, C.; Bittl, R.; Farrell, S.R.; Thorpe, C. Determination of the distance between the two neutral flavin radicals in augmenter of liver regeneration by pulsed ELDOR. J. Am. Chem. Soc. 2006, 128, 76–77. [Google Scholar] [CrossRef] [PubMed]
- Elsässer, C.; Brecht, M.; Bittl, R. Pulsed electron-electron double resonance on multinuclear metal clusters: Assignment of spin projection factors based on the dipolar interaction. J. Am. Chem. Soc. 2002, 124, 12606–12611. [Google Scholar] [CrossRef] [PubMed]
- Bennati, M.; Weber, A.; Antonic, J.; Perlstein, D.L.; Robblee, J.; Stubbe, J. Pulsed ELDOR spectroscopy measures the distance between the two tyrosyl radicals in the R2 subunit of the E. coli ribonucleotide reductase. J. Am. Chem. Soc. 2003, 125, 14988–14989. [Google Scholar] [CrossRef] [PubMed]
- Fielding, A.J.; Brodhun, F.; Koch, C.; Pievo, R.; Denysenkov, V.; Feussner, I.; Bennati, M. Multifrequency electron paramagnetic resonance characterization of PpoA, a CYP450 fusion protein that catalyzes fatty acid dioxygenation. J. Am. Chem. Soc. 2011, 133, 9052–9062. [Google Scholar] [CrossRef] [PubMed]
- Yulikov, M.; Lueders, P.; Warsi, M.F.; Chechik, V.; Jeschke, G. Distance measurements in Au nanoparticles functionalized with nitroxide radicals and Gd(3+)-DTPA chelate complexes. Phys. Chem. Chem. Phys. PCCP 2012, 14, 10732–10746. [Google Scholar] [CrossRef]
- Jeschke, G. Determination of the nanostructure of polymer materials by electron paramagnetic resonance spectroscopy. Macromol. Rapid Commun. 2002, 23, 227–246. [Google Scholar] [CrossRef]
- Bordignon, E.; Steinhoff, H.-J. Membrane Protein Structure and Dynamics Studied by Site-Directed Spin-Labeling ESR. In ESR Spectroscopy in Membrane Biophysics; Biological Magnetic Resonance; Springer: New York, NY, USA, 2007; Volume 27, pp. 129–164. [Google Scholar]
- Hubbell, W.L.; López, C.J.; Altenbach, C.; Yang, Z. Technological advances in site-directed spin labeling of proteins. Curr. Opin. Struct. Biol. 2013, 23, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, G. Conformational dynamics and distribution of nitroxide spin labels. Prog. Nucl. Magn. Reson. Spectrosc. 2013, 72, 42–60. [Google Scholar] [CrossRef] [PubMed]
- Hideg, K.; Kalai, T.; Sar, C.P. Recent results in chemistry and biology of nitroxides. J. Heterocycl. Chem. 2005, 42, 437–450. [Google Scholar] [CrossRef]
- Merbouh, N.; Bobbitt, J.M.; Brückner, C. Preperation of tetramethylpiperdine-1-oxoammonium salts and their use as oxidants in organic chemistry, a review. Org. Prep. Proced. Int. 2004, 36, 1–31. [Google Scholar] [CrossRef]
- Hawker, C.J.; Bosman, A.W.; Harth, E. New polymer synthesis by nitroxide mediated living radical polymerizations. Chem. Rev. 2001, 101, 3661–3688. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.; Kalyanaraman, B.; Hyde, J.S. Trapping of nitric oxide by nitronyl nitroxides: An electron spin resonance investigation. Biochem. Biophys. Res. Commun. 1993, 192, 926–934. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Bottle, S.; Khan, N.; Grinberg, O.; Reid, D.; Micallef, A.; Swartz, H. Development of isoindoline nitroxides for EPR oximetry in viable systems. Appl. Magn. Reson. 2002, 22, 357–368. [Google Scholar] [CrossRef]
- Rozantsev, E.G.; Ulrich, H. Reactions of Radicals Not Involving the Free Valences. In Free Nitroxyl Radicals; Ulrich, H., Ed.; Springer: New York, NY, USA, 1970; pp. 53–66. [Google Scholar]
- Brik, M.-E. Chemistry of persistent free bi- and polyradicals. Heterocycles 1995, 41, 2827–2873. [Google Scholar] [CrossRef]
- Naik, N.; Braslau, R. Synthesis and applications of optically active nitroxides. Tetrahedron 1998, 54, 667–696. [Google Scholar] [CrossRef]
- Sale, K.; Song, L.; Liu, Y.-S.; Perozo, E.; Fajer, P. Explicit treatment of spin labels in modeling of distance constraints from dipolar EPR and DEER. J. Am. Chem. Soc. 2005, 127, 9334–9335. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, T.F.; McGoff, M.S.; Sengupta, I.; Jaroniec, C.P.; Horne, W.S.; Saxena, S. High-resolution structure of a protein spin-label in a solvent-exposed β-sheet and comparison with DEER spectroscopy. Biochemistry 2012, 51, 6350–6359. [Google Scholar] [CrossRef] [PubMed]
