Synthesis of Novel 2-(Substituted amino)alkylthiopyrimidin-4(3H)-ones as Potential Antimicrobial Agents
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
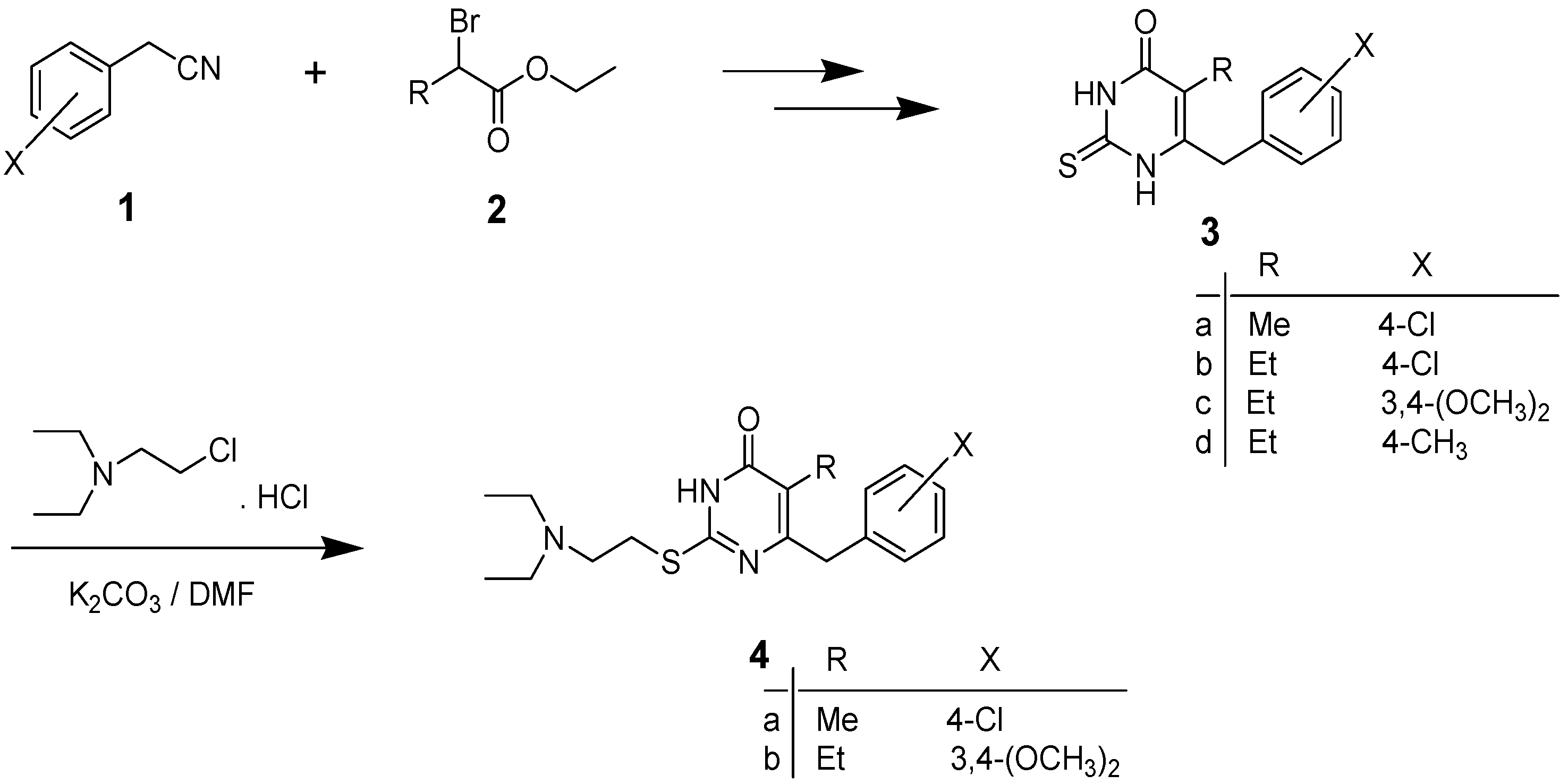
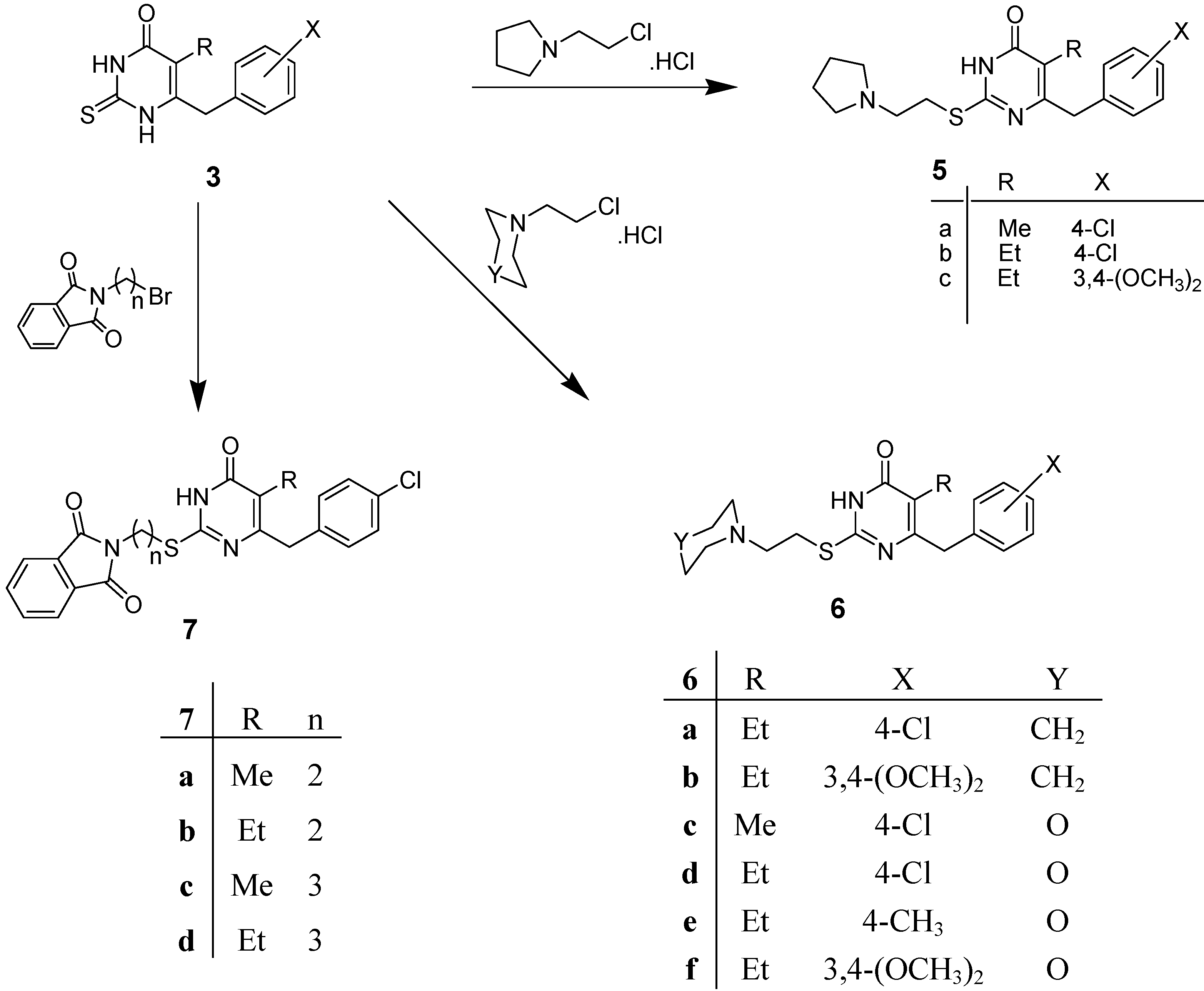
2.2. Antimicrobial Testing
| Comp. No. | Diameter of Growth Inhibition Zone (mm) a | ||||
|---|---|---|---|---|---|
| Staphylococcus aureus | Bacillus subtilis | Bacillus cereus | Candida albicans | Aspergillus niger | |
| 3a | 22 | 17 | 21 | 15 | - |
| 3b | 30 | 18 | 18 | 22 | - |
| 3c | - | - | - | - | - |
| 3d | - | - | - | - | - |
| 4a | 30 | 12 | 12 | - | 13 |
| 4b | - | - | - | 21 | 25 |
| 5a | 30 | 15 | 13 | 13 | - |
| 5b | 27 | 13 | 15 | 32 | 13 |
| 5c | - | - | - | 35 | 31 |
| 6a | 23 | - | - | 30 | 21 |
| 6b | 23 | - | - | 20 | 25 |
| 6c | 24 | - | - | - | - |
| 6d | 27 | 18 | 12 | 15 | - |
| 6e | - | 12 | 12 | - | - |
| 6f | 21 | 15 | 13 | 25 | 24 |
| 7a | 21 | - | - | 22 | - |
| 7b | 25 | 13 | 12 | 12 | - |
| 7c | - | - | - | 13 | 18 |
| 7d | 24 | 13 | 16 | 12 | - |
| Ampicillin | 35 | 38 | 35 | ||
| Clotrimazole | 38 | 40 | |||
| Compound No. | The minimal inhibitory concentration (MIC µg/mL) a | |
|---|---|---|
| Staphylococcus aureus | Candida albicans | |
| 3a | 100 | 100 |
| 3b | 25 | 25 |
| 4a | 25 | ND |
| 5a | 25 | 100 |
| 5b | 25 | 25 |
| 6b | 100 | 50 |
| 6d | 25 | 100 |
| 6f | 50 | 50 |
| 7b | 50 | 100 |
| 7d | 50 | 100 |
| Ampicillin | 6.0 | |
| Clotrimazole | 6.0 | |
3. Experimental
3.1. General
3.2. General Procedure for Preparation of 2-(Substituted amino)ethylthiopyrimidines 4b, 5c and 6b–f
3.3. General Procedure for Preparation of 2-[2-(N-Phthalimido)ethyl]thiopyrimidin-4(3H)-ones 7a,b and 2-[3-(N-Phthalimido)propyl]thiopyrimidin-4(3H)-ones 7c,d
3.4. Determination of the Antimicrobial Activity by the Agar Disc-Diffusion Method [43]
3.5. Determination of Minimal Inhibitory Concentration (MIC) [44]
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Mitsuya, H.; Yarchoan, R.; Broder, S. Molecular targets for AIDS therapy. Science 1990, 249, 1533–1544. [Google Scholar]
