3.2. Synthesis
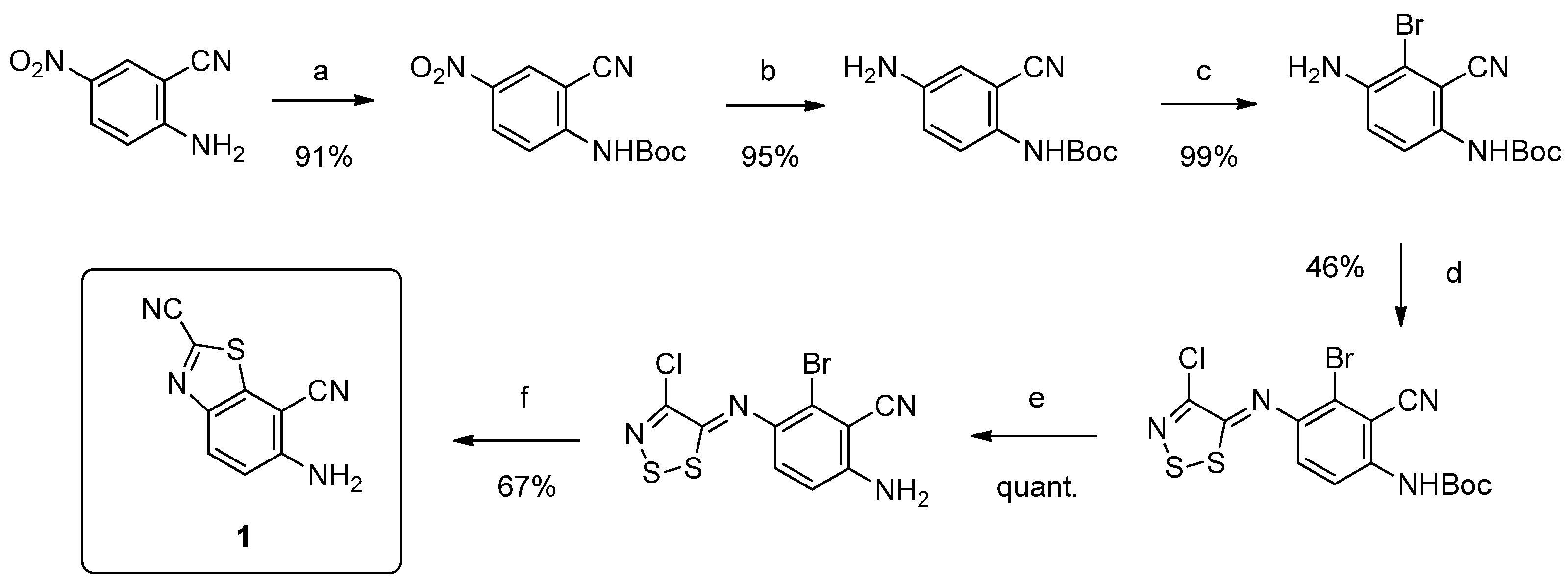
6-Aminobenzo[d]thiazole-2,7-dicarbonitrile (1) and (E)-N'-(2,7-dicyanobenzo[d]thiazol-6-yl)-N,N-dimethylformimidamide (
2) were prepared and characterized following the procedure described in Reference [
9].
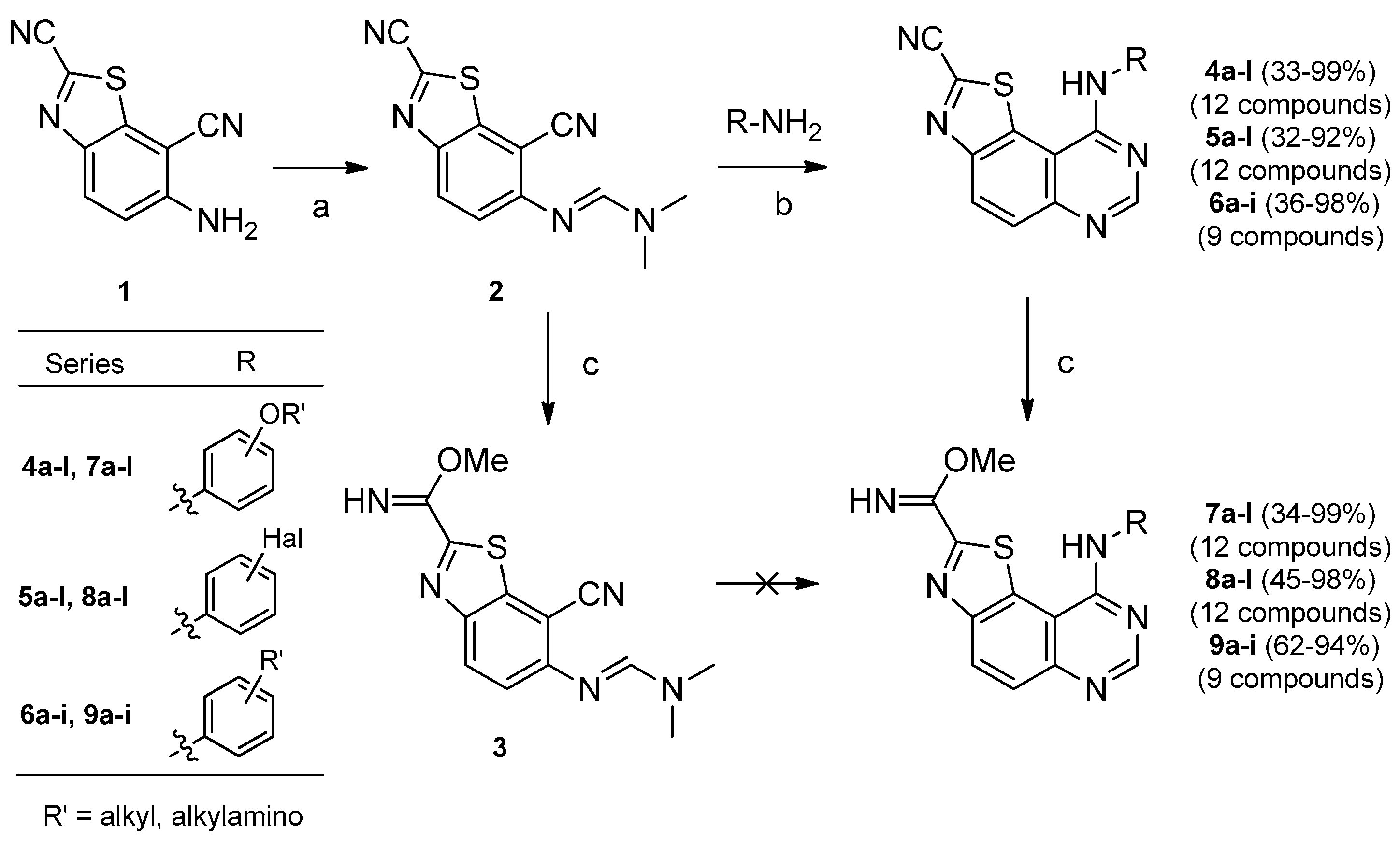
(E)-Methyl 7-cyano-6-([(dimethylamino)methylene]amino)benzo[d]thiazole-2-carbimidate (3). A stirred mixture of carbonitrile 2 (0.17 mmol) and NaOH (2.5N sol., 50 μL) in methanol (2.5 mL) was heated under microwaves (1200 W) at 80 °C for 45 min. The solvent was removed in vacuo and the crude residue purified by flash chromatography (DCM-EtOAc, 9:1) to afford the imidate 3 as a yellow solid (0.032 g, 66% yield); mp = 163–165 °C. 1H-NMR (300 MHz, DMSO-d6) δ 9.34 (s, 1H, NH), 8.22 (m, 2H), 7.49 (d, 1H, J = 9.0 Hz), 3.92 (s, 3H), 3.13 (s, 3H), 3.05 (s, 3H) ); 13C-NMR (75 MHz, DMSO-d6) δ 159.2, 156.0, 155.8, 155.2, 146.8, 139.7, 128.8, 119.4, 116.8, 97.1, 54.1, 34.2; HRMS calcd for C13H14N5OS [M + H]+: 288.0919, found 288.0930.
3.2.1. Synthesis of Thiazolo[5,4-f]quinazoline-2-carbonitriles 4a–l, 5a–l and 6a–i
A mixture of (E)-N'-(2,7-dicyanobenzo[d]thiazol-6-yl)-N,N-dimethylformimidamide 2 (0.05 g, 0.19 mmol) and the appropriate amine (0.29 mmol, 1.5 equiv) in acetic acid (2 mL) was heated under microwaves (600 W) at 118 °C. On completion (followed by TLC), the reaction was cooled to ambient temperature. The solvent was removed in vacuo and the crude residue was purified by flash chromatography to afford the expected compounds 4a–k, 5a–l and 6a–h.
Series 4a–k: Compounds Bearing 9N-Phenyl Groups with Electron-Donating Substituents (e.g., OH, OR and Derivatives)
9-(4-Methoxyphenylamino)thiazolo[5,4-f]quinazoline-2-carbonitrile (4a) and 9-(benzo[d][1,3]dioxol-5-ylamino)thiazolo[5,4-f]quinazoline-2-carbonitrile (
4b) were synthesized in Reference [
9].
9-(2,3-Dihydrobenzo[b][1,4]dioxin-6-ylamino)thiazolo[5,4-f]quinazoline-2-carbonitrile (4c). Prepared from 2 and 1,4-benzodioxan-6-amine. Flash chromatography eluent (DCM-EtOAc, 8:2). Yield: 33%; yellow solid; mp = 180–190 °C (dec). IR (cm−1) νmax 3055, 2978, 2932, 2875, 2230, 1709, 1638, 1609, 1578, 1496, 1460, 1376, 1299, 1281, 1239, 1200, 1151, 1063, 916, 885, 814. 1H-NMR (300 MHz, DMSO-d6) δ 8.37 (d, 1H, J = 8.4 Hz), 7.85 (m, 1H), 7.76 (m, 1H), 6.88 (d, 1H, J = 8.4 Hz), 6.56 (m, 2H), 4.25 (s, 4H). HRMS calcd for C18H12N5O2S [M + H]+: 362.0712, found 362.0696.