- Polyhach, Y.; Bordignon, E.; Jeschke, G. Rotamer libraries of spin labelled cysteines for protein studies. Phys. Chem. Chem. Phys. 2011, 13, 2356–2366. [Google Scholar] [CrossRef] [PubMed]
- Sarver, J.L.; Townsend, J.E.; Rajapakse, G.; Jen-Jacobson, L.; Saxena, S. Simulating the dynamics and orientations of spin-labeled side chains in a protein-DNA complex. J. Phys. Chem. B 2012, 116, 4024–4033. [Google Scholar] [CrossRef] [PubMed]
- Klose, D.; Klare, J.P.; Grohmann, D.; Kay, C.W.M.; Werner, F.; Steinhoff, H.-J. Simulation vs. Reality: A Comparison of In Silico Distance Predictions with DEER and FRET Measurements. PLoS One 2012, 7, e39492. [Google Scholar] [CrossRef] [PubMed]
- Fajer, P.G.; Brown, L.; Song, L. Practical Pulsed Dipolar ESR (DEER). In ESR Spectroscopy in Membrane Biophysics; Hemminga, M.A., Berliner, L.J., Eds.; Biological Magnetic Resonance; Springer: New York, NY, USA, 2007; Volume 27, pp. 95–128. [Google Scholar]
- Islam, S.M.; Stein, R.A.; Mchaourab, H.S.; Roux, B. Structural refinement from restrained-ensemble simulations based on EPR/DEER data: Application to T4 lysozyme. J. Phys. Chem. B 2013, 117, 4740–4754. [Google Scholar] [CrossRef] [PubMed]
- Hubbell, W.L.; Gross, A.; Langen, R.; Lietzow, M.A. Recent advances in site-directed spin labeling of proteins. Curr. Opin. Struct. Biol. 1998, 8, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Fawzi, N.L.; Fleissner, M.R.; Anthis, N.J.; Kálai, T.; Hideg, K.; Hubbell, W.L.; Clore, G.M. A rigid disulfide-linked nitroxide side chain simplifies the quantitative analysis of PRE data. J. Biomol. NMR 2011, 51, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Kálai, T. Synthesis and reactions of a symmetric paramagnetic pyrrolidine diene. Synthesis 1999, 1999, 973–980. [Google Scholar] [CrossRef]
- Fleissner, M.R.; Bridges, M.D.; Brooks, E.K.; Cascio, D.; Kálai, T.; Hideg, K.; Hubbell, W.L. Structure and dynamics of a conformationally constrained nitroxide side chain and applications in EPR spectroscopy. Proc. Natl. Acad. Sci. USA 2011, 108, 16241–16246. [Google Scholar] [CrossRef] [PubMed]
- Shelke, S.A.; Sigurdsson, S.T. Site-Directed Nitroxide Spin Labeling of Biopolymers. In Structural Information from Spin-Labels and Intrinsic Paramagnetic Centres in the Biosciences; Timmel, C.R., Harmer, J.R., Eds.; Structure and Bonding; Springer: Berlin/Heidelberg, Germany, 2013; Volume 152, pp. 121–162. [Google Scholar]
- Schreier, S.; Bozelli, J.C.; Marín, N.; Vieira, R.F.F.; Nakaie, C.R. The spin label amino acid TOAC and its uses in studies of peptides: Chemical, physicochemical, spectroscopic, and conformational aspects. Biophys. Rev. 2012, 4, 45–66. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, M.; Barbosa, S.R.; Poletti, E.F.; Zukerman-Schpector, J.; Marchetto, R.; Schreier, S.; Paiva, A.C.; Nakaie, C.R. Fmoc-POAC: [(9-fluorenylmethyloxycarbonyl)-2,2,5,5-tetramethylpyrrolidine-N-oxyl-3-amino-4-carboxylic acid]: A novel protected spin labeled β-amino acid for peptide and protein chemistry. Chem. Pharm. Bull. 2001, 49, 1027–1029. [Google Scholar] [CrossRef] [PubMed]
- Marsh, D.; Jost, M.; Peggion, C.; Toniolo, C. TOAC spin labels in the backbone of alamethicin: EPR studies in lipid membranes. Biophys. J. 2007, 92, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Stoller, S.; Sicoli, G.; Baranova, T.Y.; Bennati, M.; Diederichsen, U. TOPP: A novel nitroxide-labeled amino acid for EPR distance measurements. Angew. Chem. Int. Ed. 2011, 50, 9743–9746. [Google Scholar] [CrossRef]
- Reginsson, G.W.; Schiemann, O. Spin labeling of DNA and RNA. In Encyclopedia of Biophysics; Roberts, G.C.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 2429–2431. [Google Scholar]
- Allerson, C.R.; Chen, S.L.; Verdine, G.L. A chemical method for site-specific modification of RNA: The convertible nucleoside approach. J. Am. Chem. Soc. 1997, 119, 7423–7433. [Google Scholar] [CrossRef]
