New Natural Diterpene-Type Abietane from Tetradenia riparia Essential Oil with Cytotoxic and Antioxidant Activities
Abstract
:1. Introduction
2. Results and Discussion
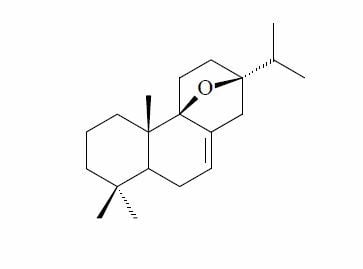
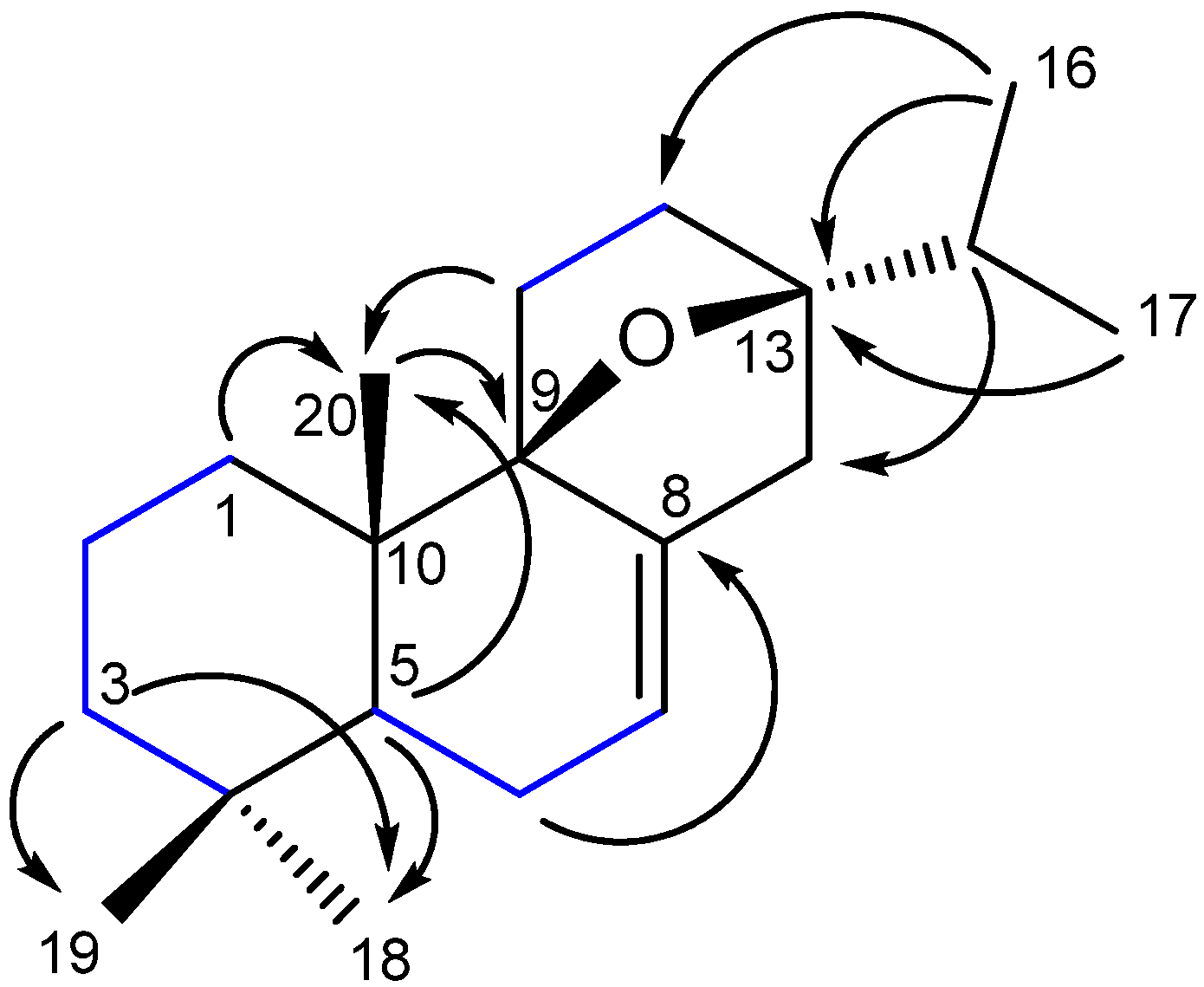
2.1. Identification of the New 9β,13β-Epoxy-7-abietene (1) and an 6,7-Dehydroroyleanone (2) Compounds
| Position | 1H [δ(ppm), mult, J in Hz] | 13C [δ(ppm)] |
|---|---|---|
| 1α | 2.02 m | 33.3 |
| 1β | 1.52 m | 33.3 |
| 2α | 1.61 m | 18.5 |
| 2β | 1.46 m | 18.5 |
| 3α | 1.42 m | 42.7 |
| 3β | 1.18 m | 42.7 |
| 4 | - | 33.3 |
| 5 | 1.38 dd (5.0; 10) | 45.7 |
| 6α | 2.05 m | 24.6 |
| 6β | 1.92 m | 24.6 |
| 7 | 5.25 m | 113.8 |
| 8 | - | 142.4 |
| 9 | - | 90.8 |
| 10 | - | 36.6 |
| 11α | 1.96 m | 30.1 |
| 11β | 1.56 m | 30.1 |
| 12 | 1.58 m | 33.1 |
| 13 | - | 87.9 |
| 14 | 2.11 m | 38.0 |
| 15 | 2.09 sep. (7) | 32.8 |
| 16 | 0.96 d (7) | 18.0 |
| 17 | 0.93 d (7) | 18.2 |
| 18 | 0.87 s | 33.5 |
| 19 | 0.95 s | 22.3 |
| 20 | 1.00 s | 15.5 |
 ) and selected HMBC (H→C) correlations of 1.
) and selected HMBC (H→C) correlations of 1.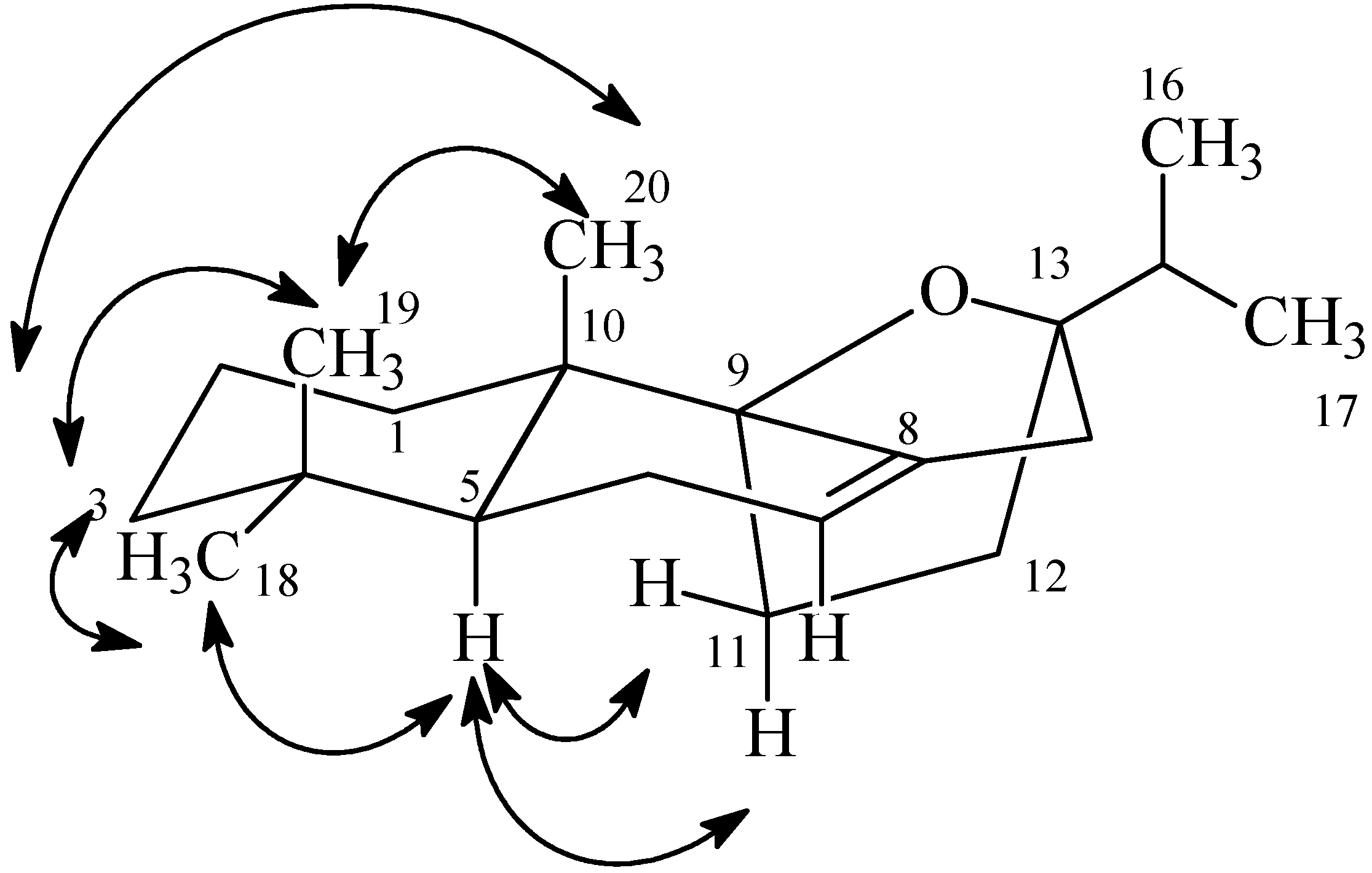

| Isolated compounds | Growth inhibition (%) of cell line * | ||
|---|---|---|---|
| MDA-MB-435 | SF-295 | HCT-8 | |
| T. riparia essential oil | 59.48 ± 0.51 a | 78.06 ± 0.67 b | 85.00 ± 0.46 a |
| 9β,13β-epoxy-7-abietene | 45.43 ± 1.36 b | 94.80 ± 0.82 a | 86.54 ± 1.37 a |
| 6,7-dehydroroyleanone | 3.34 ± 0.11 c | 15.30 ± 0.07 c | 12.08 ± 0.31 b |
2.2. Cytotoxic Analysis
2.3. Antioxidant Analysis
| Compounds | IC50 (µg mL−1) | ||
|---|---|---|---|
| DPPH | β-Carotene-linoleic acid | ABTS | |
| T. riparia essential oil | 15.63 ± 0.25 a | 130.1 ± 5.76 a | 1524 ± 123 a |
| 6,7-Dehydroroyleanone | 0.010 ± ˂0.001 b | 109.6 ± 3.83 b | 1024 ± 54 b |