9-(2,3-Dihydrobenzofuran-5-ylamino)thiazolo[5,4-f]quinazoline-2-carbonitrile (4d). Prepared from 2 and 2,3-dihydro-1-benzofuran-5-amine. Flash chromatography eluent (EtOAc). Yield: 95%; yellow solid; mp = 216–218 °C. IR (cm−1) νmax 2894, 2853, 2228, 1643, 1609, 1579, 1484, 1467, 1376, 1353, 1306, 1269, 1219, 1192, 1164, 1123, 978, 941, 881, 814; 1H-NMR (300 MHz, DMSO-d6) δ 8.49 (d, 1H, J = 8.7 Hz), 8.10 (s, 1H), 7.75 (d, 1H, J = 8.7 Hz), 7.22 (m, 1H), 7.04 (s, 1H), 6.78 (m, 1H), 4.53 (t, 2H, J = 8.7 Hz), 3.20 (t, 1H, J = 8.7 Hz); HRMS calcd for C18H12N5OS [M + H]+: 346.0763, found 346.0762.
9-(3,4-Dimethoxyphenylamino)thiazolo[5,4-f]quinazoline-2-carbonitrile (4e). Prepared from 2 and 3,4-dimethoxyaniline. Flash chromatography eluent (DCM-EtOAc, 8:2). Yield: 74%; yellow solid; mp > 260 °C. IR (cm−1) νmax 3267, 2839, 2226, 1644, 1610, 1583, 1507, 1460, 1443, 1379, 1308, 1260, 1227, 1201, 1166, 1150, 1129, 1020, 967, 935, 861, 839; 1H-NMR (300 MHz, DMSO-d6) δ 8.34 (m, 1H), 7.79 (m, 1H), 7.71 (m, 1H), 6.90 (d, 1H, J = 8.1 Hz), 6.54 (m, 2H), 4.26 (s, 6H); HRMS calcd for C18H14N5O2S [M + H]+: 364.0868, found 364.0850.
9-(2,4-Dimethoxyphenylamino)thiazolo[5,4-f]quinazoline-2-carbonitrile (4f). Prepared from 2 and 2,4-dimethoxyaniline. Flash chromatography eluent (DCM-EtOAc, 5:5). Yield: 59%; orange solid; mp = 255–257 °C. IR (cm−1) νmax 3401, 3081, 2948, 2837, 2233, 1600, 1565, 1540, 1525, 1505, 1443, 1417, 1329, 1278, 1231, 1203, 1159, 1132, 1088, 1030, 959, 915; 1H-NMR (300 MHz, DMSO-d6) δ 8.51 (d, 1H, J = 9.0 Hz), 7.98 (s, 1H), 7.83 (d, 1H, J = 9.0 Hz), 6.95 (m, 1H), 6.67 (s, 1H), 6.58 (d, 1H, J = 9.0 Hz), 3.78 (s, 3H), 3.73 (s, 3H); HRMS calcd for C18H14N5O2S [M + H]+: 364.0868, found 364.0856.
9-(3,5-Dimethoxyphenylamino)thiazolo[5,4-f]quinazoline-2-carbonitrile (4g). Prepared from 2 and 3,5-dimethoxyaniline. Flash chromatography eluent (DCM-EtOAc, 5:5). Yield: 98%; yellow solid; mp = 248–250 °C. IR (cm−1) νmax 3242, 2940, 2837, 2223, 1711, 1647, 1578, 1455, 1419, 1383, 1357, 1306, 1265, 1205, 1144, 1058, 1046, 968, 943, 917, 833, 806; 1H-NMR (300 MHz, DMSO-d6) δ 8.51 (d, 1H, J = 9.0 Hz), 8.06 (s, 1H), 7.79 (d, 1H, J = 9.0 Hz), 6.31 (m, 3H), 3.74 (s, 6H); HRMS calcd for C18H14N5O2S [M + H]+: 364.0868, found 364.0856.
9-(4-Methoxy-3-nitrophenylamino)thiazolo[5,4-f]quinazoline-2-carbonitrile (4h). Prepared from 2 and 3-nitro-4-methoxyaniline. Flash chromatography eluent (DCM-EtOAc, 7:3). Yield: 61%; yellow solid, mp = 200–260 °C (dec). IR (cm−1) νmax 2226, 1644, 1523, 1459, 1346, 1267, 1191, 1155, 1075, 1015, 970, 928, 890, 822; 1H-NMR (300 MHz, DMSO-d6) δ 8.47 (d, 1H, J = 9.0 Hz), 8.26 (s, 1H), 7.97 (d, 1H, J = 9.0 Hz), 7.70 (s, 2H), 7.32 (d, 1H, J = 9.0 Hz), 3.93 (s, 3H); HRMS calcd for C17H11N6O3S [M + H]+: 379.0613, found 379.0614.
9-(4-Hydroxyphenylamino)thiazolo[5,4-f]quinazoline-2-carbonitrile (4i). Prepared from 2 and 4-aminophenol. Flash chromatography eluent (EtOAc). Yield: 80%; orange solid; mp = 236–238 °C. IR (cm−1) νmax 3072, 2225, 1641, 1615, 1577, 1503, 1464, 1378, 1350, 1307, 1230, 1212, 1159, 1097, 972, 832; 1H-NMR (300 MHz, DMSO-d6) δ 8.48 (d, 1H, J = 8.7 Hz), 8.08 (m, 1H), 7.76 (d, 1H, J = 8.7 Hz), 7.15 (m, 2H), 6.79 (m, 2H); HRMS calcd for C16H10N5OS [M + H]+: 320.0606, found 320.0619.
9-(3-Hydroxy-4-methoxyphenylamino)thiazolo[5,4-f]quinazoline-2-carbonitrile (4j). Prepared from 2 and 5-amino-2-methoxyphenol. Flash chromatography eluent (EtOAc). Yield: 54%; yellow solid; mp = 246–248 °C. IR (cm−1) νmax 2921, 2851, 2227, 1724, 1647, 1616, 1583, 1509, 1460, 1334, 1287, 1263, 1218, 1172, 1148, 1120, 1036, 973, 953, 864, 833; 1H-NMR (300 MHz, DMSO-d6) δ 8.49 (d, 1H, J = 8.7 Hz), 8.05 (m, 1H), 7.77 (d, 1H, J = 8.7 Hz), 7.94 (d, 2H, J = 8.7 Hz), 6.65 (m, 1H), 3.77 (s, 3H); HRMS calcd for C17H12N5O2S [M + H]+: 350.0712, found 350.0715.
9-(4-Hydroxy-3-nitrophenylamino)thiazolo[5,4-f]quinazoline-2-carbonitrile (4k). Prepared from 2 and 4-amino-2-nitrophenol. Flash chromatography eluent (DCM-EtOAc, 5:5). Yield: 60%; brown solid; mp > 260 °C. IR (cm−1) νmax 3334, 3081, 2926, 2225, 1627, 1591, 1569, 1525, 1465, 1419, 1395, 1305, 1237, 1171, 1132, 1070, 966, 930, 834, 819; 1H-NMR (300 MHz, DMSO-d6) δ 8.52 (d, 1H, J = 8.7 Hz), 8.28 (m, 1H), 8.03 (m, 1H), 7.75 (d, 1H, J = 8.7 Hz), 7.61 (m, 1H), 7.15 (d, 1H, J = 9.0 Hz); HRMS calcd for C16H9N6O3S [M + H]+: 365.0457, found 365.0441.
9-(3,4,5-Trimethoxyphenylamino)thiazolo[5,4-f]quinazoline-2-carbonitrile (4l). Prepared from 2 and 3,4,5-trimethoxyaniline. Yield: 94%; pale yellow solid; mp = 230–232 °C. IR (cm−1) νmax 3255, 3089, 3001, 2947, 2837, 2230, 1735, 1637, 1613, 1581, 1498, 1458, 1412, 1381, 1352, 1307, 1270, 1229, 1193, 1165, 1122, 1037, 1002, 991, 970, 952, 852, 830; 1H-NMR (300 MHz, DMSO-d6) δ 8.51 (d, 1H, J = 9.0 Hz), 8.05 (s, 1H), 7.80 (d, 1H, J = 9.0 Hz), 6.46 (s, 2H), 3.77 (s, 6H), 3.67 (s, 3H); HRMS calcd for C19H16N5O3S [M + H]+: 394.0974, found 394.0987.
Series 5a–l: Compounds Bearing 9N-Phenyl Groups with Halogen Substituents (e.g., Cl, Br and F)
9-(4-Chlorophenylamino)thiazolo[5,4-f]quinazoline-2-carbonitrile (5a). Prepared from 2 and 4-chloroaniline. Flash chromatography eluent (DCM-EtOAc, 5:5). Yield: 89%; yellow solid; mp > 260 °C. IR (cm−1) νmax 2850, 2229, 1643, 1609, 1583, 1550, 1491, 1480, 1457, 1377, 1355, 1307, 1270, 1214, 1164, 1130, 1092, 1010, 980, 831; 1H-NMR (300 MHz, DMSO-d6) δ 8.51 (d, 1H, J = 8.7 Hz), 8.18 (s, 1H), 7.76 (d, 1H, J = 8.7 Hz), 7.38 (m, 2H), 7.04 (m, 2H); HRMS calcd for C16H9N5SCl [M + H]+: 338.0267, found 338.0274.
9-(3-Chlorophenylamino)thiazolo[5,4-f]quinazoline-2-carbonitrile (5b). Prepared from 2 and 3-chloroaniline. Flash chromatography eluent (DCM-EtOAc, 7:3). Yield: 74%; pale yellow solid; mp > 260 °C. IR (cm−1) νmax 2849, 2226, 1643, 1611, 1577, 1461, 1377, 1354, 1306, 1218, 1161, 1128, 1070, 974, 875, 833; 1H-NMR (300 MHz, DMSO-d6) δ 8.49 (d, 1H, J = 9.0 Hz), 8.21 (s, 1H), 7.74 (d, 1H, J = 9.0 Hz), 7.40–7.35 (m, 2H), 7.19–7.11 (m, 2H); HRMS calcd for C16H9N5SCl [M + H]+: 338.0267, found 338.0259.
9-(2,4-Dichlorophenylamino)thiazolo[5,4-f]quinazoline-2-carbonitrile (5c). Prepared from 2 and 2,4-dichloroaniline. Flash chromatography eluent (DCM-EtOAc, 5:5). Yield: 32%; yellow solid, mp = 260 °C. IR (cm−1) νmax 3063, 2231, 1736, 1644, 1611, 1577, 1459, 1380, 1355, 1310, 1242, 1173, 1098, 1051, 983, 830, 818; 1H-NMR (300 MHz, DMSO-d6) δ 8.56 (d, 1H, J = 9.0 Hz), 8.21 (s, 1H), 7.80 (d, 1H, J = 9.0 Hz), 7.63 (s, 1H), 7.39 (d, 1H, J = 8.1 Hz), 7.25 (d, 1H, J = 8.1 Hz); HRMS calcd for C16H8N5SCl2 [M + H]+: 371.9877, found 371.9877.