- Miyasaka, T.; Tanaka, H.; Baba, M.; Hayakawa, H.; Walker, R.T.; Balzarini, J.; de Clercq, E. A novel lead for specific anti-HIV-1 agents: 1-[(2-hydroxyethoxy)methyl]-6-(phenylthio)thymine. J. Med. Chem. 1989, 32, 2507–2509. [Google Scholar] [CrossRef]
- Hopkins, A.L.; Ren, J.; Tanaka, H.; Baba, M.; Okamato, M.; Stuart, D.I.; Stammers, D.K. Design of MKC-442 (emivirine) analogues with improved activity against drug-resistant HIV mutants. J. Med. Chem. 1999, 42, 4500–4505. [Google Scholar]
- Malik, V.; Singh, P.; Kumar, S. Unique chlorine effect in regioselective one-pot synthesis of 1-alkyl-/allyl-3-(o-chlorobenzyl) uracils: Anti-HIV activity of selected uracil derivatives. Tetrahedron 2006, 62, 5944–5951. [Google Scholar] [CrossRef]
- Gazivoda, T.; Raic-Malic, S.; Marjanovic, M.; Kralj, M.; Pavelic, K.; Balzarini, J.; de Clercq, E.; Mintas, M. The novel C-5 aryl, alkenyl and alkynyl substituted uracil derivatives of L-ascorbic acid: Synthesis, cytostatic, and antiviral activity evaluations. Bioorg. Med. Chem. 2007, 15, 749–758. [Google Scholar] [CrossRef]
- Novikov, M.S.; Buckheit, R.W., Jr.; Temburnikar, K.; Khandazhinskaya, A.L.; Ivanov, A.V.; Seley-Radtke, K.L. 1-Benzyl derivatives of 5-(arylamino)uracils as anti-HIV-1 and anti-EBV agents. Bioorg. Med. Chem. 2010, 18, 8310–8314. [Google Scholar] [CrossRef]
- Novikov, M.S.; Valuev-Elliston, V.T.; Babkov, D.A.; Paramonova, M.P.; Ivanov, A.V.; Gavryushov, S.A.; Khandazhinskaya, A.L.; Kochetkov, S.N.; Pannecouque, C.; Andrei, G.; et al. N1,N3-disubstituted uracils as nonnucleoside inhibitors of HIV-1 reverse transcriptase. Bioorg. Med. Chem. 2013, 21, 1150–1158. [Google Scholar] [CrossRef]
- Sakakibara, N.; Hamasaki, T.; Baba, M.; Demizu, Y.; Kurihara, M.; Irie, K.; Iwai, M.; Asada, E.; Kato, Y.; Maruyama, T. Synthesis and evaluation of novel 3-(3,5-dimethylbenzyl)uracil analogs as potential anti-HIV-1 agents. Bioorg. Med. Chem. 2013, 21, 5900–5906. [Google Scholar] [CrossRef]