- Sicoli, G.; Wachowius, F.; Bennati, M.; Höbartner, C. Probing secondary structures of spin-labeled RNA by pulsed EPR spectroscopy. Angew. Chem. Int. Ed. 2010, 49, 6443–6447. [Google Scholar] [CrossRef]
- Barhate, N.; Cekan, P.; Massey, A.P.; Sigurdsson, S.T. A nucleoside that contains a rigid nitroxide spin label: A fluorophore in disguise. Angew. Chem. Int. Ed. 2007, 46, 2655–2658. [Google Scholar] [CrossRef]
- Höbartner, C.; Sicoli, G.; Wachowius, F.; Gophane, D.B.; Sigurdsson, S.T. Synthesis and Characterization of RNA Containing a Rigid and Nonperturbing Cytidine-Derived Spin Label. J. Org. Chem. 2012, 77, 7749–7754. [Google Scholar] [CrossRef] [PubMed]
- Gannett, P.M.; Darian, E.; Powell, J.H.; Johnson, E.M. A short procedure for synthesis of 4-ethynyl-2,2,6,6-tetramethyl-3,4-dehydro-piperidine-1-oxyl nitroxide. Synth. Commun. 2001, 31, 2137–2141. [Google Scholar] [CrossRef]
- Piton, N.; Mu, Y.; Stock, G.; Prisner, T.F.; Schiemann, O.; Engels, J.W. Base-specific spin-labeling of RNA for structure determination. Nucleic Acids Res. 2007, 35, 3128–3143. [Google Scholar] [CrossRef] [PubMed]
- Krstić, I.; Hänsel, R.; Romainczyk, O.; Engels, J.W.; Dötsch, V.; Prisner, T.F. Long-range distance measurements on nucleic acids in cells by pulsed EPR spectroscopy. Angew. Chem. 2011, 123, 5176–5180. [Google Scholar] [CrossRef]
- Azarkh, M.; Okle, O.; Singh, V.; Seemann, I.T.; Hartig, J.S.; Dietrich, D.R.; Drescher, M. Long-range distance determination in a DNA model system inside Xenopus laevis oocytes by in-cell spin-label EPR. ChemBioChem 2011, 12, 1992–1995. [Google Scholar] [CrossRef] [PubMed]
- Azarkh, M.; Okle, O.; Eyring, P.; Dietrich, D.R.; Drescher, M. Evaluation of spin labels for in-cell EPR by analysis of nitroxide reduction in cell extract of Xenopus laevis oocytes. J. Magn. Reson. 2011, 212, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.; Varani, G. A new method to detect long-range protein-RNA contacts: NMR detection of electron-proton relaxation induced by nitroxide spin-labeled RNA. J. Am. Chem. Soc. 1998, 120, 10992–10993. [Google Scholar] [CrossRef]
- Duss, O.; Yulikov, M.; Jeschke, G.; Allain, F.H.-T. EPR-aided approach for solution structure determination of large RNAs or protein-RNA complexes. Nat. Commun. 2014. [Google Scholar] [CrossRef]
- Ding, P.; Wunnicke, D.; Steinhoff, H.-J.; Seela, F. Site-directed spin-labeling of DNA by the azide-alkyne “click” reaction: nanometer distance measurements on 7-deaza-2'-deoxyadenosine and 2'-deoxyuridine nitroxide conjugates spatially separated or linked to a “dA-dT” base pair. Chem. Weinh. Bergstr. Ger. 2010, 16, 14385–14396. [Google Scholar]
- Jakobsen, U.; Shelke, S.A.; Vogel, S.; Sigurdsson, S.T. Site-directed spin-labeling of nucleic acids by click chemistry: Detection of abasic sites in duplex DNA by EPR spectroscopy. J. Am. Chem. Soc. 2010, 132, 10424–10428. [Google Scholar] [CrossRef] [PubMed]
- Edwards, T.E.; Sigurdsson, S.T. Site-specific incorporation of nitroxide spin-labels into 2'-positions of nucleic acids. Nat. Protoc. 2007, 2, 1954–1962. [Google Scholar] [CrossRef] [PubMed]
- Schiemann, O.; Weber, A.; Edwards, T.E.; Prisner, T.F.; Sigurdsson, S.T. Nanometer distance measurements on RNA using PELDOR. J. Am. Chem. Soc. 2003, 125, 3434–3435. [Google Scholar] [CrossRef] [PubMed]
- Qin, P.Z.; Haworth, I.S.; Cai, Q.; Kusnetzow, A.K.; Grant, G.P.G.; Price, E.A.; Sowa, G.Z.; Popova, A.; Herreros, B.; He, H. Measuring nanometer distances in nucleic acids using a sequence-independent nitroxide probe. Nat. Protoc. 2007, 2, 2354–2365. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Kusnetzow, A.K.; Hubbell, W.L.; Haworth, I.S.; Gacho, G.P.C.; van Eps, N.; Hideg, K.; Chambers, E.J.; Qin, P.Z. Site-directed spin labeling measurements of nanometer distances in nucleic acids using a sequence-independent nitroxide probe. Nucleic Acids Res. 2006, 34, 4722–4730. [Google Scholar] [CrossRef] [PubMed]
- Pauly, H.; Rossbach, J. Ueber die bildung von pyrrolin- und pyrrolidin-derivaten aus triacetonamin. Berichte Dtsch. Chem. Ges. 1899, 32, 2000–2014. [Google Scholar] [CrossRef]