9-(3,4-Dichlorophenylamino)thiazolo[5,4-f]quinazoline-2-carbonitrile (5d). Prepared from 2 and 3,4-dichloroaniline. Flash chromatography eluent (DCM-EtOAc, 7:3). Yield: 42%; yellow solid; mp > 260 °C. IR (cm−1) νmax 2851, 2225, 1644, 1612, 1579, 1456, 1378, 1355, 1308, 1270, 1241, 1168, 1122, 1026, 971, 879, 834, 816; 1H-NMR (300 MHz, DMSO-d6) δ 8.55 (d, 1H, J = 8.7 Hz), 8.30 (s, 1H), 7.78 (d, 1H, J = 8.7 Hz), 7.63–7.53 (m, 2H), 7.30 (m, 2H); HRMS calcd for C16H8N5SCl2 [M + H]+: 371.9877, found 371.9882.
9-(4-Fluorophenylamino)thiazolo[5,4-f]quinazoline-2-carbonitrile (5e). Prepared from 2 and 4-fluoroaniline. Flash chromatography eluent (DCM-EtOAc, 5:5). Yield: 92%; yellow solid; mp > 260 °C. IR (cm−1) νmax 3049, 2840, 2226, 1722, 1643, 1610, 1581, 1557, 1502, 1377, 1355, 1305, 1269, 1227, 1208, 1166, 1130, 1090, 981, 846, 829, 818; 19F-NMR (282 MHz, DMSO-d6) δ −120.31; 1H-NMR (300 MHz, DMSO-d6) δ 8.51 (d, 1H, J = 9.0 Hz), 8.16 (s, 1H), 7.76 (d, 1H, J = 9.0 Hz), 7.26–7.08 (m, 4H); HRMS calcd for C16H9N5SF [M + H]+: 322.0563, found 322.0551.
9-(4-Bromo-2-fluorophenylamino)thiazolo[5,4-f]quinazoline-2-carbonitrile (
5f) and 9-(3-chloro-4-fluorophenylamino)thiazolo[5,4-f]quinazoline-2-carbonitrile (
5g) were synthesized in Reference [
9].
9-(4-Chloro-2-fluorophenylamino)thiazolo[5,4-f]quinazoline-2-carbonitrile (5h). Prepared from 2 and 4-chloro-2-fluoroaniline. Flash chromatography eluent (DCM-EtOAc, 8:2). Yield: 56%; yellow solid, mp > 260 °C. IR (cm−1) νmax 2231, 1638, 1614, 1583, 1476, 1413, 1380, 1356, 1309, 1273, 1200, 1170, 1120, 982, 901, 838, 820; 1H-NMR (300 MHz, DMSO-d6) δ 8.55 (d, 1H, J = 9.0 Hz), 8.24 (s, 1H), 7.79 (d, 1H, J = 9.0 Hz), 7.45 (d, 1H, J = 9.0 Hz), 7.34 (t, 1H, J = 8.4 Hz), 7.26 (d, 1H, J = 9.0 Hz); HRMS calcd for C16H8N5SClF [M + H]+: 356.0173, found 356.0160.
9-(2-Fluoro-4-methoxyphenylamino)thiazolo[5,4-f]quinazoline-2-carbonitrile (5i). Prepared from 2 and 2-fluoro-4-methoxyaniline. Flash chromatography eluent (DCM-EtOAc, 5:5). Yield: 85%; yellow solid; mp > 260 °C. IR (cm−1) νmax 2844, 2226, 1731, 1649, 1613, 1583, 1507, 1493, 1460, 1445, 1379, 1356, 1305, 1263, 1212, 1168, 1153, 1129, 1090, 1027, 980, 947, 841, 830, 818; 19F-NMR (282 MHz, DMSO-d6) δ −120.02; 1H-NMR (300 MHz, DMSO-d6) δ 8.51 (d, 1H, J = 9.0 Hz), 8.15 (s, 1H), 7.77 (d, 1H, J = 9.0 Hz), 7.30 (s, 1H), 6.92 (m, 1H), 6.80 (d, 2H, J = 9.0 Hz); HRMS calcd for C17H11N5OSF [M + H]+: 352.0668, found 352.0658.
9-(3-Fluoro-4-hydroxyphenylamino)thiazolo[5,4-f]quinazoline-2-carbonitrile (5j). Prepared from 2 and 4-amino-2-fluorophenol. Flash chromatography eluent (DCM-EtOAc, 5:5). Yield: 58%; orange solid, mp > 260 °C. IR (cm−1) νmax 3375, 2228, 1731, 1649, 1619, 1578, 1512, 1470, 1373, 1347, 1292, 1241, 1204, 1150, 1111, 978, 943, 856, 836; 19F-NMR (282 MHz, DMSO-d6) δ −136.8; 1H-NMR (300 MHz, DMSO-d6) δ 8.46 (d, 1H, J = 9.0 Hz), 8.15 (s, 1H), 7.69 (d, 1H, J = 9.0 Hz), 6.97–6.81 (m, 3H); HRMS calcd for C16H9N5OSF [M + H]+: 338.0512, found 338.0516.
9-(2,4-Difluorophenylamino)thiazolo[5,4-f]quinazoline-2-carbonitrile (5k). Prepared from 2 and 2,4-difluoroaniline. Flash chromatography eluent (DCM-EtOAc, 7:3). Yield: 68%; yellow solid; mp > 260 °C. IR (cm−1) νmax 2228, 1645, 1611, 1583, 1557, 1488, 1460, 1378, 1357, 1311, 1276, 1260, 1172, 1138, 1091, 962, 854, 831, 818; 19F-NMR (282 MHz, DMSO-d6) δ −117.6, −118.7; 1H-NMR (300 MHz, DMSO-d6) δ 8.54 (d, 1H, J = 9.0 Hz), 8.22 (s, 1H), 7.78 (d, 1H, J = 9.0 Hz), 7.35–7.24 (m, 2H), 7.06 (t, 1H, J = 7.8 Hz); HRMS calcd for C16H8N5SF2 [M + H]+: 340.0468, found 340.0458.
9-(4-(Trifluoromethyl)phenylamino)thiazolo[5,4-f]quinazoline-2-carbonitrile (5l). Prepared from 2 and 4-aminobenzotrifluoride. Flash chromatography eluent (DCM-EtOAc, 7:3). Yield: 61%; yellow solid, mp > 260 °C. IR (cm−1) νmax 2851, 2229, 1649, 1604, 1582, 1512, 1457, 1382, 1318, 1272, 1252, 1221, 1165, 1117, 1101, 1062, 1011, 979, 863, 830; 19F-NMR (282 MHz, DMSO-d6) δ −60.01; 1H-NMR (300 MHz, DMSO-d6) δ 8.53 (d, 1H, J = 9.0 Hz), 8.22 (s, 1H), 7.77 (d, 1H, J = 9.0 Hz), 7.70 (d, 2H, J = 8.4 Hz), 7.39 (d, 2H, J = 8.4 Hz); HRMS calcd for C17H9N5SF3 [M + H]+: 372.0521, found 372.0531.
Series 6a–h: Compounds Bearing 9N-Phenyl Groups with Alkyl, Amines and Nitrogen Containing Substituents
9-(Phenylamino)thiazolo[5,4-f]quinazoline-2-carbonitrile (6a). Prepared from 2 and aniline. Flash chromatography eluent (DCM-EtOAc, 5:5). Yield: 67%; yellow solid; mp > 260 °C. IR (cm−1) νmax 3395, 3057, 2228, 1731, 1644, 1608, 1577, 1491, 1459, 1378, 1352, 1301, 1255, 1214, 1147, 1128, 1106, 1071, 967, 896, 827; 1H-NMR (300 MHz, DMSO-d6) δ 8.51 (d, 1H, J = 9.0 Hz), 8.11 (s, 1H), 7.78 (d, 1H, J = 9.0 Hz), 7.40 (t, 2H, J = 7.5 Hz), 7.20 (m, 2H), 7.11 (t, 1H, J = 7.5 Hz); HRMS calcd for C16H10N5S [M + H]+: 304.0657, found 304.0657.
9-(p-Tolylamino)thiazolo[5,4-f]quinazoline-2-carbonitrile (6b). Prepared from 2 and 4-toluidine. Flash chromatography eluent (DCM-EtOAc, 7:3). Yield: 64%; yellow solid; mp = 260 °C. IR (cm−1) νmax 3016, 2853, 2228, 1731, 1641, 1605, 1581, 1554, 1505, 1458, 1376, 1353, 1304, 1268, 1215, 1165, 1130, 976, 831, 811; 1H-NMR (300 MHz, DMSO-d6) δ 8.49 (d, 1H, J = 9.0 Hz), 8.07 (s, 1H), 7.76 (d, 1H, J = 9.0 Hz), 7.20–7.17 (m, 2H), 7.12–7.05 (m, 2H), 2.32 (s, 3H); HRMS calcd for C17H12N5S [M + H]+: 318.0813, found 318.0811.
9-(4-tert-Butylbenzylamino)thiazolo[5,4-f]quinazoline-2-carbonitrile (6c). Prepared from 2 and 4-tert-butylaniline. Flash chromatography eluent (DCM-EtOAc, 7:3). Yield: 99%; yellow solid, mp = 154–156 °C. IR ((cm−1) νmax 2958, 2235, 1693, 1649, 1582, 1505, 1466, 1408, 1349, 1288, 1219, 1155, 1125, 989, 968, 894, 831; 1H-NMR (300 MHz, DMSO-d6) δ 8.49 (d, 1H, J = 9.0 Hz), 8.07 (s, 1H), 7.76 (d, 1H, J = 9.0 Hz), 7.40 (d, 2H, J = 7.8 Hz), 7.12 (m, 2H), 1.31 (s, 9H); HRMS calcd for C20H18N5S [M + H]+: 360.1283, found 360.1273.
9-(3-Ethynylphenylamino)thiazolo[5,4-f]quinazoline-2-carbonitrile (6d). Prepared from 2 and 3-ethynylaniline. Flash chromatography eluent (DCM-EtOAc, 7:3). Yield: 84%; yellow solid; mp = 182–184 °C. IR (cm−1) νmax 3295, 3062, 2846, 2225, 1642, 1612, 1581, 1566, 1458, 1404, 1376, 1348, 1306, 1263, 1229, 1165, 1148, 1126, 971, 910, 888, 835; 1H-NMR (300 MHz, DMSO-d6) δ 8.53 (d, 1H, J = 8.7 Hz), 8.20 (s, 1H), 7.78 (d, 1H, J = 8.7 Hz), 7.40–7.35 (m, 2H), 7.29 (m, 1H), 7.20 (m, 1H), 4.17 (s, 1H); HRMS calcd for C18H10N5S [M + H]+: 328.0657, found 328.0659.