- Novikov, M.S.; Babkov, D.A.; Paramonova, M.P.; Khandazhinskaya, A.L.; Ozerov, A.A.; Chizhov, A.O.; Andrei, G.; Snoeck, R.; Balzarini, J.; Seley-Radtke, K.L. Synthesis and anti-HCMV activity of 1-[ω-(phenoxy)alkyl]uracil derivatives and analogues thereof. Bioorg. Med. Chem. 2013, 21, 4151–4157. [Google Scholar] [CrossRef]
- Isobe, Y.; Tobe, M.; Inoue, Y.; Isobe, M.; Tsuchiya, M.; Hayashi, H. Structure and activity relationships of novel uracil derivatives as topical anti-inflammatory agents. Bioorg. Med. Chem. 2003, 11, 4933–4940. [Google Scholar] [CrossRef]
- Evaldsson, C.; Ryden, I.; Uppugunduri, S. Anti-inflammatory effects of exogenous uridine in an animal model of lung inflammation. Inter. Immunopharmcol. 2007, 7, 1025–1032. [Google Scholar] [CrossRef]
- Keche, A.P.; Hatnapure, G.D.; Tale, R.H.; Rodge, A.H.; Birajdar, S.S.; Kamble, V.M. A novel pyrimidine derivatives with aryl urea, thiourea and sulfonamide moieties: Synthesis, anti-inflammatory and antimicrobial evaluation. Bioorg. Med. Chem. Lett. 2012, 22, 3445–3448. [Google Scholar] [CrossRef]
- Agarwal, A.; Srivastava, K.; Puri, S.K.; Chuahan, P.M. Synthesis of 2,4,6-trisubstituted pyrimidines as antimalarial agents. Bioorg. Med. Chem. 2005, 13, 4645–4650. [Google Scholar] [CrossRef]
- Agarwal, A.; Srivastava, K.; Puri, S.K.; Chuahan, P.M. Antimalarial activity of 2,4,6-trisubstituted pyrimidines. Bioorg. Med. Chem. Lett. 2005, 15, 1881–1883. [Google Scholar] [CrossRef]
- Singh, K.; Kaur, H.; Chibale, K.; Balzarini, J. Synthesis of 4-aminoquinoline-pyrimidine hybrids as potent antimalarials and their mode of action studies. Eur. J. Med. Chem. 2013, 66, 314–323. [Google Scholar] [CrossRef]
- Xie, F.; Zhao, H.; Zhao, L.; Lou, L.; Hu, Y. Synthesis and biological evaluation of novel 2,4,5-substituted pyrimidine derivatives for anticancer activity. Bioorg. Med. Chem. Lett. 2009, 19, 275–278. [Google Scholar]
- El-Deeb, I.M.; Lee, S.H. Design and synthesis of new anticancer pyrimidines with multiple-kinase inhibitory effect. Bioorg. Med. Chem. 2010, 18, 3860–3874. [Google Scholar] [CrossRef]