| BHT | - | 133.5 ± 7.36 a | - |
| Quercetin | 2.05 ± 0.02 c | - | 190 ± 38 c |
3. Experimental
3.1. General
3.2. GC Analysis
3.3. Plant Material
3.4. Essential Oil Fractionation of the Leaves
3.5. Cytotoxicity In Vitro
3.6. Antioxidant Activity
4. Conclusions
Acknowledgments
Conflicts of Interest
References and Notes
- Weaver, D.K.; Dunkel, F.V.; van Puyvelde, L.; Richards, D.C.; Frizgerald, G.W. Toxicity and protectant potential of the essential oil of Tetradenia riparia (Lamiales, Lamiaceae) against Zabrotes subfasciatus (Col., Bruchidae) infesting dried pinto beans (Fabales, Leguminosae). J. Appl. Entomol. 1994, 118, 179–196. [Google Scholar] [CrossRef]
- Campbell, W.E.; Gammon, D.W.; Smith, P.; Abrahams, M.; Purves, T. Composition and antimalarial activity in vitro of the essential oil of Tetradenia riparia. Planta Med. 1997, 63, 270–272. [Google Scholar] [CrossRef]
- Van Puyvelde, L.; Nyirankuliza, S.; Panebianco, R.; Boily, Y.; Geizer, I.; Sebikali, B.; de Kimpe, N.; Schamp, N. Active principles of Tetradenia riparia. I. Antimicrobial activity of 8(14),15-sandaracopimaradiene-7α,18-diol. J. Ethnopharmacol. 1986, 17, 269–275. [Google Scholar] [CrossRef]
- Van Puyvelde, L.; Lefebvre, R.; Mugabo, P.; de Kimpe, N.; Schamp, N. Active principle of Tetradenia riparia. II. Antispasmodic activity of 8(14),15-sandaracopimaradiene-7α,18-diol. Planta Med. 1987, 53, 156–158. [Google Scholar] [CrossRef]
- Van Puyvelde, L.; de Kimpe, N. Tetradenolide, an α-Pirone from Tetradenia riparia. Phytochemistry 1998, 49, 1157–1158. [Google Scholar] [CrossRef]
- Zelnik, R.; Rabenhorst, E.; Matida, A.K.; Gottlieb, H.E.; Lavie, D.; Panizza, S. Ibosol, a new diterpenoid from Iboza riparia. Phytochemistry 1978, 17, 1795–1797. [Google Scholar] [CrossRef]