9-(4-Cyanophenylamino)thiazolo[5,4-f]quinazoline-2-carbonitrile (6e). Prepared from 2 and 4-aminobenzonitrile. Flash chromatography eluent (DCM-EtOAc, 5:5). Yield: 36%; yellow solid; mp > 260 °C. IR (cm−1) νmax 3293, 2225, 2218, 1722, 1628, 1590, 1562, 1495, 1461, 1387, 1261, 1228, 1132, 966, 847, 814; 1H-NMR (300 MHz, DMSO-d6) δ 8.54 (d, 1H, J = 9.0 Hz), 8.28 (s, 1H), 7.77 (m, 3H), 7.40 (d, 2H, J = 9.0 Hz); HRMS calcd for C17H9N6S [M + H]+: 329.0609, found 329.0612.
9-(3-Cyanophenylamino)thiazolo[5,4-f]quinazoline-2-carbonitrile (6f). Prepared from 2 and 3-aminobenzonitrile. Yield: 40%; yellow solid, mp > 260 °C. IR (cm−1) νmax 3240, 3171, 3088, 2228, 1623, 1591, 1555, 1509, 1465, 1393, 1273, 1229, 1149, 969, 919, 825; 1H-NMR (300 MHz, DMSO-d6) δ 8.54 (d, 1H, J = 9.0 Hz), 8.43 (s, 1H), 7.74 (m, 2H), 7.55–7.53 (m, 3H); HRMS calcd for C17H9N6S [M + H]+: 329.0609, found 329.0600.
9-(1H-Benzo[d]imidazol-6-ylamino)thiazolo[5,4-f]quinazoline-2-carbonitrile (6g). Prepared from 2 and 6-aminobenzimidazole. Flash chromatography eluent (DCM-MeOH 8:2). Yield: 98%; yellow solid; mp > 260 °C. IR (cm−1) νmax 3084, 2226, 1615, 1557, 1464, 1376, 1347, 1248, 1147, 967, 939, 809; 1H-NMR (300 MHz, DMSO-d6) δ 8.48 (d, 1H, J = 8.7 Hz), 8.15–8.10 (m, 2H), 8.02 (m, 1H), 7.75 (d, 1H, J = 8.7 Hz), 7.56 (m, 1H), 7.04 (m, 1H); HRMS calcd for C17H10N7S [M + H]+: 344.0718, found 344.0705.
9-[4-(Dimethylamino)phenylamino]thiazolo[5,4-f]quinazoline-2-carbonitrile (6h). Prepared from 2 and N,N-dimethyl-p-phenylene-diamine. Flash chromatography eluent (DCM-EtOAc, 8:2). Yield: 25%; yellow solid; mp > 260 °C. IR (cm−1) νmax 3293, 2228, 1609, 1572, 1523, 1460, 1368, 1274, 1229, 1204, 1188, 1163, 1141, 1058, 1009, 948, 842, 811; 1H-NMR (300 MHz, DMSO-d6) δ 8.52 (d, 1H, J = 9.0 Hz), 8.16 (s, 1H), 7.86 (d, 1H, J = 9.0 Hz), 7.37 (d, 2H, J = 8.7 Hz), 6.92 (d, 2H, J = 8.7 Hz), 3.00 (s, 6H); HRMS calcd for C18H15N6S [M + H]+: 347.1079, found 347.1066.
9-[4-(Pyrrolidin-1-yl)phenylamino]thiazolo[5,4-f]quinazoline-2-carbonitrile (6i). Prepared from 2 and 4-(pyrrolidin-1-yl)aniline. Flash chromatography eluent (DCM-EtOAc, 8:2). Yield: 48%; yellow solid; mp > 260 °C. IR (cm−1) νmax 3303, 2842, 2233, 1709, 1629, 1613, 1583, 1522, 1466, 1388, 1347, 1275, 1219, 1185, 1166, 1060, 1014, 989, 828, 809; 1H-NMR (300 MHz, DMSO-d6) δ 8.54 (d, 1H, J = 9.0 Hz), 8.15 (s, 1H), 7.88 (d, 1H, J = 9.0 Hz), 7.35 (d, 2H, J = 8.1 Hz), 6.73 (d, 2H, J = 8.1 Hz), 3.17 (m, 4H), 1.99 (m, 4H); HRMS calcd for C20H17N6S [M + H]+: 373.1235, found 373.1218.
3.2.2. Synthesis of Methyl Imidates 7a–l, 8a–l and 9a–i
General procedure: a stirred mixture of carbonitriles 4a–l, 5a–l and 6a–i (0.13 mmol) and NaOCH3 (0.5 M sol. in MeOH, 130 μL) in methanol (4 mL) was heated under microwaves at 65 °C (600W) for 30 min. The solvent was removed in vacuo and the crude residue purified by flash chromatography (DCM-EtOAc) to afford imidates 7a–l, 8a–l and 9a–i.
Series 7a–l: Compounds Bearing 9N-Phenyl Groups with Electron-Donating Substituents (e.g., OH, OR and Derivatives)
Methyl 9-(4-methoxyphenylamino)thiazolo[5,4-f]quinazoline-2-carbimidate (
7a) and
methyl 9-(benzo[d][1,3]dioxol-5-ylamino)thiazolo[5,4-f]quinazoline-2-carbimidate (
7b) were synthesized in Reference [
9].
Methyl 9-(2,3-dihydrobenzo[b][1,4]dioxin-6-ylamino)thiazolo[5,4-f]quinazoline-2-carbimidate (7c). Prepared from carbonitrile 4c. Flash chromatography eluent (EtOAc). Yield: 80%; yellow solid; mp = 192–194 °C. IR (cm−1) νmax 3575, 3063, 1647, 1578, 1499, 1439, 1347, 1302, 1241, 1199, 1156, 1122, 1062, 948, 915, 836; 1H-NMR (300 MHz, DMSO-d6) δ 8.40 (d, 1H, J = 9 Hz), 8.01 (s, 1H), 7.69 (d, 1H, J = 9 Hz), 6.87 (d, 1H, J = 2.1 Hz), 6.67 (m, 2H), 4.24 (s, 4H), 3.94 (s, 3H); HRMS calcd for C19H16N5O3S [M + H]+: 394.0974, found 394.0954.
Methyl 9-(2,3-dihydrobenzofuran-5-ylamino)thiazolo[5,4-f]quinazoline-2-carbimidate (7d). Prepared from carbonitrile 4d. Flash chromatography eluent (EtOAc). Yield: 66%; yellow solid; mp = 200–202 °C. IR (cm−1) νmax 3291, 3053, 2911, 1641, 1611, 1573, 1508, 1482, 1437, 1355, 1333, 1287, 1226, 1196, 1158, 1092, 1067, 985, 942, 821; 1H-NMR (300 MHz, DMSO-d6) δ 8.39 (d, 1H, J = 9 Hz), 8.01 (s, 1H), 7.69 (d, 1H, J = 9 Hz), 7.12 (m, 1H), 6.92 (m, 1H), 6.78–6.73 (m, 1H), 4.53 (t, 2H, J = 8.7 Hz), 3.95 (s, 3H), 3.19 (t, 2H, J = 8.7 Hz); HRMS calcd for C19H16N5O2S [M + H]+: 378.1025, found 378.1006.
Methyl 9-(3,4-dimethoxyphenylamino)thiazolo[5,4-f]quinazoline-2-carbimidate (7e). Prepared from carbonitrile 4e. Flash chromatography eluent (EtOAc). Yield: 89%; yellow solid; mp = 216–218 °C. IR (cm−1) νmax 3289, 2921, 2852, 1651, 1613, 1583, 1505, 1466, 1432, 1376, 1348, 1309, 1261, 1226, 1195, 1164, 1146, 1128, 1075, 1027, 968, 945, 924, 854, 832; 1H-NMR (300 MHz, DMSO-d6) δ 8.44 (d, 1H, J = 9 Hz), 7.92 (s, 1H), 7.72 (d, 1H, J = 9 Hz), 6.99 (d, 1H, J = 2.1 Hz), 6.83 (d, 1H, J = 8.4 Hz), 6.75 (dd, 1H, J1 = 2.1 Hz, J2 = 8.4 Hz), 3.94 (s, 3H), 3.76 (s, 6H); HRMS calcd for C19H18N5O3S [M + H]+: 396.1130, found 396.1119.
Methyl 9-(2,4-dimethoxyphenylamino)thiazolo[5,4-f]quinazoline-2-carbimidate (7f). Prepared from carbonitrile 4f. Flash chromatography eluent (EtOAc). Yield: 71%; pale green solid; mp = 244–246 °C. IR (cm−1) νmax 3380, 3277, 2999, 2942, 2828, 1654, 1608, 1566, 1545, 1526, 1506, 1455, 1431, 1332, 1276, 1204, 1152, 1123, 1097, 1063, 1026, 993, 963, 942, 916; 1H-NMR (300 MHz, DMSO-d6) δ 9.33 (s, 1H, NH), 8.41 (d, 1H, J = 9.0 Hz), 7.84 (s, 1H), 7.73 (d, 1H, J = 9.0 Hz), 6.88 (m, 1H), 6.68 (m, 1H), 6.58 (d, 1H, J = 7.8 Hz), 3.94 (s, 3H), 3.79 (s, 3H), 3.72 (s, 3H); HRMS calcd for C19H18N5O3S [M + H]+: 396.1130, found 396.1124.
Methyl 9-(3,5-dimethoxyphenylamino)thiazolo[5,4-f]quinazoline-2-carbimidate (7g). Prepared from carbonitrile 4g. Flash chromatography eluent (EtOAc). Yield: 58%; pale yellow solid; mp = 258–260 °C. IR (cm−1) νmax 3237, 2955, 2929, 1731, 1660, 1579, 1495, 1440, 1368, 1347, 1301, 1250, 1189, 1149, 1107, 1058, 973, 953, 856, 824; 1H-NMR (300 MHz, DMSO-d6) δ 9.34 (s, 1H, NH), 8.43 (d, 1H, J = 9.0 Hz), 7.96 (s, 1H), 7.74 (d, 1H, J = 9.0 Hz), 6.25 (m, 3H), 3.94 (s, 3H), 3.74 (s, 6H); HRMS calcd for C19H18N5O3S [M + H]+: 396.1130, found 396.1128.