- Prachayasittikul, S.; Worachartcheewan, A.; Nantasenamat, C.; Chinworrungsee, M.; Sornsongkhram, N.; Ruchirawat, S.; Prachayasittikul, V. Synthesis and structure-activity relationship of 2-thiopyrimidine-4-one analogs as antimicrobial and anticancer agents. Eur. J. Med. Chem. 2011, 46, 738–742. [Google Scholar] [CrossRef]
- Tsoukala, E.; Agelis, G.; Dolinsek, J.; Botic, T.; Cencic, A.; Komiotis, D. An efficient synthesis of 3-fluoro-5-thio-xylofuranosyl nucleosides of thymine, uracil and 5-fluorouracil as potential antitumor or/and antiviral agents. Bioorg. Med. Chem. 2007, 15, 3241–3247. [Google Scholar] [CrossRef]
- Manta, S.; Tsoukala, E.; Tzioumaki, N.; Kiritsis, C.; Balzarini, J.; Komiotis, D. Synthesis of 4,6-dideoxy-3-fluoro-β-d-glucopyranosyl analogues of 5-fluorouracil, N6-benzyl adenine, uracil, thymine, N4-benzoyl cytosine and evaluation of their antitumor activities. Bioorg Chem. 2010, 38, 48–55. [Google Scholar] [CrossRef]
- Lauria, A.; Patella, C.; Abbate, I.; Martorana, A.; Almerico, A.M. An unexpected Dimroth rearrangement leading to annelated thieno[3,2-d][1,2,3]triazolo[1,5-a]pyrimidines with potent antitumor activity. Eur. J. Med. Chem. 2013, 65, 381–388. [Google Scholar] [CrossRef]
- Matyugina, E.; Khandazhinskaya, A.; Chernousova, L.; Andreevskaya, S.; Smirnova, T.; Chizhov, A.; Karpenko, I.; Kochetkov, S.; Alexandrova, L. The synthesis and antituberculosis activity of 5'-nor carbocyclic uracil derivatives. Bioorg. Med. Chem. 2012, 20, 6680–6686. [Google Scholar] [CrossRef]
- Tobe, M.; Isobe, Y.; Goto, Y.; Obara, F.; Tsuchiya, M.; Matsui, J.; Hirota, K.; Hayashi, H. Synthesis and biological evaluation of CX-659S and related compounds for their inhibitory effects on the delayed-type hypersensitivity reaction. Bioorg. Med. Chem. 2000, 8, 2037–2047. [Google Scholar] [CrossRef]
- Bhabak, K.P.; Bhowmick, D. Synthesis and structural characterization of some trisulfide analoges of thiouracil-based antithyroid drugs. J. Mol. Struct. 2012, 1022, 16–24. [Google Scholar] [CrossRef]
