Biomimetic Adhesive Materials Containing Cyanoacryl Group for Medical Application
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Dopamine Methacrylamide (DMA)
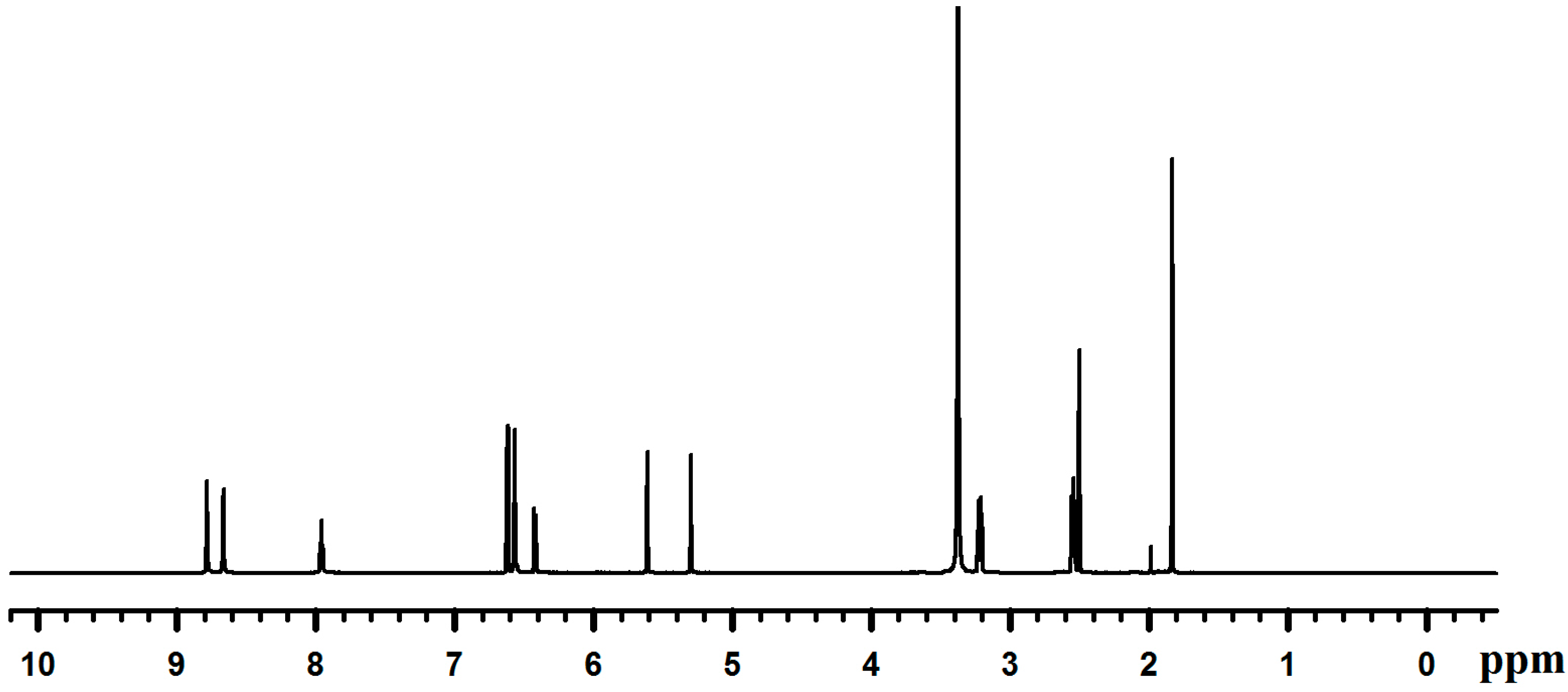
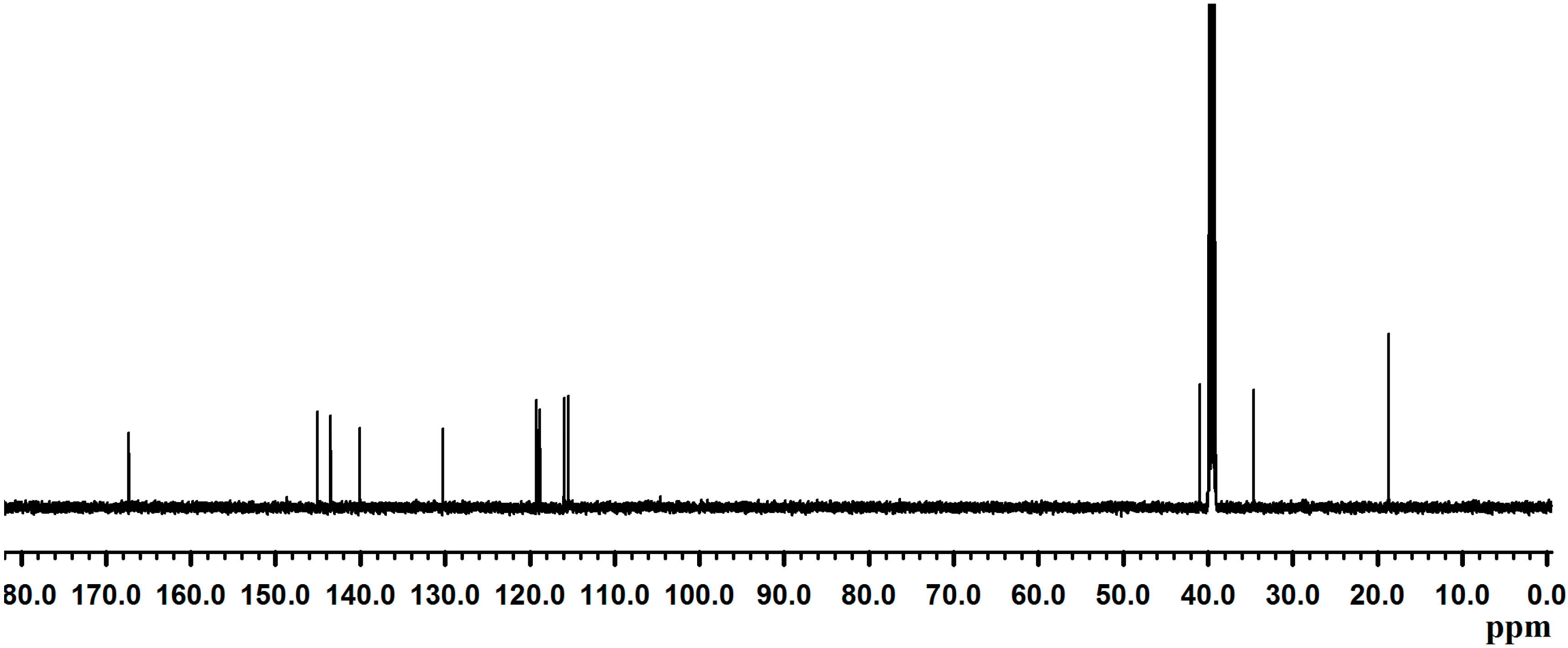
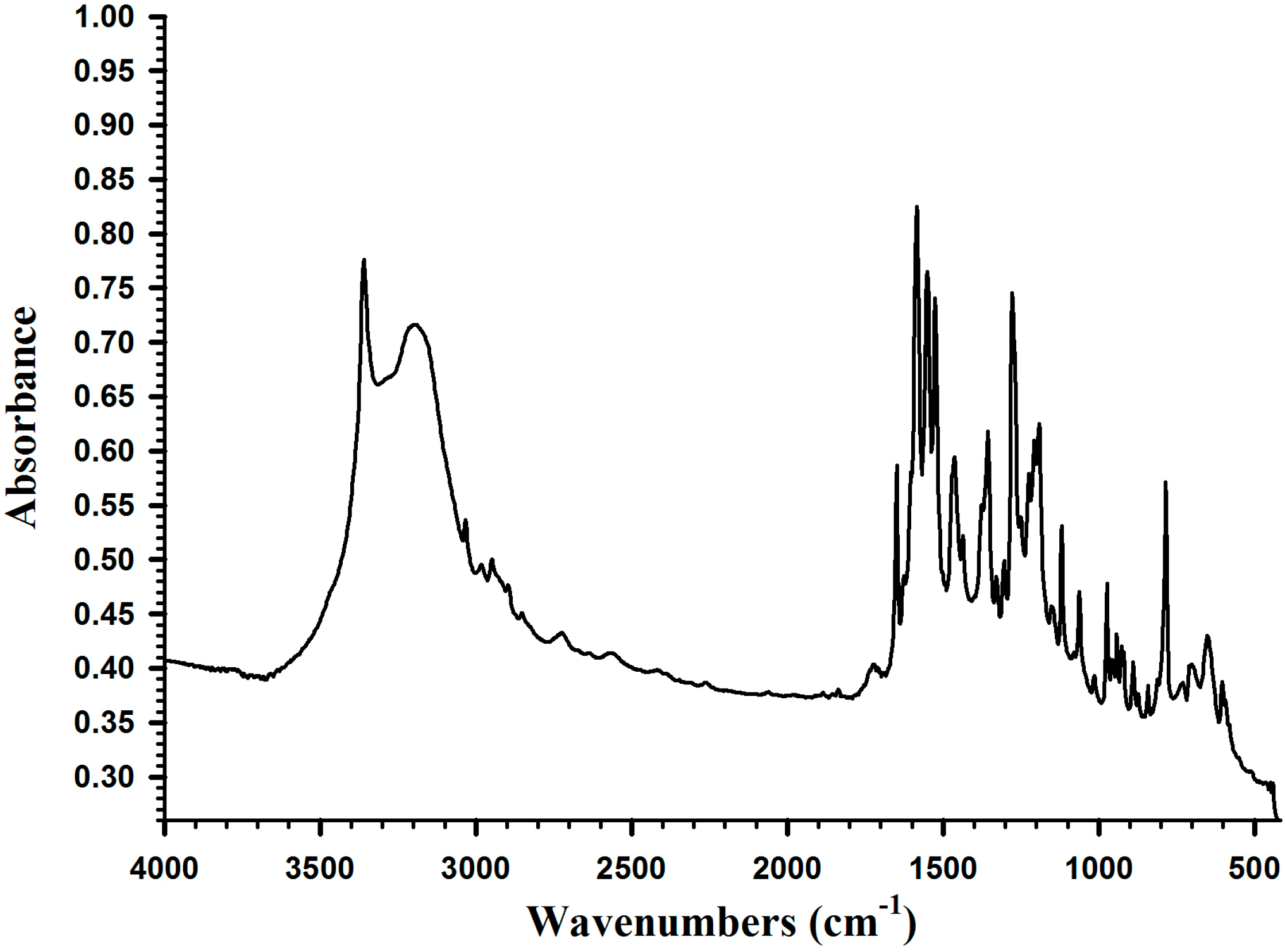
2.2. Synthesis of Poly(dopamine methacrylamide-co-Methoxyethyl acrylate), PDM, and Poly(dopamine methacrylamide-co-Methoxyethyl acrylate-co-2-ethyl cyano acrylate), PDMC
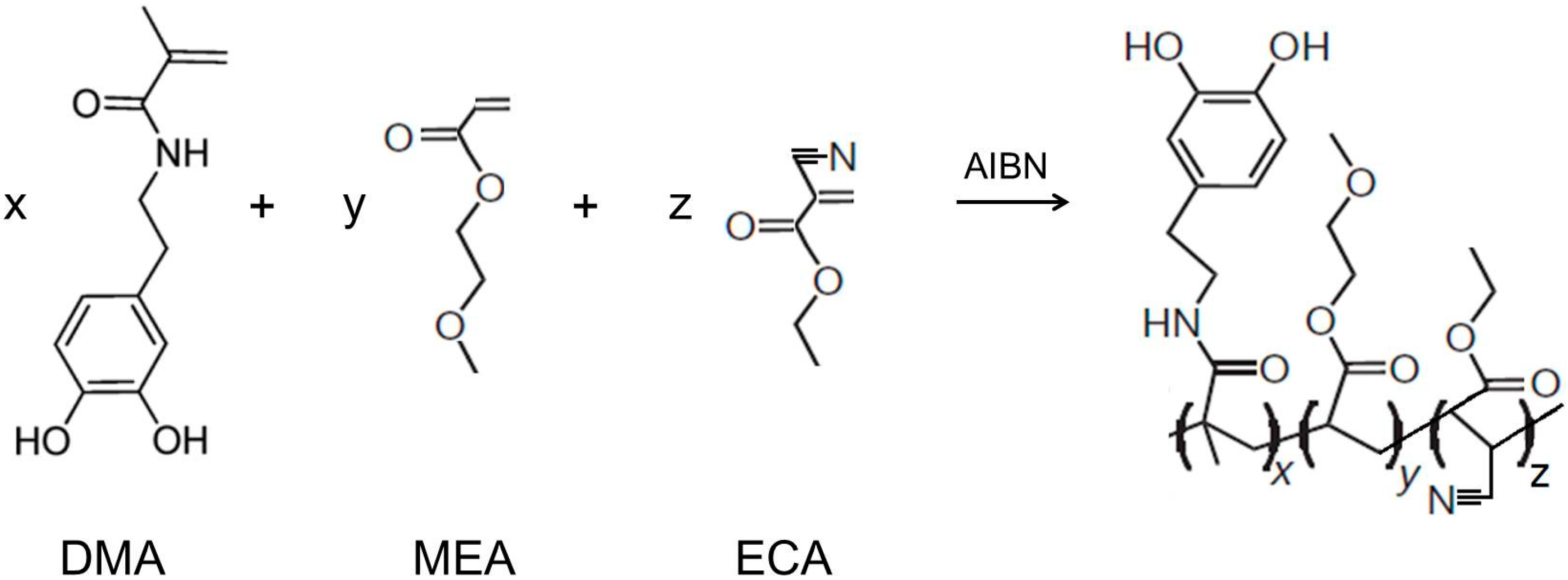
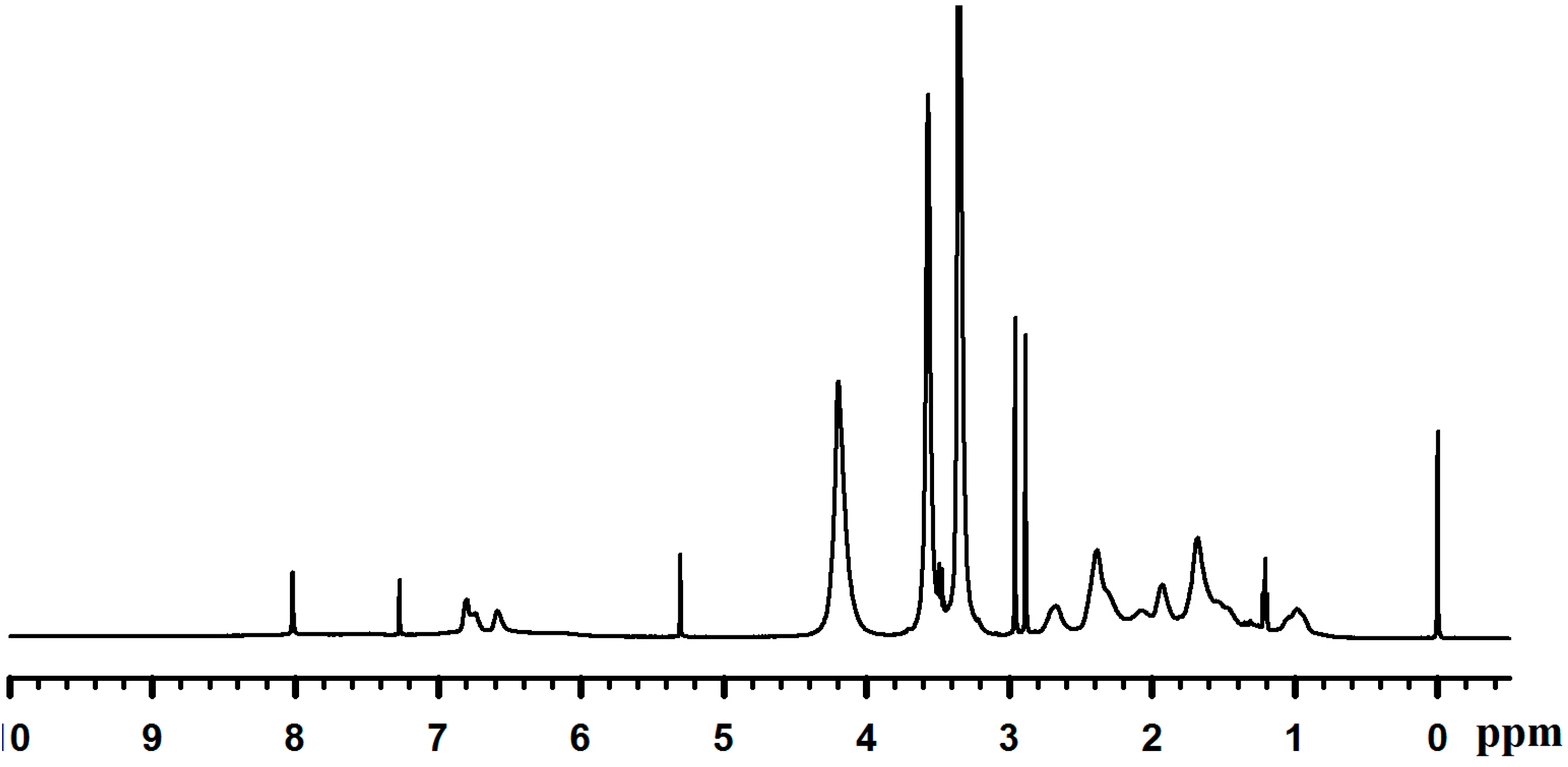
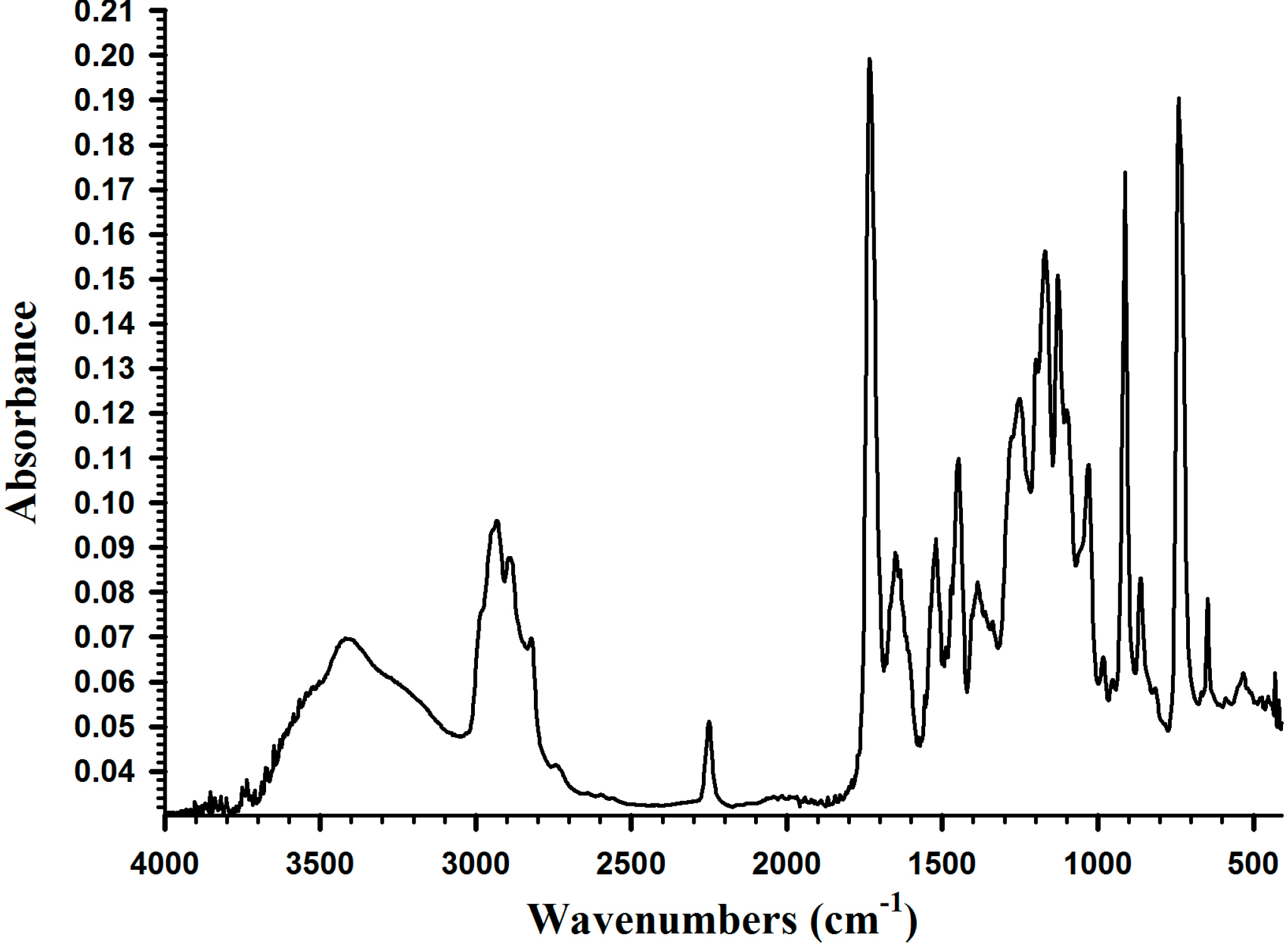
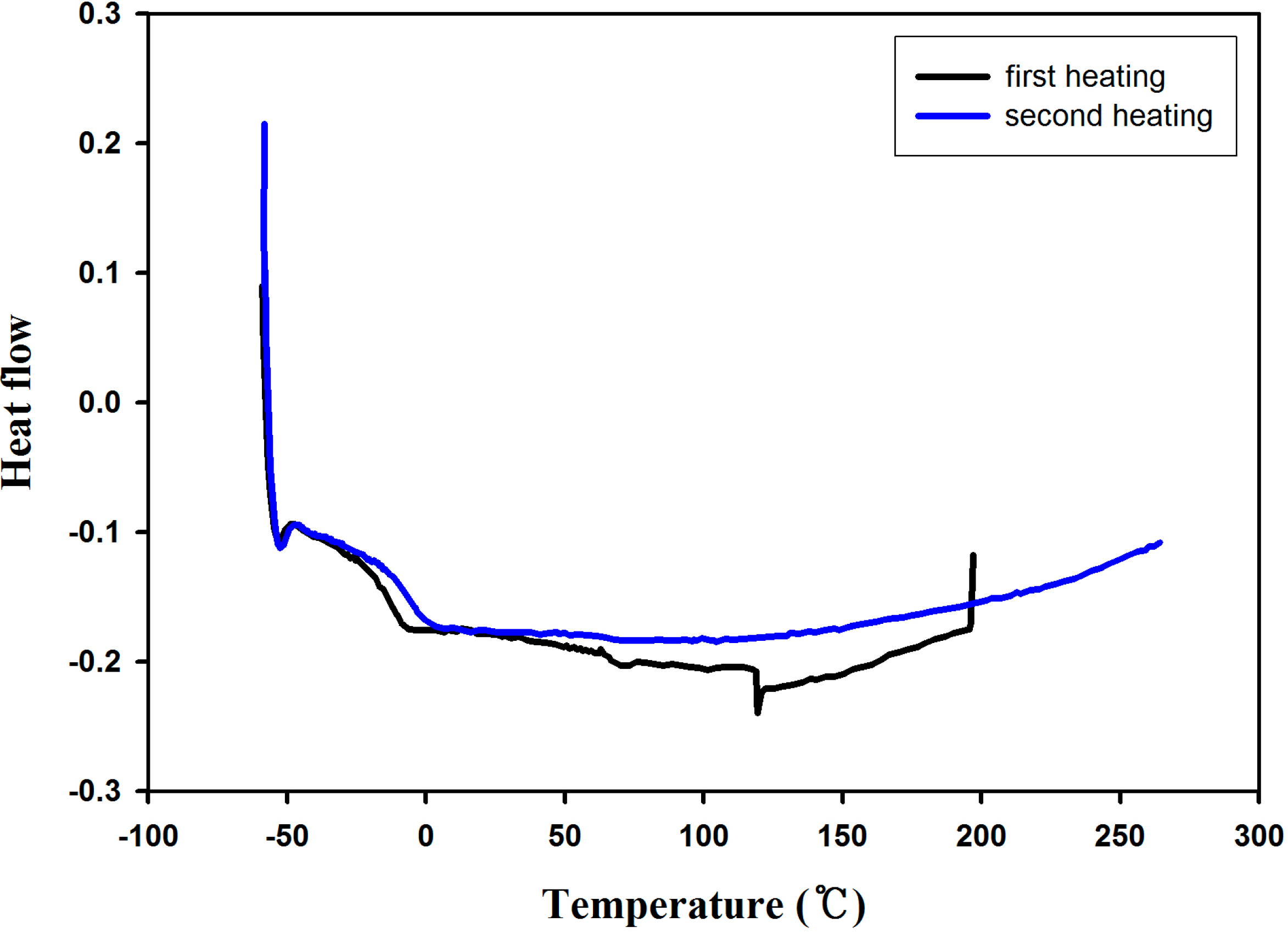
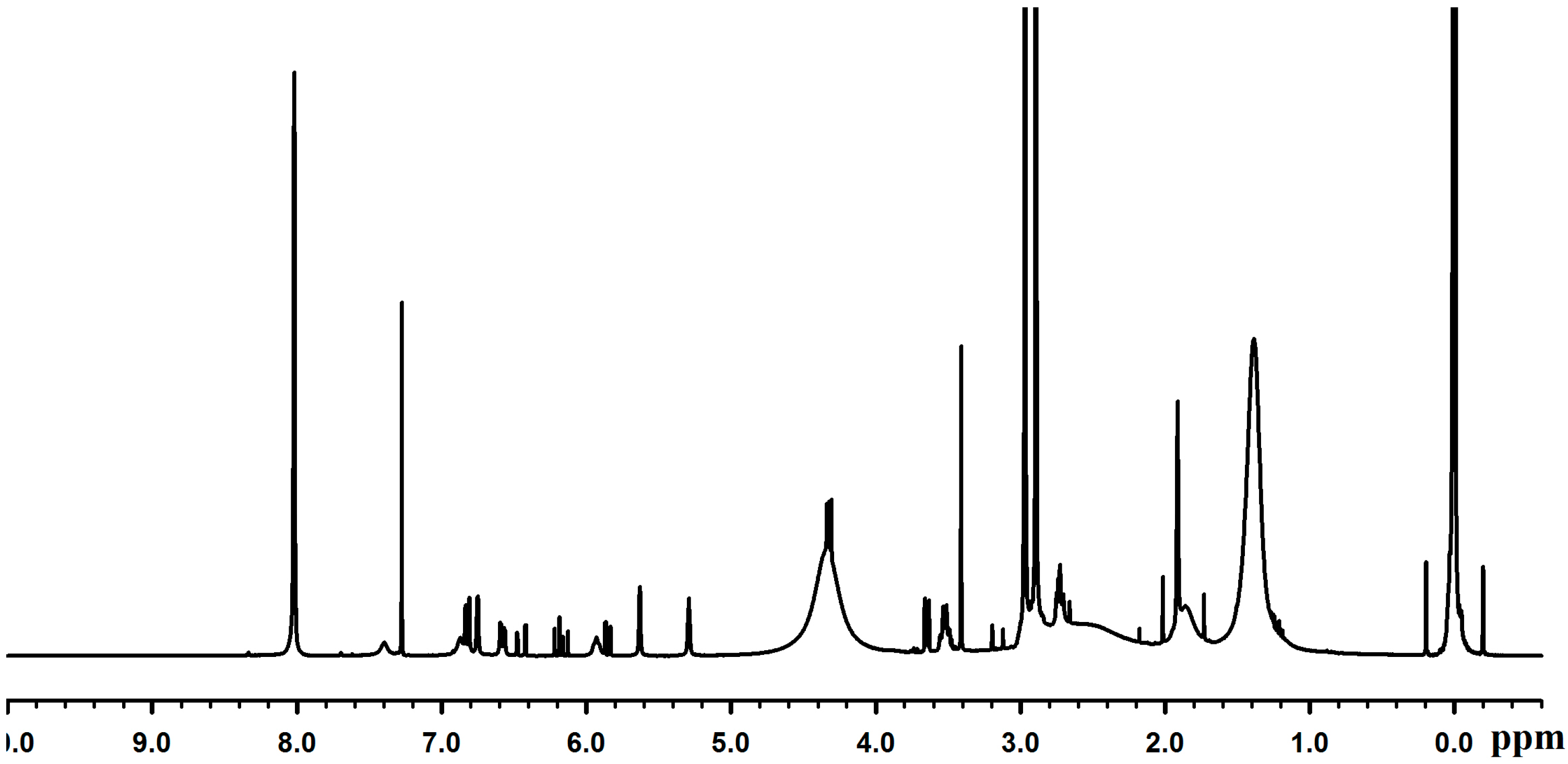

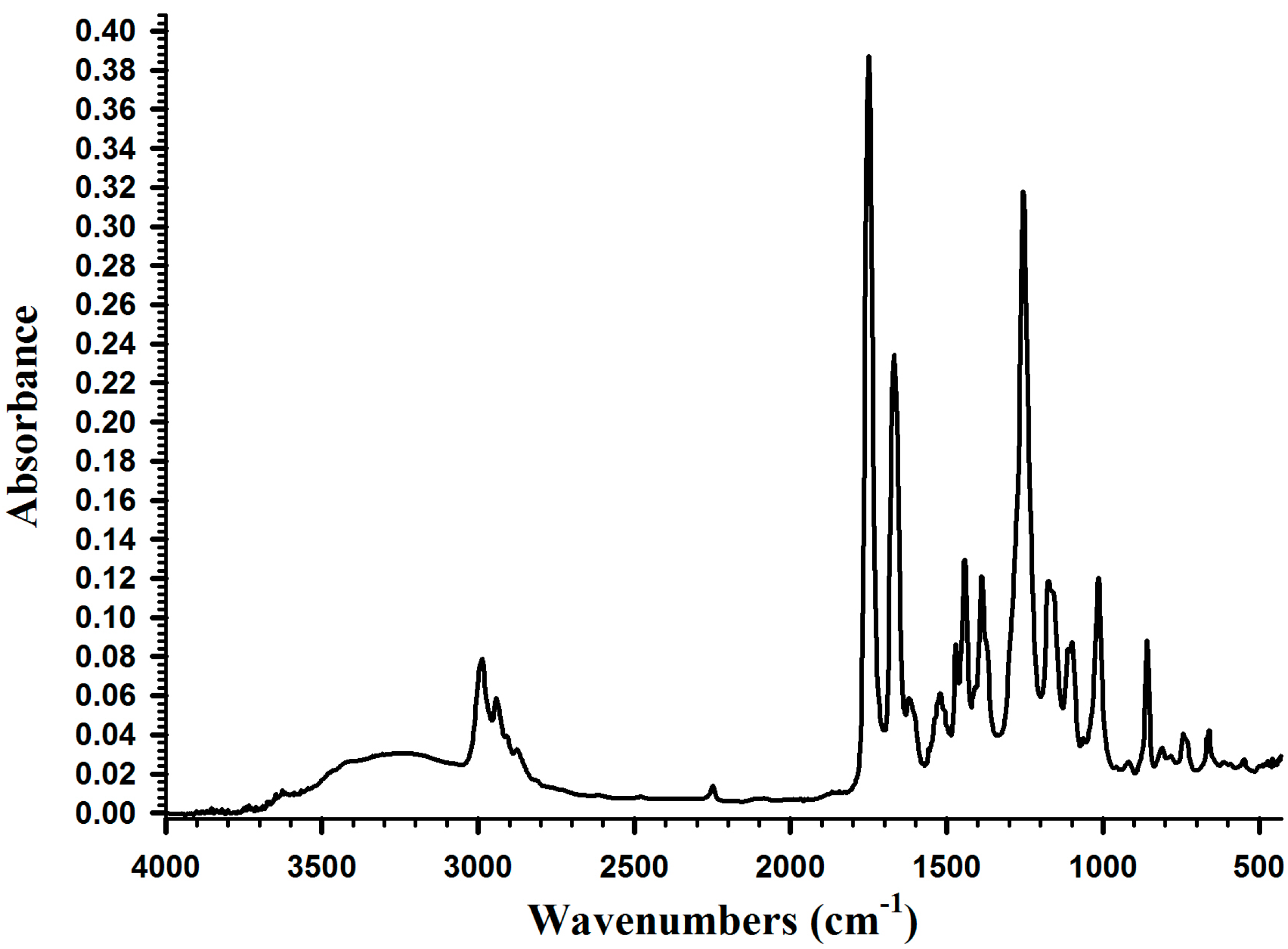
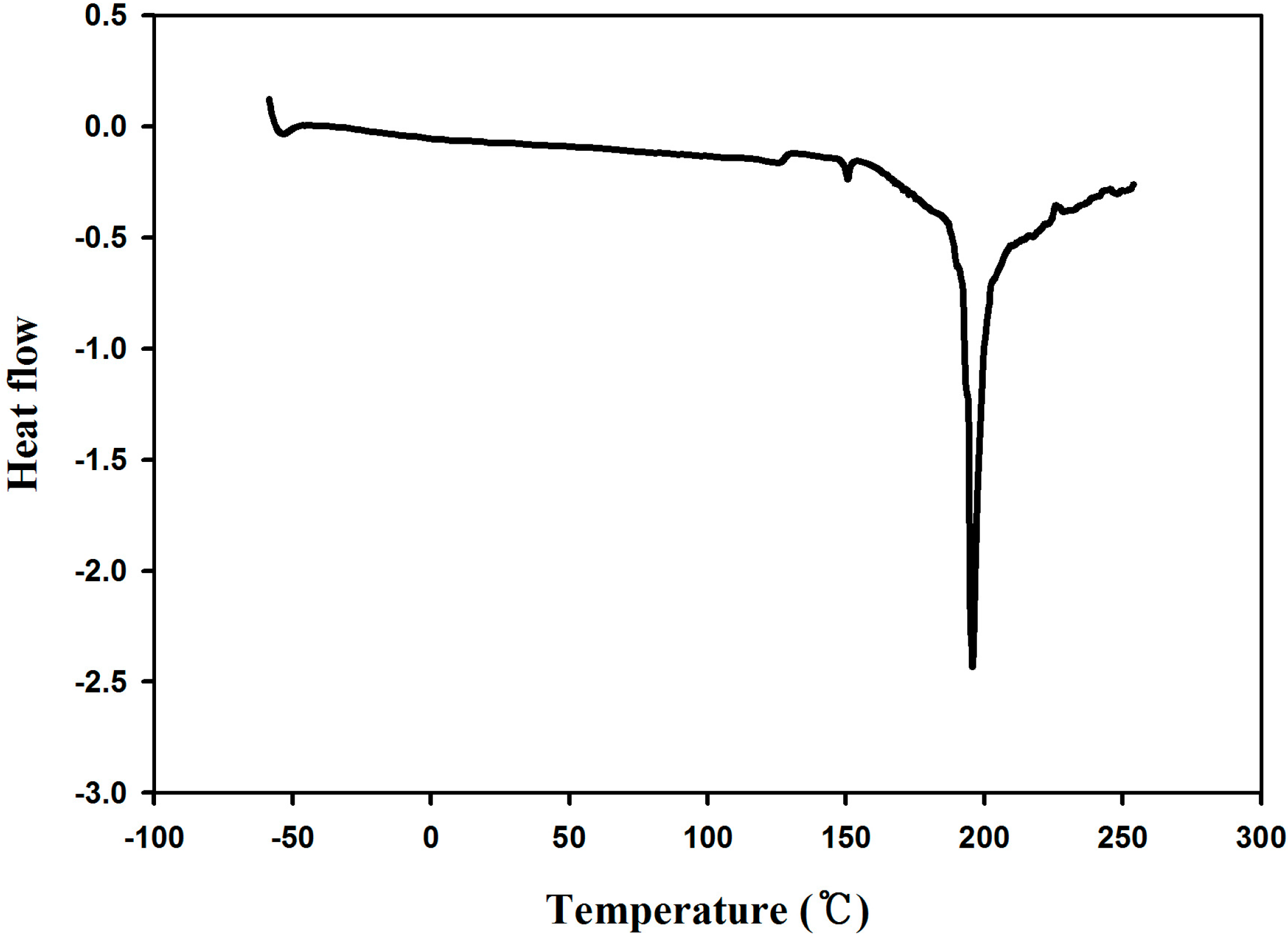
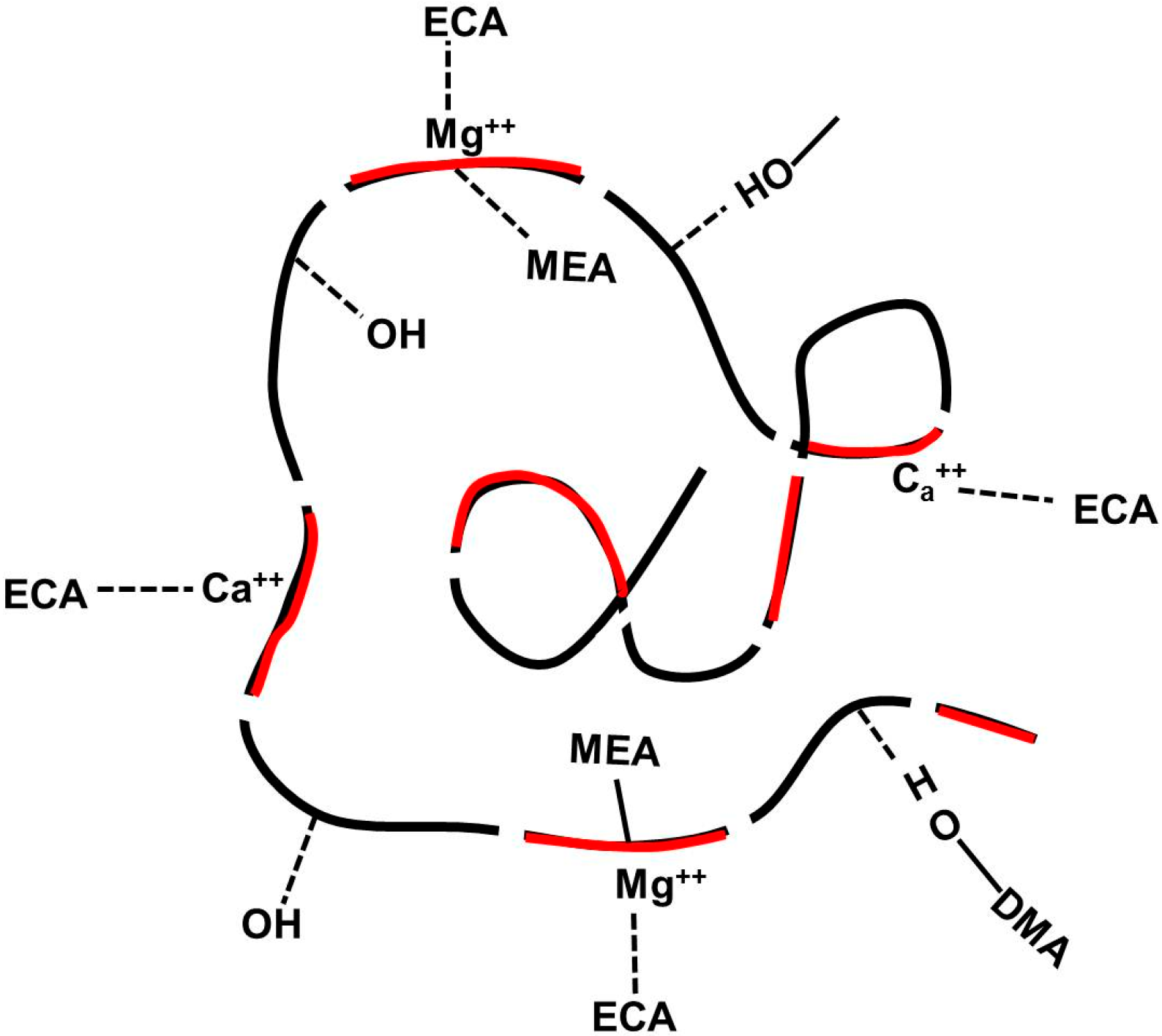
2.3. Adhesion Properties

| CC | PDM | PDMC | EPE | Ca2+/Mg2+ |
|---|---|---|---|---|
| CC-1 | 1 | 0 | 1 | 0.1 |
| CC-2 | 0 | 1 | 1 | 0.1 |
| CC-3 | 1 | 1 | 1 | 0.1 |
| CC-4 | 1 | 2 | 1 | 0.1 |
| CC-5 | 1 | 2 | 1 | 0.0 |
| CC | Stress at Break (kPa) | Modulus (MPa) | Contact Angle (deg) |
|---|---|---|---|
| CC-1 | 75 ± 6.8 | 18 ± 2.5 | 76 ± 2.3 |