- Valmorbida, J.; Boaro, C.F.S.; Marques, M.O.M.; Ferri, A.F. Rendimento e composição química de óleos essenciais de Mentha piperita L. cultivada em solução nutritiva com diferentes concentrações de potássio. Rev. Bras. Pl. Med. 2006, 8, 56–61. [Google Scholar]
- Gazim, Z.C.; Amorim, A.C.L.; Hovell, A.M.C.; Rezende, C.M.; Nascimento, I.A.; Ferreira, G.A.; Cortez, D.A.G. Seasonal variation, chemical composition, and analgesic and antimicrobial activities of the essential oil from leaves of Tetradenia riparia (Hochst.) Codd in Southern Brazil. Molecules 2010, 15, 5509–5524. [Google Scholar] [CrossRef]
- Van Puyvelde, L.; de Kimpe, N.; Dubé, S.; Chagnon-Dubé, M.; Boily, Y.; Borremans, F.; Schamp, N.; Anteunis, M.J. 1',2'-Dideacetylboronolide, an α-pyrone from Iboza riparia. Phytochemistry 1981, 20, 2753–2755. [Google Scholar] [CrossRef]
- Davies-Coleman, M.T.; Rivett, D.E.A. Structure of the 5,6-dihydro-α-pyrone, umuravumbolide. Phytochemistry 1995, 38, 791–792. [Google Scholar] [CrossRef]
- Van Puyvelde, L.; Ntawukiliyayo, J.D.; Portaels, F. In vitro inhibition of mycobacteria by Rwandese medicinal plants. Phytother. Res. 1994, 8, 65–69. [Google Scholar] [CrossRef]
- Omolo, M.O.; Okinyo, D.; Ndiege, I.O.; Lwande, W.; Hassanali, A. Repellency of essential oils of some Kenyan plants against Anopheles gambiae. Phytochemistry 2004, 65, 2797–2802. [Google Scholar] [CrossRef]
- Gazim, Z.C.; Demarchi, I.G.; Lonardoni, M.V.C.; Amorim, A.C.L.; Hovell, A.M.C.; Rezende, C.M.; Ferreira, G.A.; Lima, E.L.; Cosmo, F.A.; Cortez, D.A.G. Acaricidal activity of the essential oil from Tetradenia riparia (Lamiaceae) on the cattle tick Rhipicephalus (Boophilus) microplus (Acari; Ixodidae). Exp. Parasitol. 2011, 129, 175–178. [Google Scholar] [CrossRef]