- Sriharsha, S.N.; Satish, S.; Shashikanth, S.; Raveesha, K.A. Design, synthesis and antibacterial activity of novel 1,3-thiazolidine pyrimidine nucleoside analogues. Bioorg. Med. Chem. 2006, 14, 7476–7481. [Google Scholar] [CrossRef]
- Semenov, V.E.; Voloshina, A.D.; Toroptzova, E.M.; Kulik, N.V.; Zobov, V.V.; Giniyatullin, R.K.; Mikhailov, A.S.; Nikolaev, A.E.; Akamsin, V.D.; Reznik, V.S. Antibacterial and antifungal activity of acyclic and macrocyclic uracil derivatives with quaternized nitrogen atoms in spacers. Eur. J. Med. Chem. 2006, 41, 1093–1101. [Google Scholar] [CrossRef]
- Svenstrup, N.; Kuhl, A.; Ehlert, K.; Habich, D. Improved synthesis of antibacterial 3-substituted 6-aniliouracils. Bioorg. Med. Chem. Lett. 2008, 18, 3215–3218. [Google Scholar] [CrossRef]
- Al-Abdullah, E.S.; Al-Obaid, A.M.; Al-Deeb, O.A.; Habib, E.E.; El-Emam, E.E. Synthesis of novel 6-phenyl-2,4-disubstituted pyrimidine-5-carbonitriles as potential antimicrobial agents. Eur. J. Med. Chem. 2011, 46, 4642–4647. [Google Scholar] [CrossRef]
- Krim, J.; Grunewald, C.; Taourirte, M.; Engels, J.W. Efficient microwave-assisted synthesis, antibacterial activity and high fluorescence of 5-benzimidazolyl-2'-deoxyuridines. Bioorg. Med. Chem. 2012, 20, 480–486. [Google Scholar] [CrossRef]
- Al-Deeb, O.A.; Al-Turkistani, A.A.; Al-Abdullah, E.S.; El-Brollosy, N.R.; Habib, E.E.; El-Emam, A.A. Pyrimidine-5-carbonitriles-part III: Synthesis and antimicrobial activity of novel 6-(2-substituted propyl)-2,4-disubstituted pyrimidine-5-carbinitriles. Heterocycl. Commun. 2013, 19, 411–419. [Google Scholar]
- Orr, G.F.; Musso, D.L.; Boswell, G.E.; Kelly, J.L.; Joyner, S.S.; Davis, S.T.; Baccanari, D.P. Inhibition of uridine phosphorylase: Synthesis and structure-activity relationships of aryl-substituted 5-benzyluracils and 1-[(2-hydroxyethoxy)methyl]-5-benzyluracils. J. Med. Chem. 1995, 38, 3850–3856. [Google Scholar] [CrossRef]
- Murray, P.E.; McNally, V.A.; Lockyer, S.D.; Williams, K.J.; Stratford, I.J.; Jaffar, M.; Freeman, S. Synthesis and enzymatic evaluation of pyridinium-substituted uracil derivatives as novel inhibitors of thymidine phosphorylase. Bioorg. Med. Chem. 2002, 10, 525–530. [Google Scholar] [CrossRef]