Methyl 9-(4-methoxy-3-nitrophenylamino)thiazolo[5,4-f]quinazoline-2-carbimidate (7h). Prepared from carbonitrile 153. Flash chromatography eluent (DCM-MeOH, 95:5). Yield: 59%; yellow solid; mp = 212–214 °C. IR (cm−1) νmax 1731, 1643, 1603, 1520, 1489, 1438, 1345, 1266, 1158, 1072, 1014, 946, 870, 821, 810; 1H-NMR (300 MHz, DMSO-d6) δ 9.32 (s, 1H, NH), 8.40 (d, 1H, J = 9.0 Hz), 8.17 (s, 1H), 7.82 (d, 1H, J = 9.0 Hz), 7.66 (d, 1H, J = 9.0 Hz), 7.55 (d, 1H, J = 8.1 Hz), 7.36 (d, 1H, J = 9.0 Hz), 3.95 (s, 3H), 3.92 (s, 3H); HRMS calcd for C18H15N6O4S [M + H]+: 411.0876, found 411.0869.
Methyl 9-(4-hydroxyphenylamino)thiazolo[5,4-f]quinazoline-2-carbimidate (7i). Prepared from carbonitrile 4i. Flash chromatography eluent (EtOAc). Yield: 81%; yellow solid; mp = 194–196 °C. IR (cm−1) νmax 2953, 2852, 1644, 1619, 1573, 1508, 1477, 1372, 1326, 1235, 1164, 1100, 1077, 968, 940, 835; 1H-NMR (300 MHz, DMSO-d6) δ 8.38 (d, 1H, J = 9 Hz), 8.02 (s, 1H), 7.69 (d, 1H, J = 9 Hz), 7.04 (m, 2H), 6.80–6.73 (m, 2H), 3.94 (s, 3H); HRMS calcd for C17H14N5O2S [M + H]+: 352.0868, found 352.0873.
Methyl 9-(3-hydroxy-4-methoxyphenylamino)thiazolo[5,4-f]quinazoline-2-carbimidate (7j). Prepared from carbonitrile 4j. Flash chromatography eluent (EtOAc). Quantitative yield; yellow solid; mp = 216–218 °C. IR (cm−1) νmax 3289, 2921, 2852, 1643, 1611, 1578, 1505, 1441, 1379, 1348, 1281, 1245, 1154, 1128, 1077, 1027, 957, 834; 1H-NMR (300 MHz, DMSO-d6) δ 8.40 (d, 1H, J = 9 Hz), 7.99 (s, 1H), 7.71 (d, 1H, J = 9 Hz), 6.94 (d, 1H, J = 8.4 Hz), 6.65–6.55 (m, 2H), 3.94 (s, 3H), 3.77 (s, 3H); HRMS calcd for C18H16N5O3S [M + H]+: 382.0974, found 382.0957.
Methyl 9-(4-hydroxy-3-nitrophenylamino)thiazolo[5,4-f]quinazoline-2-carbimidate (7k). Prepared from carbonitrile 4k. Flash chromatography eluent (EtOAc). Yield: 34%; orange solid; mp = 208–201 °C. IR (cm−1) νmax 2957, 2911, 1724, 1622, 1560, 1520, 1476, 1379, 1310, 1243, 1156, 1070, 971, 945, 820; 1H-NMR (300 MHz, DMSO-d6) δ 8.30 (d, 1H, J = 8.7 Hz), 8.18 (m, 1H), 7.67 (m, 2H), 7.16 (d, 1H, J = 8.7 Hz), 3.96 (s, 3H); HRMS calcd for C17H13N6O4S [M + H]+: 397.0719, found 397.0710.
Methyl 9-(3,4,5-trimethoxyphenylamino)thiazolo[5,4-f]quinazoline-2-carbimidate (7l). Prepared from carbonitrile 4l. Flash chromatography eluent (EtOAc). Yield: 87%; yellow solid; mp = 252–254 °C. IR (cm−1) νmax 3291, 2941, 2833, 1640, 1583, 1496, 1434, 1415, 1337, 1228, 1164, 1143, 1116, 1073, 993, 975, 954, 861, 844, 822; 1H-NMR (300 MHz, DMSO-d6) δ 9.34 (s, 1H, NH), 8.42 (d, 1H, J = 9.0 Hz), 7.94 (s, 1H), 7.74 (d, 1H, J = 9.0 Hz), 6.37 (s, 2H), 3.94 (s, 3H), 3.77 (s, 6H), 3.67 (s, 3H); HRMS calcd for C20H20N5O4S [M + H]+: 426.1236, found 426.1240.
Series 8a–l: Compounds Bearing 9N-Phenyl Groups with Halogen Substituents (e.g., Cl, Br and F)
Methyl 9-(4-chlorophenylamino)thiazolo[5,4-f]quinazoline-2-carbimidate (8a). Prepared from carbonitrile 5a. Flash chromatography eluent (EtOAc). Yield: 62%; yellow solid; mp > 260 °C. IR (cm−1) νmax 2948, 1644, 1604, 1557, 1509, 1481, 1435, 1401, 1356, 1285, 1240, 1159, 1094, 1074, 992, 943, 816; 1H-NMR (300 MHz, DMSO-d6) δ 8.42 (d, 1H, J = 9 Hz), 8.08 (s, 1H), 7.70 (d, 1H, J = 9 Hz), 7.41 (d, 2H, J = 8.1 Hz), 7.20 (m, 2H), 3.95 (s, 3H); HRMS calcd for C17H13N5OSCl [M + H]+: 370.0529, found 370.0521.
Methyl 9-(3-chlorophenylamino)thiazolo[5,4-f]quinazoline-2-carbimidate (8b). Prepared from carbonitrile 5b. Flash chromatography eluent (EtOAc). Yield: 78%; pale yellow solid; mp = 234–236 °C. IR (cm−1) νmax 3293, 2950, 1639, 1593, 1550, 1507, 1470, 1437, 1355, 1286, 1157, 1070, 994, 968, 943, 876, 821; 1H-NMR (300 MHz, DMSO-d6) δ 9.32 (s, 1H, NH), 8.40 (d, 1H, J = 9.0 Hz), 8.11 (s, 1H), 7.67 (d, 1H, J = 9.0 Hz), 7.37 (t, 1H, J = 7.8 Hz), 7.23 (m, 1H), 7.12–7.08 (m, 2H), 3.94 (s, 3H); HRMS calcd for C17H13N5OSCl [M + H]+: 370.0529, found 370.0524.
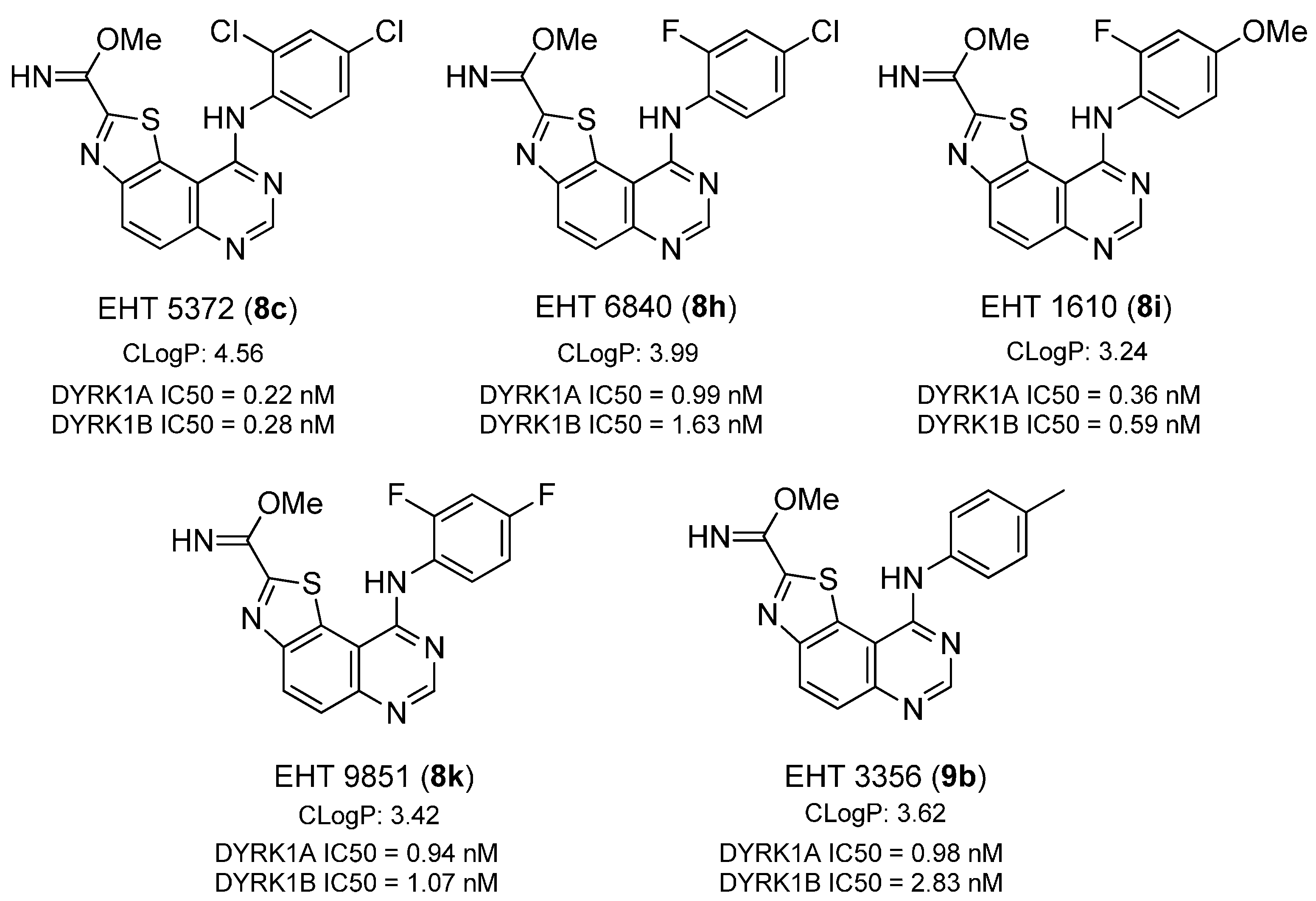
Methyl 9-(2,4-dichlorophenylamino)thiazolo[5,4-f]quinazoline-2-carbimidate (8c). Prepared from carbonitrile 5c. Flash chromatography eluent (EtOAc). Yield: 81%; yellow solid; mp > 260 °C. IR (cm−1) νmax 2953, 1727, 1641, 1586, 1507, 1488, 1464, 1394, 1354, 1284, 1158, 1099, 1073, 1054, 941, 860, 816; 1H-NMR (300 MHz, DMSO-d6) δ 9.34 (s, 1H, NH), 8.45 (d, 1H, J = 9.0 Hz), 8.10 (s, 1H), 7.71 (d, 1H, J = 9.0 Hz), 7.62 (s, 1H), 7.38 (d, 1H, J = 8.1 Hz), 7.18 (d, 1H, J = 8.1 Hz), 3.93 (s, 3H); HRMS calcd for C17H12N5OSCl2 [M + H]+: 404.0140, found 404.0146.