- Sosnovsky, G.; Cai, Z. A study of the Favorskii rearrangement with 3-bromo-4-oxo-2,2,6,6-tetramethylpiperidine-1-oxyl. J. Org. Chem. 1995, 60, 3414–3418. [Google Scholar] [CrossRef]
- Zhdanov, R.I. Nitroxyl Radicals and Non-Radical Reactions of Free Radicals. In Bioactive Spin Labels; Zhdanov, R.I., Ed.; Springer: Berlin, Germany, 1992; pp. 23–82. [Google Scholar]
- Couet, W.R.; Brasch, R.C.; Sosnovsky, C.; Lukszo, J.; Prakash, I.; Gnewech, C.T.; Tozer, T.N. Influerce of chemical structure of nitroxyl spin labels on their reduction by ascorbic acid. Tetrahedron 1985, 41, 1165–1172. [Google Scholar] [CrossRef]
- Morris, S.; Sosnovsky, G.; Hui, B.; Huber, C.O.; Rao, N.U.M.; Swartz, H.M. Chemical and electrochemical reduction rates of cyclic nitroxides (nitroxyls). J. Pharm. Sci. 1991, 80, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Bottle, S.E.; Chand, U.; Micallef, A.S. Hydrogen abstraction from unactivated hydrocarbons using a photochemically excited isoindoline nitroxide. Chem. Lett. 1997, 26, 857–858. [Google Scholar] [CrossRef]
- Gillies, D.G.; Sutcliffe, L.H.; Wu, X. NMR determination of EPR hyperfine coupling constants of some 5-(n-alkyl)-1,1,3,3-tetrakis(trideuteriomethyl)isoindolin-2-yloxyls. J. Chem. Soc. Faraday Trans. 1994, 90, 2345–2349. [Google Scholar] [CrossRef]
- Bottle, S.E.; Gillies, D.G.; Micallef, A.S.; Reid, D.A.; Sutcliffe, L.H. ESR measurements of the partitioning of some new spin probes in n-octanol-water. Magn. Reson. Chem. 1999, 37, 730–734. [Google Scholar] [CrossRef]
- Keddie, D.J.; Fairfull-Smith, K.E.; Bottle, S.E. The palladium-catalysed copper-free Sonogashira coupling of isoindoline nitroxides: A convenient route to robust profluorescent carbon-carbon frameworks. Org. Biomol. Chem. 2008, 6, 3135–3143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blinco, J.P.; McMurtrie, J.C.; Bottle, S.E. The first example of an azaphenalene profluorescent nitroxide. Eur. J. Org. Chem. 2007, 2007, 4638–4641. [Google Scholar] [CrossRef]
- Reid, D.A.; Bottle, S.E. The synthesis of water soluble isoindoline nitroxides and a pronitroxide hydroxylamine hydrochloride UV-Vis probe for free radicals. Chem. Commun. 1998, 1907–1908. [Google Scholar]
- Blinco, J.P.; Chalmers, B.A.; Chou, A.; Fairfull-Smith, K.E.; Bottle, S.E. Spin-coated carbon. Chem. Sci. 2013, 4, 3411–3415. [Google Scholar] [CrossRef]
- Khramtsov, V.V. Functional EPR Spectroscopy and Imaging of Nitroxides. In Supramolecular Structure and Function 9; Pifat-Mrzljak, G., Ed.; Springer: Dordrecht, The Netherlands, 2007; pp. 181–208. [Google Scholar]
- Gruian, C.; Vulpoi, A.; Steinhoff, H.-J.; Simon, S. Structural changes of methemoglobin after adsorption on bioactive glass, as a function of surface functionalization and salt concentration. J. Mol. Struct. 2012, 1015, 20–26. [Google Scholar] [CrossRef]
- Griffith, O.H.; McConnell, H.M. A Nitroxide-maleimide Spin Label. Proc. Natl. Acad. Sci. USA 1966, 55, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Hankovszky, H.O.; Hideg, K.; Jerkovich, G. Synthesis of 3-substituted 2,5-dihydro-2,2,5,5-tetramethyl-1H-pyrrol-1-yloxyl radicals, useful for spin-labelling of biomolecules. Synthesis 1989, 1989, 526–529. [Google Scholar] [CrossRef]
- Berliner, L.J.; McConnell, H.M. A spin-labeled substrate for alpha-chymotrypsin. Proc. Natl. Acad. Sci. USA 1966, 55, 708–712. [Google Scholar] [CrossRef] [PubMed]
- Adackaparayil, M.; Smith, J.H. Preparation and reactivity of a new spin label reagent. J. Org. Chem. 1977, 42, 1655–1656. [Google Scholar] [CrossRef]
- Hankovszky, O.H.; Hideg, K.; Goldammer, E.V.; Matuszak, E.; Kolkenbrock, H.; Tschesche, H.; Wenzel, H.R. New nitroxide reagents for the selective spin-labelling at the guanidino moiety of arginine residues in peptides and proteins. BBA-Protein Struct. Mol. Enzymol. 1987, 916, 152–155. [Google Scholar] [CrossRef]