- Nickavar, B.; Kamalinejad, M.; Izadpanah, H. In vitro free radical scavenging activity of five Salvia species. Pak. J. Pharm. Sci. 2007, 20, 291–294. [Google Scholar]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils-a review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Fan, P.; Lou, H. Effects of polyphenols from grape seeds on oxidative damage to cellular DNA. Mol. Cell. Biochem. 2004, 267, 67–74. [Google Scholar] [CrossRef]
- Bronzetti, G.; Cini, M.; Andreoli, E.; Caltavuturo, L.; Panunzio, M.; Croce, C.D. Protective effects of vitamin and selenium compound in yeast. Mutat. Res. Gen. Toxicol. Enviroment. Mutagen. 2001, 429, 105–115. [Google Scholar]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bodesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer—Drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef]
- Berridge, M.V.; Tan, A.S.; McCoy, K.D.; Wang, R. The biochemical and cellular basis of cell proliferation assays that use tetrazolium salts. Biochemica 1996, 4, 14–19. [Google Scholar]
- Kusumoto, N.; Ashitani, T.; Hayasaka, Y.; Murayama, T.; Ogiyama, K.; Takahashi, K. Antitermitic activities of abietane-type diterpenes from Taxodium distichum cones. J. Chem. Ecol. 2009, 35, 635–642. [Google Scholar] [CrossRef]
- Mossman, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Suhaj, M. Spice antioxidants isolation and their antiradical activity: A review. J. Food Compost. Anal. 2006, 19, 531–537. [Google Scholar] [CrossRef]
- USDA. Agricultural Research Service, Germplasm Resources Information Network, National Genetic Resources Program. Dr. Duke’s Phytochemical and Ethnobotanical Databases. Available online: http://www.ars-grin.gov/duke/ (accessed on 25 June 2013).