Methyl 9-(3,4-dichlorophenylamino)thiazolo[5,4-f]quinazoline-2-carbimidate (8d). Prepared from carbonitrile 5d. Flash chromatography eluent (DCM-EtOAc, 5:5). Yield: 45%; yellow solid; mp = 228–230 °C. IR (cm−1) νmax 3296, 2920, 1640, 1608, 1588, 1551, 1507, 1491, 1469, 1437, 1397, 1356, 1284, 1158, 1129, 1073, 1023, 942, 860, 821; 1H-NMR (300 MHz, DMSO-d6) δ 8.42 (d, 1H, J = 9 Hz), 8.19 (s, 1H), 7.69 (d, 1H, J = 9 Hz), 7.56 (d, 2H, J = 9 Hz), 7.22 (m, 1H), 3.95 (s, 3H); HRMS calcd for C17H12N5OSCl2 [M + H]+: 404.0140, found 404.0135.
Methyl 9-(4-fluorophenylamino)thiazolo[5,4-f]quinazoline-2-carbimidate (8e). Prepared from carbonitrile 5e. Flash chromatography eluent (EtOAc). Yield: 77%; yellow solid; mp > 260 °C. IR (cm−1) νmax 3416, 3298, 3226, 3150, 2950, 1731, 1641, 1611, 1574, 1558, 1506, 1490, 1434, 1400, 1355, 1329, 1285, 1226, 1157, 1103, 1072, 994, 968, 943, 819; 19F-NMR (282 MHz, DMSO-d6) δ −120.8; 1H-NMR (300 MHz, DMSO-d6) δ 9.33 (s, 1H, NH), 8.41 (d, 1H, J = 9.0 Hz), 7.93 (s, 1H), 7.68 (d, 1H, J = 9.0 Hz), 7.19 (m, 4H), 3.95 (s, 3H); HRMS calcd for C17H13N5OSF [M + H]+: 354.0825, found 354.0811.
Methyl 9-(4-bromo-2-fluorophenylamino)thiazolo[5,4-f]quinazoline-2-carbimidate (
8f) and
methyl 9-(3-chloro-4-fluorophenylamino)thiazolo[5,4-f]quinazoline-2-carbimidate (
8i) were synthesized in Reference [
9].
Methyl 9-(4-chloro-2-fluorophenylamino)thiazolo[5,4-f]quinazoline-2-carbimidate (8h). Prepared from carbonitrile 5h. Flash chromatography eluent (EtOAc). Yield: 58%; yellow solid; mp > 260 °C. IR (cm−1) νmax 2953, 1641, 1600, 1553, 1507, 1481, 1397, 1355, 1287, 1198, 1159, 1120, 1072, 944, 899, 818; 19F-NMR (282 MHz, DMSO-d6) δ −120.1; 1H-NMR (300 MHz, DMSO-d6) δ 9.34 (s, 1H, NH), 8.45 (d, 1H, J = 9.0 Hz), 8.14 (s, 1H), 7.71 (d, 1H, J = 9.0 Hz), 7.44 (d, 1H, J = 9.0 Hz), 7.24 (m, 2H), 3.94 (s, 3H); HRMS calcd for C17H12N5OSClF [M + H]+: 388.0435, found 388.0426.
Methyl 9-(2-fluoro-4-methoxyphenylamino)thiazolo[5,4-f]quinazoline-2-carbimidate (8i). Prepared from carbonitrile 5i. Flash chromatography eluent (EtOAc). Yield: 82%; yellow solid; mp = 224–226 °C. IR (cm−1) νmax 3150, 2950, 1645, 1601, 1570, 1488, 1435, 1355, 1322, 1285, 1269, 1203, 1155, 1096, 1069, 1032, 939, 819; 19F-NMR (282 MHz, DMSO-d6) δ −120.44; 1H-NMR (300 MHz, DMSO-d6) δ 9.33 (s, 1H, NH), 8.42 (d, 1H, J = 9.0 Hz), 8.06 (s, 1H), 7.70 (d, 1H, J = 9.0 Hz), 7.19 (m, 1H), 6.88 (m, 1H), 6.77 (m, 1H), 3.94 (s, 3H), 3.78 (s, 3H); HRMS calcd for C18H15N5O2SF [M + H]+: 384.0927, found 384.0930.
Methyl 9-(3-fluoro-4-hydroxyphenylamino)thiazolo[5,4-f]quinazoline-2-carbimidate (8j). Prepared from carbonitrile 5j. Flash chromatography eluent (EtOAc). Yield: 70%; pale brown solid; mp > 260 °C. IR (cm−1) νmax 3374, 1729, 1652, 1626, 1585, 1519, 1465, 1386, 1352, 1302, 1241, 1209, 1156, 1111, 978, 856, 827; 19F-NMR (282 MHz, DMSO-d6) δ −138.5; 1H-NMR (300 MHz, DMSO-d6) δ 8.52 (d, 1H, J = 9.0 Hz), 8.19 (s, 1H), 7.72 (d, 1H, J = 9.0 Hz), 7.04–6.91 (m, 3H), 3.95 (s, 3H); HRMS calcd for C17H13N5O2SF [M + H]+: 370.0774, found 370.0762.
Methyl 9-(2,4-difluorophenylamino)thiazolo[5,4-f]quinazoline-2-carbimidate (8k). Prepared from carbonitrile 5k. Flash chromatography eluent (EtOAc). Yield: 71%; yellow solid; mp > 260 °C; IR (cm−1) νmax 1644, 1608, 1574, 1556, 1509, 1488, 1435, 1357, 1285, 1260, 1188, 1140, 1073, 963, 943, 843, 819; 19F-NMR (282 MHz, DMSO-d6) δ −117.6, −118.8; 1H-NMR (300 MHz, DMSO-d6) δ 9.34 (s, 1H, NH), 8.45 (d, 1H, J = 9.0 Hz), 8.12 (s, 1H), 7.70 (d, 1H, J = 9.0 Hz), 7.27 (m, 2H), 7.05 (t, 1H, J = 7.8 Hz), 3.94 (s, 3H); HRMS calcd for C17H12N5OSF2 [M + H]+: 372.0731, found 372.0725.
Methyl 9-(4-(trifluoromethyl)phenylamino)thiazolo[5,4-f]quinazoline-2-carbimidate (8l). Prepared from carbonitrile 5l. Flash chromatography eluent (EtOAc). Yield: 53%; pale yellow solid; mp > 260 °C. IR (cm−1) νmax 3277, 1643, 1601, 1588, 1561, 1509, 1493, 1324, 1284, 1151, 1104, 1065, 1015, 966, 937, 829, 809; 19F-NMR (282 MHz, DMSO-d6) δ −59.95; 1H-NMR (300 MHz, DMSO-d6) δ 8.42 (d, 1H, J = 9.0 Hz), 8.11 (s, 1H), 7.69 (d, 3H, J = 7.8 Hz), 7.32 (d, 2H, J = 7.8 Hz), 3.93 (s, 3H); HRMS calcd for C18H13N5OSF3 [M + H]+: 404.0736, found 404.0742.
Series 9a–h: Compounds Bearing 9N-Phenyl Groups with Alkyl, Amines and Nitrogen Containing Substituents
Methyl 9-(phenylamino)thiazolo[5,4-f]quinazoline-2-carbimidate (9a). Prepared from carbonitrile 6a. Flash chromatography eluent (EtOAc). Yield: 52%; yellow solid; mp > 260 °C. IR (cm−1) νmax 3294, 3147, 2950, 2877, 1727, 1640, 1609, 1570, 1552, 1507, 1480, 1434, 1351, 1284, 1210, 1153, 1067, 990, 965, 939, 869, 819; 1H-NMR (300 MHz, DMSO-d6) δ 9.32 (s, 1H, NH), 8.41 (d, 1H, J = 9.0 Hz), 7.99 (s, 1H), 7.68 (d, 1H, J = 9.0 Hz), 7.38 (m, 2H), 7.09 (m, 3H), 3.94 (s, 3H); HRMS calcd for C17H14N5OS [M + H]+: 336.0919, found 336.0904.
Methyl 9-(p-tolylamino)thiazolo[5,4-f]quinazoline-2-carbimidate (9b). Prepared from carbonitrile 6b. Flash chromatography eluent (EtOAc). Yield: 88%; yellow solid; mp > 260 °C. IR (cm−1) νmax 3292, 3148, 2848, 1725, 1641, 1600, 1557, 1488, 1434, 1351, 1283, 1155, 1068, 990, 966, 939, 820, 804; 1H-NMR (300 MHz, DMSO-d6) δ 9.33 (s, 1H, NH), 8.41 (d, 1H, J = 9.0 Hz), 7.95 (s, 1H), 7.69 (d, 1H, J = 9.0 Hz), 7.20 (m, 2H), 7.11 (m, 2H), 3.94 (s, 3H), 2.32 (s, 3H); HRMS calcd for C18H16N5OS [M + H]+: 350.1076, found 350.1072.
Methyl 9-(4-tert-butylphenylamino)thiazolo[5,4-f]quinazoline-2-carbimidate (9d). Prepared from carbonitrile 6c. Flash chromatography eluent (EtOAc). Yield: 69%; yellow solid; ; mp > 260 °C. IR (cm−1) νmax 3267, 2939, 1731, 1644, 1599, 1580, 1493, 1342, 1269, 1248, 1161, 1114, 1066, 988, 965, 941, 899, 838; 1H-NMR (300 MHz, DMSO-d6) δ 9.31 (s, 1H, NH), 8.39 (d, 1H, J = 9.0 Hz), 8.17 (s, 1H), 7.84 (d, 1H, J = 9.0 Hz), 7.41 (d, 2H, J = 7.8 Hz), 7.12 (m, 2H), 3.93 (s, 3H), 1.31 (s, 9H); HRMS calcd for C22H22N5OS (M + H+): 392.1545, found 392.1539.
Methyl 9-(3-ethynylphenylamino)thiazolo[5,4-f]quinazoline-2-carbimidate (9d). Prepared from carbonitrile 6d. Flash chromatography eluent (EtOAc). Yield: 68%; yellow solid; mp = 220–222 °C. IR (cm−1) νmax 3293, 2950, 1731, 1644, 1613, 1552, 1489, 1437, 1353, 1286, 1157, 1070, 968, 941, 871, 822; 1H-NMR (300 MHz, DMSO-d6) δ 8.43 (d, 1H, J = 9 Hz), 8.11 (s, 1H), 7.70 (d, 1H, J = 9 Hz), 7.37 (t, 1H, J = 7.8 Hz), 7.26–7.16 (m, 3H), 4.16 (s, 1H), 3.95 (s, 3H); HRMS calcd for C19H14N5OS [M + H]+: 360.0919, found 360.0908.