| CC-2 | 96 ± 10.5 | 40 ± 5.2 | 87 ± 4.3 |
| CC-3 | 138 ± 15.6 | 27 ± 3.2 | 95 ± 3.7 |
| CC-4 | 165 ± 13.5 | 33 ± 3.5 | 110 ± 3.5 |
| CC-5 | 125 ± 10.5 | 28 ± 3.0 | 110 ± 5.8 |
| Super Glue | 275 ± 3.5 | 42 ± 4.8 | 116 ± 6.9 |
3. Experimental Section
3.1. Materials
3.2. Syntheses
3.2.1. Synthesis of Dopamine Methacrylamide (DMA), DOPA Analog Monomer
3.2.2. Synthesis of Poly(dopamine methacrylamide-co-Methoxyethyl acrylate), [Poly(DMA-co-MEA)]
3.2.3. Synthesis of Poly(dopamine methacrylamide-co-Methoxyethyl acrylate-co-2-ethyl cyano acrylate), [Poly(DMA-co-MEA-co-ECA), PDMC]
3.3. Polymer Characterization Procedure
3.4. Preparation of Blends and Complex Coacervates
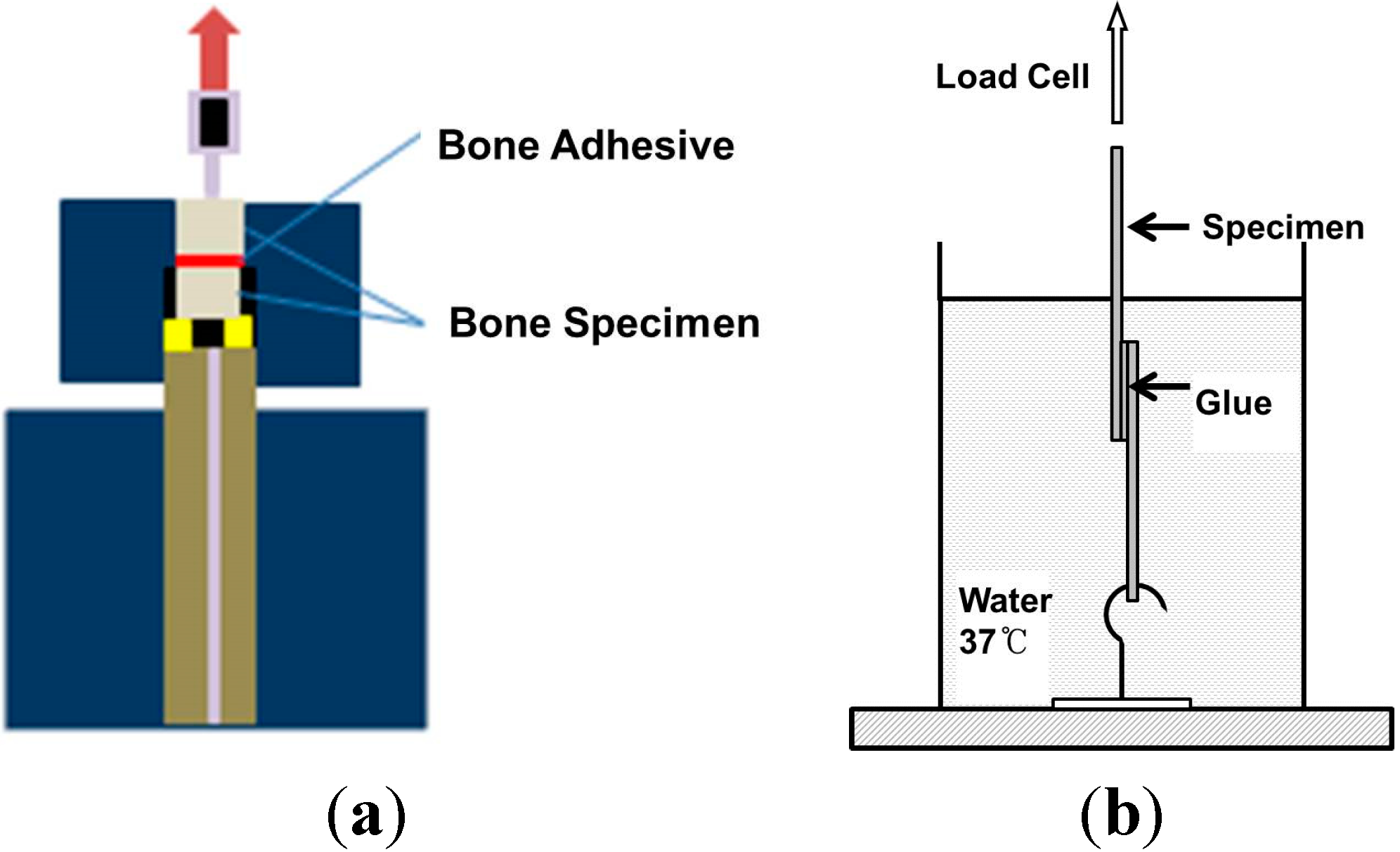
3.5. Bone Adhesion Property Test
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Kamino, K. Barnacle underwater attachment. Biol. Adhes. 2006, 145–166. [Google Scholar]
- Waite, J.H. Nature’s underwater adhesive specialist. Int. J. Adhes. Adhes. 1987, 7, 9–14. [Google Scholar] [CrossRef]
- Tay, F.R.; Pashley, D.H. Dental adhesives of the future. J. Adhes. Dent. 2002, 4, 91–103. [Google Scholar] [PubMed]