- Lorenzi, M.; Puppo, C.; Lebrun, R.; Lignon, S.; Roubaud, V.; Martinho, M.; Mileo, E.; Tordo, P.; Marque, S.R.A.; Gontero, B.; et al. Tyrosine-targeted spin labeling and EPR spectroscopy: An alternative strategy for studying structural transitions in proteins. Angew. Chem. Int. Ed. 2011, 50, 9108–9111. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click chemistry: Diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. Engl. 2001, 40, 2004–2021. [Google Scholar] [CrossRef] [PubMed]
- Kálai, T.; Hubbell, W.; Hideg, K. Click reactions with nitroxides. Synthesis 2009, 2009, 1336–1340. [Google Scholar] [CrossRef]
- Kálai, T.; Jekő, J.; Hideg, K. Synthesis of a paramagnetic boronic acid as a useful synthetic building block and carbohydrate affinity spin probe. Tetrahedron Lett. 2004, 45, 8395–8398. [Google Scholar] [CrossRef]
- Rajca, A.; Kathirvelu, V.; Roy, S.K.; Pink, M.; Rajca, S.; Sarkar, S.; Eaton, S.S.; Eaton, G.R. A spirocyclohexyl nitroxide amino acid spin label for pulsed EPR spectroscopy distance measurements. Chem. Weinh. Bergstr. Ger. 2010, 16, 5778–5782. [Google Scholar]
- McNulty, J.C.; Thompson, D.A.; Carrasco, M.R.; Millhauser, G.L. Dap-SL: A new site-directed nitroxide spin labeling approach for determining structure and motions in synthesized peptides and proteins. FEBS Lett. 2002, 529, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.-K.; Murali, A.; DeRose, V.J. A distance ruler for RNA using EPR and site-directed spin labeling. Chem. Biol. 2004, 11, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Kálai, T.; Kuppusamy, M.L.; Balog, M.; Selvendiran, K.; Rivera, B.K.; Kuppusamy, P.; Hideg, K. Synthesis of N-substituted 3,5-bis(arylidene)-4-piperidones with high antitumor and antioxidant activity. J. Med. Chem. 2011, 54, 5414–5421. [Google Scholar] [CrossRef] [PubMed]
- Kósa, C.; Danko, M.; Hrdlovič, P. Preparation and spectral characterization of fluorescence probes based on 4-N,N-dimethylamino benzoic acid and sterically hindered amines. J. Fluoresc. 2012, 22, 1371–1381. [Google Scholar] [CrossRef] [PubMed]
- Mravljak, J.; Ojsteršek, T.; Pajk, S.; Sollner Dolenc, M. Coumarin-based dual fluorescent spin-probes. Tetrahedron Lett. 2013, 54, 5236–5238. [Google Scholar] [CrossRef]
- Dzuba, S.A.; Maryasov, A.G.; Salikhov, K.M.; Tsvetkov, Y.D. Superslow rotations of nitroxide radicals studied by pulse EPR spectroscopy. J. Magn. Reson. 1984, 58, 95–117. [Google Scholar]
- Lindgren, M.; Eaton, G.R.; Eaton, S.S.; Jonsson, B.-H.; Hammarström, P.; Svensson, M.; Carlsson, U. Electron spin echo decay as a probe of aminoxyl environment in spin-labeled mutants of human carbonic anhydrase II†. J. Chem. Soc. Perkin Trans. 2 1997, 2549–2554. [Google Scholar]
- Sato, H.; Kathirvelu, V.; Fielding, A.; Blinco, J.P.; Micallef, A.S.; Bottle, S.E.; Eaton, S.S.; Eaton, G.R. Impact of molecular size on electron spin relaxation rates of nitroxyl radicals in glassy solvents between 100 and 300 K. Mol. Phys. 2007, 105, 2137–2151. [Google Scholar] [CrossRef] [Green Version]
- Kathirvelu, V.; Smith, C.; Parks, C.; Mannan, M.A.; Miura, Y.; Takeshita, K.; Eaton, S.S.; Eaton, G.R. Relaxation rates for spirocyclohexyl nitroxyl radicals are suitable for interspin distance measurements at temperatures up to about 125 K. Chem. Commun. 2009, 454–456. [Google Scholar]
- Khan, N.; Blinco, J.P.; Bottle, S.E.; Hosokawa, K.; Swartz, H.M.; Micallef, A.S. The evaluation of new and isotopically labeled isoindoline nitroxides and an azaphenalene nitroxide for EPR oximetry. J. Magn. Reson. 2011, 211, 170–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abe, J.; Ueki, S.; Arata, T.; Nakazawa, S.; Yamauchi, S.; Ohba, Y. Improved sensitivity by isotopic substitution in distance measurements based on double quantum coherence EPR. Appl. Magn. Reson. 2012, 42, 473–485. [Google Scholar] [CrossRef]
- Jeschke, G.; Zimmermann, H.; Godt, A. Isotope selection in distance measurements between nitroxides. J. Magn. Reson. 2006, 180, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Fielding, A.J.; Carl, P.J.; Eaton, G.R.; Eaton, S.S. Multifrequency EPR of four triarylmethyl radicals. Appl. Magn. Reson. 2005, 28, 231–238. [Google Scholar] [CrossRef]