- Focher, F.; Ubiali, D.; Pregnolato, M.; Zhi, C.; Gambino, J.; Wright, G.E.; Spadari, S. Novel nonsubstrate inhibitors of human thymidine phosphorylase, a potential target for tumor-dependent angiogenesis. J. Med. Chem. 2000, 43, 2601–2607. [Google Scholar] [CrossRef]
- El-Brollosy, N.R.; Al-Omar, M.A.; Al-Deeb, O.A.; El-Emam, A.A.; Nielsen, C. Synthesis of novel uracil non-nucleosides analogues of 3,4-dihydro-2-alkylthio-6-benzyl-4-oxopyrimidines and 6-benzyl-1-ethyloxymethyl-5-isopropyluracil. J. Chem. Res. 2007, 263–267. [Google Scholar]
- El-Brollosy, N.R.; Jorgensen, P.T.; Dahan, B.; Boel, A.M.; Pedersen, E.B.; Nielsen, C. Synthesis of Novel N-1 (allyloxymethyl) Analogues of 6-Benzyl-1-(ethoxymethyl)-5-isopropyluracil (MKC-442, Emivirine) with Improved Activity Against HIV-1 and its Mutants. J. Med. Chem. 2002, 45, 5721–5726. [Google Scholar] [CrossRef]
- El-Brollosy, N.R.; Pedersen, E.B.; Nielsen, C. Synthesis of novel MKC-442 analogues with potent activities against HIV-1. Arch. Pharm. Pharm. Med. Chem. 2003, 336, 236–241. [Google Scholar] [CrossRef]
- El-Essawy, F.A.; El-Brollosy, N.R.; Pedersen, E.B.; Nielsen, C. Synthesis of new uracil non-nucleoside derivatives as potential inhibitors of HIV-1. J. Heterocyl. Chem. 2003, 40, 213–217. [Google Scholar] [CrossRef]
- Wamberg, M.; Pedersen, E.B.; El-Brollosy, N.R.; Nielsen, C. Synthesis of 6-arylvinyl analogues of the HIV drugs SJ-3366 and Emivirine. Bioorg. Med. Chem. 2004, 12, 1141–1149. [Google Scholar] [CrossRef]
- El-Brollosy, N.R.; Nielsen, C.; Pedersen, E.B. Synthesis of N-1-(indanyloxymethyl) and N-1-(4-Hydroxybut-2-enyloxymethyl) analogues of the HIV drug Emivirine and GCA-186. Monatsh. Chem. 2005, 136, 1247–1254. [Google Scholar] [CrossRef]
- Sorensen, E.R.; El-Brollosy, N.R.; Jorgensen, P.T.; Pedersen, E.B.; Nielsen, C. Synthesis of 6-(3,5-dichlorobenzyl) derivatives as isosteric analogues of the HIV drug 6-(3,5-dimethylbenzyl)-1-(ethoxymethyl)-5-isopropyluracil (GCA-186). Arch. Pharm. Chem. Life Sci. 2005, 338, 299–304. [Google Scholar] [CrossRef]