- Walker, G. The biochemical composition of the cement of two barnacle species, balanus hameri and balanus crenatus. J. Mar. Biol. Assoc. UK 1972, 52, 429–435. [Google Scholar] [CrossRef]
- Saroyan, J.; Lindner, E.; Dooley, C. Repair and reattachment in the balanidae as related to their cementing mechanism. Biol. Bull. 1970, 139, 333–350. [Google Scholar] [CrossRef]
- Kamino, K.; Inoue, K.; Maruyama, T.; Takamatsu, N.; Harayama, S.; Shizuri, Y. Barnacle cement proteins importance of disulfide bonds in their insolubility. J. Biol. Chem. 2000, 275, 27360–27365. [Google Scholar] [PubMed]
- Heiss, C.; Schnettler, R. Bioresorbable bone adhesives. Historical perspective and current status. Unfallchirurg 2005, 108, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Liang, Z.; Hall, M.; Söderhäll, K. A transglutaminase involved in the coagulation system of the freshwater crayfish, pacifastacus leniusculus. Tissue localisation and cDNA cloning. Fish Shellfish Immunol. 2001, 11, 623–637. [Google Scholar] [CrossRef]
- Barlow, D.; Dickinson, G.; Orihuela, B.; Rittschof, D.; Wahl, K. In situ ATR-FTIR characterization of primary cement interfaces of the barnacle Balanus amphitrite. Biofouling 2009, 25, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Cheung, P.; Ruggieri, G.; Nigrelli, R. A new method for obtaining barnacle cement in the liquid state for polymerization studies. Mar. Biol. 1977, 43, 157–163. [Google Scholar] [CrossRef]
- Dickinson, G.H.; Vega, I.E.; Wahl, K.J. Barnacle cement: A polymerization model based on evolutionary concepts. J. Exp. Biol. 2009, 212, 3499–3510. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, W.J. Carboxypeptidase activity of the zinc metalloprotease in the cement precursor secretion of the barnacle, chthamalus fragilis darwin. Comp. Biochem. Physiol. B: Biochem. Mol. Biol. 1997, 117, 565–570. [Google Scholar] [CrossRef]
- Lee, H.; Scherer, N.F.; Messersmith, P.B. Single-molecule mechanics of mussel adhesion. Proc. Natl. Acad. Sci. USA 2006, 103, 12999–13003. [Google Scholar] [CrossRef] [PubMed]
- Sedó, J.; Saiz-Poseu, J.; Busqué, F.; Ruiz-Molina, D. Catechol-based biomimetic functional materials. Adv. Mater. 2012, 25, 653–701. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Grubbs, R.H. Rapidly cross-linkable DOPA containing terpolymer adhesives and PEG-based cross-linkers for biomedical applications. Macromolecules 2012, 45, 9666–9673. [Google Scholar] [CrossRef]