- Owenius, R.; Eaton, G.R.; Eaton, S.S. Frequency (250 MHz to 9.2 GHz) and viscosity dependence of electron spin relaxation of triarylmethyl radicals at room temperature. J. Magn. Reson. 2005, 172, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Liu, Y.; Borbat, P.; Zweier, J.L.; Freed, J.H.; Hubbell, W.L. Pulsed ESR dipolar spectroscopy for distance measurements in immobilized spin labeled proteins in liquid solution. J. Am. Chem. Soc. 2012, 134, 9950–9952. [Google Scholar] [CrossRef] [PubMed]
- Shevelev, G.Y.; Krumkacheva, O.A.; Lomzov, A.A.; Kuzhelev, A.A.; Rogozhnikova, O.Y.; Trukhin, D.V.; Troitskaya, T.I.; Tormyshev, V.M.; Fedin, M.V.; Pyshnyi, D.V.; et al. Physiological-temperature distance measurement in nucleic acid using triarylmethyl-based spin labels and pulsed dipolar EPR spectroscopy. J. Am. Chem. Soc. 2014, 136, 9874–9877. [Google Scholar] [CrossRef] [PubMed]
- Reginsson, G.W.; Kunjir, N.C.; Sigurdsson, S.T.; Schiemann, O. Trityl radicals: Spin labels for nanometer-distance measurements. Chem.-Eur. J. 2012, 18, 13580–13584. [Google Scholar] [CrossRef] [PubMed]
- Reddy, T.J.; Iwama, T.; Halpern, H.J.; Rawal, V.H. General synthesis of persistent trityl radicals for EPR imaging of biological systems. J. Org. Chem. 2002, 67, 4635–4639. [Google Scholar] [CrossRef] [PubMed]
- Bobko, A.A.; Dhimitruka, I.; Zweier, J.L.; Khramtsov, V.V. Trityl radicals as persistent dual function pH and oxygen probes for in vivo electron paramagnetic resonance spectroscopy and imaging: Concept and experiment. J. Am. Chem. Soc. 2007, 129, 7240–7241. [Google Scholar] [CrossRef] [PubMed]
- Grassetti, D.R.; Murray, J.F. Determination of sulfhydryl groups with 2,2'- or 4,4'-dithiodipyridine. Arch. Biochem. Biophys. 1967, 119, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Kunjir, N.C.; Reginsson, G.W.; Schiemann, O.; Sigurdsson, S.T. Measurements of short distances between trityl spin labels with CW EPR, DQC and PELDOR. Phys. Chem. Chem. Phys. PCCP 2013, 15, 19673–19685. [Google Scholar] [CrossRef]
- Di Valentin, M.; Albertini, M.; Zurlo, E.; Gobbo, M.; Carbonera, D. Porphyrin triplet state as a potential spin label for nanometer distance measurements by PELDOR spectroscopy. J. Am. Chem. Soc. 2014, 136, 6582–6585. [Google Scholar] [CrossRef] [PubMed]
- Dolphin, D. The Porphyrins. Volume IV. Physical Chemistry, Part B; Academic Press: New York, NY, USA, 1978. [Google Scholar]
- Lubitz, W.; Lendzian, F.; Bittl, R. Radicals, radical pairs and triplet states in photosynthesis. Acc. Chem. Res. 2002, 35, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.; Ruthstein, S.; Saxena, S. Paramagnetic metal ions in pulsed ESR distance distribution measurements. Acc. Chem. Res. 2014, 47, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Roessler, M.M.; King, M.S.; Robinson, A.J.; Armstrong, F.A.; Harmer, J.; Hirst, J. Direct assignment of EPR spectra to structurally defined iron-sulfur clusters in complex I by double electron-electron resonance. Proc. Natl. Acad. Sci. USA 2010, 107, 1930–1935. [Google Scholar] [CrossRef] [PubMed]
- Bode, B.E.; Plackmeyer, J.; Bolte, M.; Prisner, T.F.; Schiemann, O. PELDOR on an exchange coupled nitroxide copper(II) spin pair. J. Organomet. Chem. 2009, 694, 1172–1179. [Google Scholar] [CrossRef]
- Potapov, A.; Song, Y.; Meade, T.J.; Goldfarb, D.; Astashkin, A.V.; Raitsimring, A. Distance measurements in model bis-Gd(III) complexes with flexible “bridge”. Emulation of biological molecules having flexible structure with Gd(III) labels attached. J. Magn. Reson. 2010, 205, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Potapov, A.; Yagi, H.; Huber, T.; Jergic, S.; Dixon, N.E.; Otting, G.; Goldfarb, D. Nanometer-scale distance measurements in proteins using Gd3+ spin labeling. J. Am. Chem. Soc. 2010, 132, 9040–9048. [Google Scholar] [CrossRef] [PubMed]
- Gordon-Grossman, M.; Kaminker, I.; Gofman, Y.; Shai, Y.; Goldfarb, D. W-Band pulse EPR distance measurements in peptides using Gd3+-dipicolinic acid derivatives as spin labels. Phys. Chem. Chem. Phys. 2011, 13, 10771–10780. [Google Scholar] [CrossRef] [PubMed]