- Rodriguez, B. Spectral assignments and reference data. Magn. Reson. Chem. 2003, 41, 741–746. [Google Scholar] [CrossRef]
- Mourão, F.; Umeo, S.H.; Takemura, O.S.; Linde, G.A.; Colauto, N.B. Antioxidant activity of Agaricus brasiliensis basidiocarps on different maturation phases. Braz. J. Microbiol. 2011, 42, 197–202. [Google Scholar] [CrossRef]
- Molyneux, P. The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J. Sci. Technol. 2004, 26, 211–219. [Google Scholar]
- Kumaran, A.; Karunakaran, R.J. Antioxidant and free radical scavenging activity of an aqueous extract of Coleus aromaticus. Food Chem. 2006, 97, 109–114. [Google Scholar] [CrossRef]
- Ozgen, M.; Reese, R.N.; Tulio, A.Z.; Scheerens, J.C.; Miller, A.R. Modified 2,2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) method to measure antioxidant capacity of Selected small fruits and comparison to ferric reducing antioxidant power (FRAP) and 2,2'-diphenyl-1-picrylhydrazyl (DPPH) methods. J. Agric. Food Chem. 2006, 54, 1151–1157. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 1 and 2 are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gazim, Z.C.; Rodrigues, F.; Amorin, A.C.L.; Rezende, C.M.d.; Soković, M.; Tešević, V.; Vučković, I.; Krstić, G.; Cortez, L.E.R.; Colauto, N.B.; et al. New Natural Diterpene-Type Abietane from Tetradenia riparia Essential Oil with Cytotoxic and Antioxidant Activities. Molecules 2014, 19, 514-524. https://doi.org/10.3390/molecules19010514
Gazim ZC, Rodrigues F, Amorin ACL, Rezende CMd, Soković M, Tešević V, Vučković I, Krstić G, Cortez LER, Colauto NB, et al. New Natural Diterpene-Type Abietane from Tetradenia riparia Essential Oil with Cytotoxic and Antioxidant Activities. Molecules. 2014; 19(1):514-524. https://doi.org/10.3390/molecules19010514
Chicago/Turabian StyleGazim, Zilda Cristiani, Felipe Rodrigues, Ana Carolina Lourenço Amorin, Cláudia Moraes de Rezende, Marina Soković, Vele Tešević, Ivan Vučković, Gordana Krstić, Lucia Elaine Ranieri Cortez, Nelson Barros Colauto, and et al. 2014. "New Natural Diterpene-Type Abietane from Tetradenia riparia Essential Oil with Cytotoxic and Antioxidant Activities" Molecules 19, no. 1: 514-524. https://doi.org/10.3390/molecules19010514
APA StyleGazim, Z. C., Rodrigues, F., Amorin, A. C. L., Rezende, C. M. d., Soković, M., Tešević, V., Vučković, I., Krstić, G., Cortez, L. E. R., Colauto, N. B., Linde, G. A., & Cortez, D. A. G. (2014). New Natural Diterpene-Type Abietane from Tetradenia riparia Essential Oil with Cytotoxic and Antioxidant Activities. Molecules, 19(1), 514-524. https://doi.org/10.3390/molecules19010514