- Lee, B.P.; Huang, K.; Nunalee, F.N.; Shull, K.R.; Messersmith, P.B. Synthesis of 3,4-dihydroxyphenylalanine (DOPA) containing monomers and their co-polymerization with PEG-diacrylate to form hydrogels. J. Biomater. Sci. Polym. Ed. 2004, 15, 449–464. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Yu, J.; Broomell, C.; Israelachvili, J.N.; Waite, J.H. Hydrophobic enhancement of dopa-mediated adhesion in a mussel foot protein. J. Am. Chem. Soc. 2012, 135, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Burkett, J.R.; Wojtas, J.L.; Cloud, J.L.; Wilker, J.J. A method for measuring the adhesion strength of marine mussels. J. Adhes. 2009, 85, 601–615. [Google Scholar] [CrossRef]
- Conlan, S.L.; Mutton, R.J.; Aldred, N.; Clare, A.S. Evaluation of a fully automated method to measure the critical removal stress of adult barnacles. Biofouling 2008, 24, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Chu, B.; Zhou, Z. Physical chemistry of polyoxyalkylene block copolymer surfactants. In Nonionic Surfactants: Polyoxyalkylene Block Copolymers; Nace, V.M., Ed.; Marcel Dekker: New York, NY, USA, 1996; Volume 60, p. 67. [Google Scholar]