- Yagi, H.; Banerjee, D.; Graham, B.; Huber, T.; Goldfarb, D.; Otting, G. Gadolinium tagging for high-precision measurements of 6 nm distances in protein assemblies by EPR. J. Am. Chem. Soc. 2011, 133, 10418–10421. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Meade, T.J.; Astashkin, A.V.; Klein, E.L.; Enemark, J.H.; Raitsimring, A. Pulsed dipolar spectroscopy distance measurements in biomacromolecules labeled with Gd(III) markers. J. Magn. Reson. 2011, 210, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Goldfarb, D. Gd3+ spin labeling for distance measurements by pulse EPR spectroscopy. Phys. Chem. Chem. Phys. PCCP 2014, 16, 9685–9699. [Google Scholar] [CrossRef]
- Matalon, E.; Huber, T.; Hagelueken, G.; Graham, B.; Frydman, V.; Feintuch, A.; Otting, G.; Goldfarb, D. Gadolinium(III) spin labels for high-sensitivity distance measurements in transmembrane helices. Angew. Chem. Int. Ed. 2013, 52, 11831–11834. [Google Scholar] [CrossRef]
- Graham, B.; Loh, C.T.; Swarbrick, J.D.; Ung, P.; Shin, J.; Yagi, H.; Jia, X.; Chhabra, S.; Barlow, N.; Pintacuda, G.; et al. DOTA-amide lanthanide tag for reliable generation of pseudocontact shifts in protein NMR spectra. Bioconjug. Chem. 2011, 22, 2118–2125. [Google Scholar] [CrossRef] [PubMed]
- Garbuio, L.; Bordignon, E.; Brooks, E.K.; Hubbell, W.L.; Jeschke, G.; Yulikov, M. Orthogonal Spin Labeling and Gd(III)—Nitroxide Distance Measurements on Bacteriophage T4-Lysozyme. J. Phys. Chem. B 2013, 117, 3145–3153. [Google Scholar] [CrossRef] [PubMed]
- Kaminker, I.; Yagi, H.; Huber, T.; Feintuch, A.; Otting, G.; Goldfarb, D. Spectroscopic selection of distance measurements in a protein dimer with mixed nitroxide and Gd3+ spin labels. Phys. Chem. Chem. Phys. PCCP 2012, 14, 4355–4358. [Google Scholar] [CrossRef]
- Joseph, B.; Korkhov, V.M.; Yulikov, M.; Jeschke, G.; Bordignon, E. Conformational cycle of the vitamin B12 ABC importer in liposomes detected by double electron-electron resonance (DEER). J. Biol. Chem. 2014, 289, 3176–3185. [Google Scholar] [CrossRef] [PubMed]
- Kaminker, I.; Tkach, I.; Manukovsky, N.; Huber, T.; Yagi, H.; Otting, G.; Bennati, M.; Goldfarb, D. W-band orientation selective DEER measurements on a Gd3+/nitroxide mixed-labeled protein dimer with a dual mode cavity. J. Magn. Reson. 2013, 227, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Lueders, P.; Jäger, H.; Hemminga, M.A.; Jeschke, G.; Yulikov, M. Distance measurements on orthogonally spin-labeled membrane spanning WALP23 polypeptides. J. Phys. Chem. B 2013, 117, 2061–2068. [Google Scholar] [CrossRef] [PubMed]
- Narr, E.; Godt, A.; Jeschke, G. Selective measurements of a nitroxide-nitroxide separation of 5 nm and a nitroxide-copper separation of 2.5 nm in a terpyridine-based copper(II) complex by pulse EPR spectroscopy. Angew. Chem. Int. Ed. 2002, 41, 3907–3910. [Google Scholar] [CrossRef]
- Becker, J.S.; Saxena, S. Double quantum coherence electron spin resonance on coupled Cu(II)—Cu(II) electron spins. Chem. Phys. Lett. 2005, 414, 248–252. [Google Scholar] [CrossRef]
- Yang, Z.; Ji, M.; Saxena, S. Practical aspects of copper ion-based double electron electron resonance distance measurements. Appl. Magn. Reson. 2010, 39, 487–500. [Google Scholar] [CrossRef]
- Van Wonderen, J.H.; Kostrz, D.N.; Dennison, C.; MacMillan, F. Refined distances between paramagnetic centers of a multi-copper nitrite reductase determined by pulsed EPR (i DEER) spectroscopy. Angew. Chem. Int. Ed. 2013, 52, 1990–1993. [Google Scholar] [CrossRef]
- Yang, Z.; Kurpiewski, M.R.; Ji, M.; Townsend, J.E.; Mehta, P.; Jen-Jacobson, L.; Saxena, S. ESR spectroscopy identifies inhibitory Cu2+ sites in a DNA-modifying enzyme to reveal determinants of catalytic specificity. Proc. Natl. Acad. Sci. USA 2012, 109, E993–E1000. [Google Scholar] [CrossRef] [PubMed]
- Noren, C.J.; Anthony-Cahill, S.J.; Griffith, M.C.; Schultz, P.G. A general method for site-specific incorporation of unnatural amino acids into proteins. Science 1989, 244, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.C.; Schultz, P.G. Adding new chemistries to the genetic code. Annu. Rev. Biochem. 2010, 79, 413–444. [Google Scholar] [CrossRef] [PubMed]