Methyl 9-(4-cyanophenylamino)thiazolo[5,4-f]quinazoline-2-carbimidate (9e). Prepared from carbonitrile 6e. Flash chromatography eluent (EtOAc). Yield: 52%; yellow solid; mp > 260 °C. IR (cm−1) νmax 3264, 2215, 1655, 1625, 1591, 1561, 1493, 1435, 1385, 1335, 1272, 1227, 1146, 1069, 995, 938, 848, 815; 1H-NMR (300 MHz, DMSO-d6) δ 9.32 (s, 1H, NH), 8.43 (d, 1H, J = 9.0 Hz), 8.17 (s, 1H), 7.79 (d, 2H, J = 6.9 Hz), 7.68 (d, 1H, J = 7.2 Hz), 7.32 (d, 2H, J = 6.9 Hz), 3.94 (s, 3H); HRMS calcd for C18H13N6OS [M + H]+: 361.0872, found 361.0863.
Methyl 9-(3-cyanophenylamino)thiazolo[5,4-f]quinazoline-2-carbimidate (9f). Prepared from carbonitrile 6f. Flash chromatography eluent (EtOAc). Yield: 33%; pale yellow solid; mp > 260 °C. IR (cm−1) νmax 3272, 2235, 1722, 1638, 1615, 1581, 1571, 1491, 1437, 1143, 1109, 1067, 989, 968, 943, 885, 840; 1H-NMR (300 MHz, DMSO-d6) δ 9.34 (s, 1H, NH), 8.45 (d, 1H, J = 9.0 Hz), 8.20 (s, 1H), 7.68 (d, 1H, J = 9.0 Hz), 7.61 (m, 4H), 3.95 (s, 3H); HRMS calcd for C18H13N6OS [M + H]+: 361.0872, found 361.0862.
Methyl 9-(1H-benzo[d]imidazol-6-ylamino)thiazolo[5,4-f]quinazoline-2-carbimidate (9g). Prepared from carbonitrile 6g. Flash chromatography eluent (DCM-MeOH, 8:2). Yield: 57%; yellow solid; mp > 260 °C. IR (cm−1) νmax 3094, 1641, 1615, 1573, 1479, 1380, 1343, 1294, 1199, 1141, 1070, 947, 824; 1H-NMR (300 MHz, DMSO-d6) δ 8.46–8.36 (m, 2H), 8.18 (m, 1H), 8.02 (d, 1H, J = 9 Hz), 7.73 (d, 1H, J = 8.1 Hz), 7.65 (d, 1H, J = 8.1 Hz), 7.54 (m, 1H), 3.95 (s, 3H); HRMS calcd for C18H14N7OS [M + H]+: 376.0981, found 376.0974.
Methyl 9-(4-(dimethylamino)phenylamino)thiazolo[5,4-f]quinazoline-2-carbimidate (9h). Prepared from carbonitrile 6h. Flash chromatography eluent (EtOAc). Yield: 94%; beige solid; mp > 260 °C. IR (cm−1) νmax 3288, 2945, 1629, 1608, 1577, 1520, 1496, 1444, 1337, 1291, 1275, 1209, 1183, 1167, 1068, 968, 943, 820; 1H-NMR (300 MHz, DMSO-d6) δ 9.35 (s, 1H, NH), 8.42 (d, 1H, J = 9.0 Hz), 8.07 (s, 1H), 7.77 (d, 1H, J = 9.0 Hz), 7.36 (d, 2H, J = 8.4 Hz), 6.91 (d, 2H, J = 8.4 Hz), 3.94 (s, 3H), 2.99 (s, 3H); HRMS calcd for C19H19N6OS [M + H]+: 379.1341, found 379.1330.
Methyl 9-(4-(pyrrolidin-1-yl)phenylamino)thiazolo[5,4-f]quinazoline-2-carbimidate (9i). Prepared from carbonitrile 6i. Flash chromatography eluent (DCM-EtOAc, 5:5). Yield: 75%; beige solid; mp > 260 °C. IR (cm−1) νmax 3293, 2847, 1632, 1608, 1577, 1520, 1491, 1444, 1388, 1293, 1265, 1209, 1178, 1163, 1107, 1062, 968, 927, 820, 806; 1H-NMR (300 MHz, DMSO-d6) δ 9.35 (s, 1H, NH), 8.42 (d, 1H, J = 9.0 Hz), 8.06 (s, 1H), 7.77 (d, 1H, J = 9.0 Hz), 7.34 (d, 2H, J = 8.4 Hz), 6.72 (d, 2H, J = 8.4 Hz), 3.94 (s, 3H), 3.29 (m, 4H), 1.98 (m, 4H); HRMS calcd for C21H21N6OS [M + H]+: 405.1457, found 405.1452.
3.2.3. SAR Studies
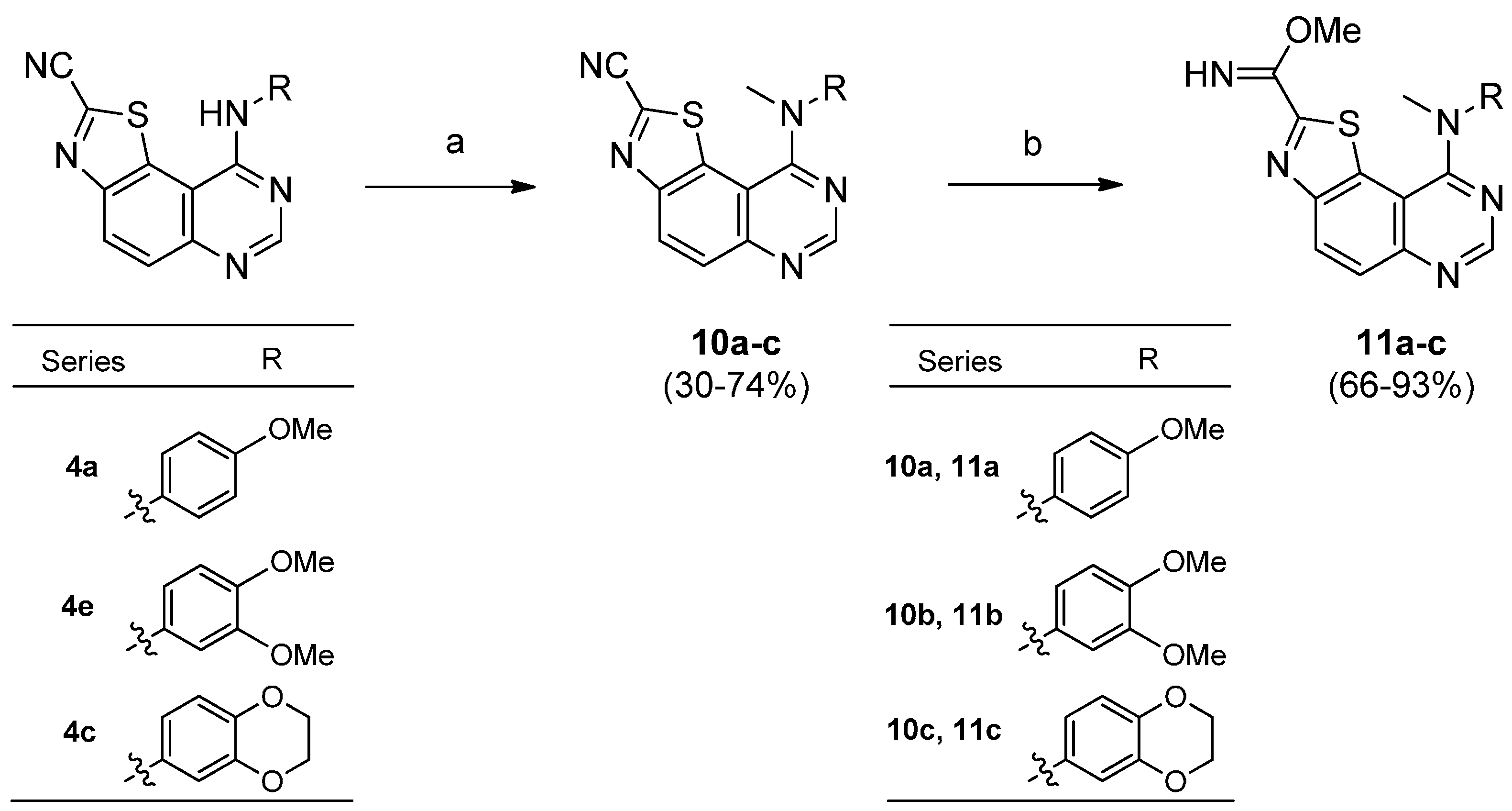
Synthesis of 9N-Methylated-thiazolo[5,4-f]quinazoline-2-carbonitriles (10a–c)
Methyl iodide (0.90 mmol) was added dropwise to a stirred suspension of carbonitrile 4a, 4c and 4e (0.60 mmol) and sodium hydride (0.90 mmol, 60% dispersion in mineral oil) in dimethylformamide (4 mL). The mixture was stirred for 1 h at 0 °C and then for 2 h at room temperature. After cooling, the resulting mixture was concentrated under reduced pressure. The crude residue obtained was purified by flash chromatography (DCM-ethyl acetate, 1:9) to give 10a–c.
9-[(4-Methoxyphenyl)(methyl)amino]thiazolo[5,4-f]quinazoline-2-carbonitrile (10a). Prepared from carbonitrile 4a. Flash chromatography eluent (EtOAc). Yield: 60%; orange solid; mp > 260 °C. IR (cm−1) νmax 3073, 2949, 2908, 2835, 2225, 1615, 1551, 1497, 1481, 1461, 1452, 1436, 1362, 1235, 1153, 1060, 1031, 974, 839, 825, 801; 1H-NMR (300 MHz, DMSO-d6) δ 8.49 (d, 1H, J = 9.0 Hz), 8.23 (s, 1H), 7.88 (d, 1H, J = 9.0 Hz), 7.48 (d, 1H, J = 8.7 Hz), 6.92 (d, 1H, J = 8.7 Hz), 3.77 (s, 3H), 3.73 (s, 3H); HRMS calcd for C18H14N5OS [M + H]+: 348.0919, found 348.0908.