- Van Meerbeek, B.; Inoue, S.; Pedigao, J. Enamel and Dentin Adhesion. In Fundamentals of Operative Dentistry, 2nd ed.; Summitt, J.B., Robbins, J.W., Schwartz, R.S., Eds.; Quintessence Publishing Co. Inc.: Hanover Park, IL, USA, 2001; pp. 178–235. [Google Scholar]
- Hillmyer, M.A.; Lipic, P.M.; Hajduk, D.A.; Almdal, K.; Bates, F.S. Self-assembly and polymerization of epoxy-resin amphiphilic block-copolymer nanocomposites. J. Am. Chem. Soc. 1997, 119, 2749–2750. [Google Scholar] [CrossRef]
- Lipic, P.M.; Bates, F.S.; Hillmyer, M.A. Nanostructured thermosets from self-assembled amphiphilic block copolymer/epoxy resin mixtures. J. Am. Chem. Soc. 1998, 120, 8963–8970. [Google Scholar] [CrossRef]
- Heiss, C.; Kraus, R.; Schlukerbier, D.; Stiller, A.C.; Wenisch, S.; Schnettler, R. Bone adhesives in trauma and orthopedic surgery. Eur. J. Trauma 2006, 2, 41–48. [Google Scholar]
- Shao, H.; Bachus, K.N.; Stewart, R.J. A water-borne adhesive modeled after the sandcastle glue of P. californica. Macromol. Biosci. 2009, 9, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Waite, J.H. Mapping chemical gradients within and along a fibrous structural tissue, mussel byssal threads. J. Biol. Chem. 2005, 280, 39332–39336. [Google Scholar] [CrossRef] [PubMed]
- Almgren, M.; Brown, W.; Hvidt, S. Self-aggregation and phase behavior of poly (ethylene oxide)-poly (propylene oxide)-poly (ethylene oxide) block copolymers in aqueous solution. Colloid Polym. Sci. 1995, 273, 2–15. [Google Scholar] [CrossRef]
- Alexandridis, P.; Alan Hatton, T. Poly (ethylene oxide)-poly (propylene oxide)-poly (ethylene oxide) block copolymer surfactants in aqueous solutions and at interfaces: Thermodynamics, structure, dynamics and modeling. Colloids Surf. A 1995, 96, 1–46. [Google Scholar] [CrossRef]
- Kabanov, A.V.; Alakhov, V.Y. Pluronic block copolymers in drug delivery: From micellar nanocontainers to biological response modifiers. Crit. Rev. Ther. Drug Carr. Syst. 2002, 19, 1–72. [Google Scholar] [CrossRef]