- El-Brollosy, N.R.; Sorensen, E.R.; Pedersen, E.B.; Sanna, G.; LaColla, P.; Loddo, R. Synthesis and antiviral evaluation of 6-(trifluoromethylbenzyl) and 6-(fluorobenzyl) analogues of HIV drugs emivirine and GCA-186. Arch. Pharm. Chem. Life Sci. 2008, 341, 9–19. [Google Scholar]
- El-Brollosy, N.R.; Al-Deeb, O.A.; El-Emam, A.A.; Pedersen, E.B.; LaColla, P.; Collu, G.; Sanna, G.; Loddo, R. Synthesis of novel uracil non-nucleoside derivatives as potential reverse transcriptase inhibitors of HIV-1. Arch. Pharm. Chem. Life Sci. 2009, 342, 663–670. [Google Scholar] [CrossRef]
- Penna, C.A.; Marino, S.G.; Gutkind, G.O.; Clavin, M.; Ferraro, G.; Martino, V. Antimicrobial activity of Eupatorium species growing in Argentina. J. Herbs. Spices Med. Plants 1998, 5, 21–28. [Google Scholar]
- Wilkins, T.D.; Holdeman, J.J.; Abramson, I.J.; Moore, W.E.C. Standardized single-disc methodfor antibiotic susceptibility testing of anaerobic bacteria. Antimicrob. Agents Chemother. 1972, 1, 451–455. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 3a–d, 4a,b, 5a–c, 6a–f and 7a–d are available from the corresponding author.
© 2013 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Attia, M.I.; El-Emam, A.A.; Al-Turkistani, A.A.; Kansoh, A.L.; El-Brollosy, N.R. Synthesis of Novel 2-(Substituted amino)alkylthiopyrimidin-4(3H)-ones as Potential Antimicrobial Agents. Molecules 2014, 19, 279-290. https://doi.org/10.3390/molecules19010279
Attia MI, El-Emam AA, Al-Turkistani AA, Kansoh AL, El-Brollosy NR. Synthesis of Novel 2-(Substituted amino)alkylthiopyrimidin-4(3H)-ones as Potential Antimicrobial Agents. Molecules. 2014; 19(1):279-290. https://doi.org/10.3390/molecules19010279
Chicago/Turabian StyleAttia, Mohamed I., Ali A. El-Emam, Abdulghafoor A. Al-Turkistani, Amany L. Kansoh, and Nasser R. El-Brollosy. 2014. "Synthesis of Novel 2-(Substituted amino)alkylthiopyrimidin-4(3H)-ones as Potential Antimicrobial Agents" Molecules 19, no. 1: 279-290. https://doi.org/10.3390/molecules19010279