- Cornish, V.W.; Benson, D.R.; Altenbach, C.A.; Hideg, K.; Hubbell, W.L.; Schultz, P.G. Site-specific incorporation of biophysical probes into proteins. Proc. Natl. Acad. Sci. USA 1994, 91, 2910–2914. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.B.F.; Xu, J.; Shen, Z.; Takimoto, J.K.; Schultz, M.D.; Schmitz, R.J.; Xiang, Z.; Ecker, J.R.; Briggs, S.P.; Wang, L. RF1 knockout allows ribosomal incorporation of unnatural amino acids at multiple sites. Nat. Chem. Biol. 2011, 7, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.B.F.; Wang, C.; Xu, J.; Schultz, M.D.; Schmitz, R.J.; Ecker, J.R.; Wang, L. Release factor one is nonessential in Escherichia coli. ACS Chem. Biol. 2012, 7, 1337–1344. [Google Scholar] [CrossRef] [PubMed]
- Loscha, K.V.; Herlt, A.J.; Qi, R.; Huber, T.; Ozawa, K.; Otting, G. Multiple-site labeling of proteins with unnatural amino acids. Angew. Chem. Int. Ed. 2012, 51, 2243–2246. [Google Scholar] [CrossRef]
- Chatterjee, A.; Sun, S.B.; Furman, J.L.; Xiao, H.; Schultz, P.G. A versatile platform for single- and multiple-unnatural amino acid mutagenesis in Escherichia coli. Biochemistry 2013, 52, 1828–1837. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.J.; Borbas, J.; Drescher, M.; Summerer, D. A genetically encoded spin label for electron paramagnetic resonance distance measurements. J. Am. Chem. Soc. 2014, 136, 1238–1241. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, Z.; Brock, A.; Schultz, P.G. Addition of the keto functional group to the genetic code of Escherichia coli. Proc. Natl. Acad. Sci. USA 2003, 100, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Fleissner, M.R.; Brustad, E.M.; Kálai, T.; Altenbach, C.; Cascio, D.; Peters, F.B.; Hideg, K.; Peuker, S.; Schultz, P.G.; Hubbell, W.L. Site-directed spin labeling of a genetically encoded unnatural amino acid. Proc. Natl. Acad. Sci. USA 2009, 106, 21637–21642. [Google Scholar] [CrossRef] [PubMed]
- Plass, T.; Milles, S.; Koehler, C.; Schultz, C.; Lemke, E.A. Genetically encoded copper-free click chemistry. Angew. Chem. Int. Ed. 2011, 50, 3878–3881. [Google Scholar] [CrossRef]
- Kálai, T.; Fleissner, M.R.; Jekő, J.; Hubbell, W.L.; Hideg, K. Synthesis of new spin labels for Cu-free click conjugation. Tetrahedron Lett. 2011, 52, 2747–2749. [Google Scholar] [CrossRef]
- Igarashi, R.; Sakai, T.; Hara, H.; Tenno, T.; Tanaka, T.; Tochio, H.; Shirakawa, M. Distance determination in proteins inside Xenopus laevis oocytes by double electron−electron resonance experiments. J. Am. Chem. Soc. 2010, 132, 8228–8229. [Google Scholar] [CrossRef] [PubMed]
- Azarkh, M.; Singh, V.; Okle, O.; Seemann, I.T.; Dietrich, D.R.; Hartig, J.S.; Drescher, M. Site-directed spin-labeling of nucleotides and the use of in-cell EPR to determine long-range distances in a biologically relevant environment. Nat. Protoc. 2013, 8, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Baldauf, C.; Schulze, K.; Lueders, P.; Bordignon, E.; Tampé, R. In-situ spin labeling of his-tagged proteins: Distance measurements under in-cell conditions. Chem.-Eur. J. 2013, 19, 13714–13719. [Google Scholar] [CrossRef] [PubMed]
- Cruickshank, P.A.S.; Bolton, D.R.; Robertson, D.A.; Hunter, R.I.; Wylde, R.J.; Smith, G.M. A kilowatt pulsed 94 GHz electron paramagnetic resonance spectrometer with high concentration sensitivity, high instantaneous bandwidth, and low dead time. Rev. Sci. Instrum. 2009, 80, 103102. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fielding, A.J.; Concilio, M.G.; Heaven, G.; Hollas, M.A. New Developments in Spin Labels for Pulsed Dipolar EPR. Molecules 2014, 19, 16998-17025. https://doi.org/10.3390/molecules191016998
Fielding AJ, Concilio MG, Heaven G, Hollas MA. New Developments in Spin Labels for Pulsed Dipolar EPR. Molecules. 2014; 19(10):16998-17025. https://doi.org/10.3390/molecules191016998
Chicago/Turabian StyleFielding, Alistair J., Maria Grazia Concilio, Graham Heaven, and Michael A. Hollas. 2014. "New Developments in Spin Labels for Pulsed Dipolar EPR" Molecules 19, no. 10: 16998-17025. https://doi.org/10.3390/molecules191016998