- Alexandridis, P.; Holzwarth, J.F.; Hatton, T.A. Micellization of poly(ethylene oxide)-poly (propylene oxide)-poly (ethylene oxide) triblock copolymers in aqueous solutions: Thermodynamics of copolymer association. Macromolecules 1994, 27, 2414–2425. [Google Scholar] [CrossRef]
- Wang, T.T.; Ryan, F.; Schonhorn, H. Effect of bonding defects on shear strength in tension of lap joints having brittle adhesives. J. Appl. Polym. Sci. 1972, 16, 1901–1909. [Google Scholar] [CrossRef]
- Lee, H.; Lee, B.P.; Messersmith, P.B. A reversible wet/dry adhesive inspired by mussels and geckos. Nature 2007, 448, 338–341. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, S.H.; Sohn, J.S. Biomimetic Adhesive Materials Containing Cyanoacryl Group for Medical Application. Molecules 2014, 19, 16779-16793. https://doi.org/10.3390/molecules191016779
Jo SH, Sohn JS. Biomimetic Adhesive Materials Containing Cyanoacryl Group for Medical Application. Molecules. 2014; 19(10):16779-16793. https://doi.org/10.3390/molecules191016779
Chicago/Turabian StyleJo, Sueng Hwan, and Jeong Sun Sohn. 2014. "Biomimetic Adhesive Materials Containing Cyanoacryl Group for Medical Application" Molecules 19, no. 10: 16779-16793. https://doi.org/10.3390/molecules191016779