9-[(3,4-Dimethoxyphenyl)(methyl)amino]thiazolo[5,4-f]quinazoline-2-carbonitrile (10b). Prepared from carbonitrile 4e. Flash chromatography eluent (EtOAc). Yield: 74%; orange solid; mp = 222–224 °C. IR (cm−1) νmax 3040, 2988, 2957, 2828, 2225, 1621, 1553, 1501, 1442, 1409, 1392, 1366, 1255, 1227, 1201, 1173, 1142, 1123, 1023, 936, 872, 803; 1H-NMR (300 MHz, DMSO-d6) δ 8.56 (d, 1H, J = 9.0 Hz), 8.29 (s, 1H), 7.85 (d, 1H, J = 9.0 Hz), 7.17 (d, 1H, J = 2.1 Hz), 7.08 (dd, 1H, J1 = 2.1 Hz, J2 = 8.7 Hz), 6.93 (d, 1H, J = 8.7 Hz), 3.77 (m, 9H); HRMS calcd for C19H16N5O2S [M + H]+: 378.1025, found 378.1008.
9-[(2,3-Dihydrobenzo[b][1,4]dioxin-6-yl)(methyl)amino]thiazolo[5,4-f]quinazoline-2-carbonitrile (10c). Prepared from carbonitrile 4c. Flash chromatography eluent (EtOAc). Yield: 30%; orange solid; mp > 260 °C. IR (KBr) νmax/cm−1 3422, 2932, 2875, 2220, 1612, 1547, 1487, 1455, 1360, 1299, 1253, 1201, 1149, 1065, 914, 877, 811. 1H-NMR (300 MHz, DMSO-d6) δ 8.54 (d, 1H, J = 9.0 Hz), 8.28 (s, 1H), 7.84 (d, 1H, J = 9.0 Hz), 7.12 (d, 1H, J = 2.4 Hz), 6.98 (dd, 1H, J1 = 2.4 Hz, J2 = 8.7 Hz), 6.82 (d, 1H, J = 8.7 Hz), 4.24 (s, 4H), 3.76 (s, 3H); HRMS calcd for C19H13N5O2S [M + H]+: 375.0819, found 375.0808.
Synthesis of Methyl Thiazolo[5,4-f]quinazoline-2-carbimidates (11a, 11b and 11c)
A stirred mixture of carbonitrile 10a, 10b or 10c (0.13 mmol) and NaOCH3 (0.5 M sol. in MeOH, 130 μL) in methanol (4 mL) was irradiated under microwaves at 65 °C for 30 min. The solvent was removed in vacuo and the crude residue purified by flash chromatography to afford imidates 11a, 11b and 11c, respectively.
Methyl 9-[(4-methoxyphenyl)(methyl)amino]thiazolo[5,4-f]quinazoline-2-carbimidate (11a). Prepared from carbonitrile 10a. Flash chromatography eluent (DCM-MeOH, 9:1). Yield: 93%; yellow solid; mp = 246–248 °C. IR (cm−1) νmax 3267, 3057, 2929, 2837, 1736, 1654, 1613, 1555, 1493, 1434, 1404, 1369, 1330, 1268, 1240, 1219, 1146, 1100, 1058, 1033, 982, 939, 886, 835, 811; 1H-NMR (300 MHz, MeOD-d4) δ 8.42 (d, 1H, J = 9.0 Hz), 8.18 (s, 1H), 7.71 (d, 1H, J = 9.0 Hz), 7.34 (d, 2H, J = 9.0 Hz), 6.91 (d, 2H, J = 9.0 Hz), 3.96 (s, 3H), 3.75 (s, 3H), 3.73 (s, 3H); HRMS calcd for C19H18N5O2S [M + H]+: 380.1181, found 380.1179.
Methyl 9-[(3,4-dimethoxyphenyl)(methyl)amino]thiazolo[5,4-f]quinazoline-2-carbimidate (11b). Prepared from carbonitrile 10b. Flash chromatography eluent (DCM-MeOH, 9:1). Yield: 73%; yellow solid; mp = 220–222 °C. IR (cm−1) νmax 3298, 2986, 2832, 1644, 1619, 1555, 1492, 1434, 1361, 1253, 1228, 1162, 1142, 1127, 1069, 1026, 953, 927, 832, 800; 1H-NMR (300 MHz, DMSO-d6) δ 8.46 (d, 1H, J = 9 Hz), 8.21 (s, 1H), 7.75 (d, 1H, J = 9 Hz), 7.03 (d, 1H, J = 2.1 Hz), 6.93 (d, 1H, J = 8.4 Hz), 6.84 (dd, 1H, J1 = 2.1 Hz, J2 = 8.4 Hz), 3.96 (s, 3H), 3.75 (s, 9H); HRMS calcd for C20H19N5O3S [M + H]+: 409.1230, found 409.1219.
Methyl 9-[(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)(methyl)amino]thiazolo[5,4-f]quinazoline-2-carbimidate (11c). Prepared from carbonitrile 10c. Flash chromatography eluent (DCM-MeOH, 9:1). Yield: 66%; yellow solid; mp = 248–250 °C. IR (cm−1) νmax 3298, 2973, 2875, 1642, 1620, 1555, 1484, 1435, 1408, 1360, 1298, 1273, 1242, 1203, 1162, 1146, 1065, 939, 878, 847; 1H-NMR (300 MHz, DMSO-d6) δ 8.45 (d, 1H, J = 9 Hz), 8.21 (s, 1H), 7.74 (d, 1H, J = 9 Hz), 6.97 (d, 1H, J = 2.1 Hz), 6.83 (m, 2H), 4.24 (s, 4H), 3.96 (s, 3H), 3.75 (s, 3H); HRMS calcd for C20H18N5O3S [M + H]+: 408.1130, found 408.1111.
Synthesis of Ethyl, Isopropyl and Benzyl Thiazolo[5,4-f]quinazoline-2-carbimidates (12a–c)
Ethyl 9-(benzo[d][1,3]dioxol-5-ylamino)thiazolo[5,4-f]quinazoline-2-carbimidate (12a). A stirred mixture of carbonitrile 4b (0.05 g, 0.14 mmol) and NaOCH2CH3 (0.5 M sol. in EtOH, 130 μL) in ethanol (4 mL) was heated under microwaves (600 W) at 80 °C for 30 min. The solvent was removed in vacuo and the crude residue purified by flash chromatography (DCM-EtOAc, 5:5) to afford ethyl imidate 12a (0.043 g 79%) as a yellow solid; mp = 193–195 °C. IR (cm−1) νmax 3286, 2892, 1722, 1654, 1626, 1579, 1497, 1484, 1465, 1372, 1334, 1242, 1230, 1184, 1159, 1128, 1036, 966, 923, 824; 1H-NMR (300 MHz, DMSO-d6) δ 8.39 (d, 1H, J = 9.0 Hz), 7.94 (s, 1H), 7.68 (d, 1H, J = 9.0 Hz), 6.94 (d, 1H, J = 8.1 Hz), 6.76–6.46 (m, 2H), 6.01 (s, 2H), 4.38 (q, 2H, J = 6.9 Hz), 1.38 (t, 3H, J = 6.9 Hz); HRMS calcd for C19H16N5O3S [M + H]+: 394.0974, found 394.0967.
Isopropyl 9-(benzo[d][1,3]dioxol-5-ylamino)thiazolo[5,4-f]quinazoline-2-carbimidate (12b). A stirred mixture of carbonitrile 4b (0.078 g, 0.22 mmol) and KOH (2.5 N sol., 78 μL) in isopropanol (3.9 mL) was heated under microwaves (600 W) at 100 °C for 2 h. The solvent was removed in vacuo and the crude residue purified by flash chromatography (DCM-EtOAc, 5:5) to afford the isopropyl imidate 12b (0.024 g, 27%) as a yellow solid, mp = 224–226 °C. IR (cm−1) νmax 3267, 2977, 2876, 1638, 1613, 1572, 1489, 1475, 1450, 1382, 1369, 1317, 1272, 1244, 1189, 1142, 1112, 1036, 924, 885, 828, 808; 1H-NMR (300 MHz, DMSO-d6) δ 8.40 (d, 1H, J = 9.0 Hz), 7.94 (s, 1H), 7.68 (d, 1H, J = 9.0 Hz), 6.94 (d, 2H, J = 8.1 Hz), 6.75–6.55 (m, 2H), 6.01 (s, 2H), 5.32–5.24 (m, 1H), 1.38 (d, 6H, J = 6.0 Hz); HRMS calcd for C20H18N5O3S [M + H]+: 408.0962, found 408.0956.
Benzyl 9-(benzo[d][1,3]dioxol-5-ylamino)thiazolo[5,4-f]quinazoline-2-carbimidate (12c). A stirred mixture of carbonitrile 4b (0.05 g, 0.14 mmol) and NaOCH2Ph (1.0 M sol. in benzylalcohol, 70 μL) in benzylalcohol (3 mL) was irradiated under microwaves at 100 °C for 30 min. The solvent was removed in vacuo and the crude residue purified by flash chromatography (EtOAc) to afford the benzyl imidate 12c (0.018 g, 28%) as a yellow solid, mp = 180–182 °C. IR (cm−1) νmax 3375, 2228, 1726, 1644, 1613, 1575, 1473, 1378, 1327, 1244, 1192, 1151, 1036, 922, 833; 1H-NMR (300 MHz, DMSO-d6) δ 8.42 (d, 1H, J = 9.0 Hz), 7.99 (s, 1H), 7.68 (d, 1H, J = 9.0 Hz), 7.51 (d, 2H, J = 7.5 Hz), 7.43–7.34 (m, 3H), 6.92 (d, 1H, J = 7.5 Hz), 6.78 (m, 1H), 6.59 (m, 1H), 6.01 (s, 2H), 5.45 (s, 2H); HRMS calcd for C24H18N5O3S [M + H]+: 456.1130, found 456.1128.
Methyl 9-(benzo[d][1,3]dioxol-5-ylamino)thiazolo[5,4-f]quinazoline-2-carboxylate (13). A mixture of methyl 9-(benzo[d][1,3]dioxol-5-ylamino)thiazolo[5,4-f]quinazoline-2-carbimidate (7b) (0.017 mmol) and 5 mL of MeOH/H2O + TFA(0.1%)(60/40) under argon was stirred at room temperature overnight. The solvent was removed in vacuo and the crude residue purified by flash chromatography (DCM-EtOAc, 5:5) to afford ester 13 (5.9 mg, 94% yield) as a yellow solid; mp = 206 °C. IR (cm−1) νmax 3287, 2902, 1648, 1617, 1575, 1528, 1499, 1483, 1452, 1432, 1385, 1322, 1272, 1196, 1125, 1043, 936, 885, 834, 817; 1H-NMR (300 MHz, DMSO-d6) δ 8.42 (d, 1H, J = 9.0 Hz), 8.03 (s, 1H), 7.95 (d, 1H, J = 9.0 Hz), 6.96 (d, 1H, J = 8.0 Hz), 6.84 (m, 1H), 6.72 (d, 1H, J = 8.0 Hz), 5.94 (s, 2H), 4.05 (s, 3H); HRMS calcd for C18H13N4O4S [M + H]+: 381.0658, found 381.0651.