Identification of Multiple Ingredients for a Traditional Chinese Medicine Preparation (Bu-yang-huan-wu-tang) by Liquid Chromatography Coupled with Tandem Mass Spectrometry
Abstract
:1. Introduction
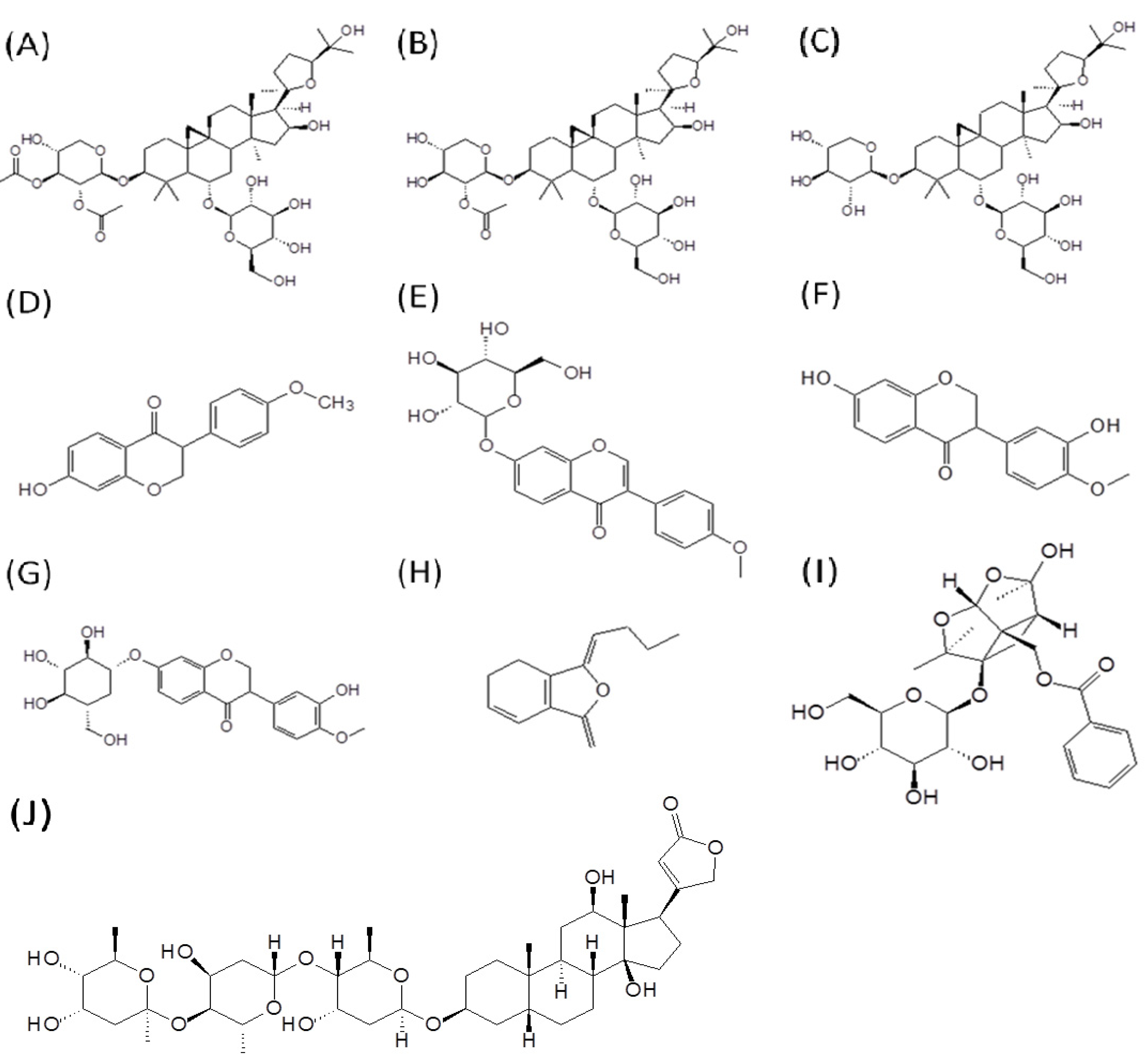
2. Results and Discussion
2.1. Optimization of Chromatographic Separation Conditions
| Compounds | RT (min) | Mass fragments | Ionization parameters | |||||
|---|---|---|---|---|---|---|---|---|
| Precursor (amu) | Product (amu) | DP (V) | FP (V) | EP (V) | CE (V) | CXP (V) | ||
| Astragaloside I | 11.21 | 886.6 [M + NH4]+ | 143.2 | 55 | 290 | 12 | 23 | 13 |
| Astragaloside II | 10.73 | 844.5 [M + NH4]+ | 143.2 | 62 | 330 | 13 | 25 | 13 |
| Astragaloside IV | 11.18 | 802.6 [M + NH4]+ | 143.2 | 65 | 370 | 14 | 28 | 10 |
| Formononetin | 8.32 | 269.2 [M + H]+ | 253.2 | 190 | 380 | 14 | 37 | 16 |
| Ononin | 8.23 | 431.3 [M + H]+ | 269.2 | 80 | 380 | 13 | 20 | 18 |
| Calycosin | 8.72 | 285.2 [M + H]+ | 270.2 | 190 | 380 | 13 | 33 | 17 |
| Calycosin-7-O-β- d-glucoside | 7.68 | 447.2 [M + H]+ | 285.1 | 80 | 380 | 12 | 23 | 19 |
| Ligustilide | 10.05 | 208.1 [M + NH4]+ | 173.2 | 70 | 380 | 14 | 25 | 11 |
| Paeoniflorin | 7.37 | 498.3 [M + NH4]+ | 179.2 | 76 | 390 | 14 | 25 | 12 |
| Digoxin (IS) | 9.12 | 798.6 [M + NH4]+ | 651.6 | 20 | 160 | 10 | 18 | 11 |
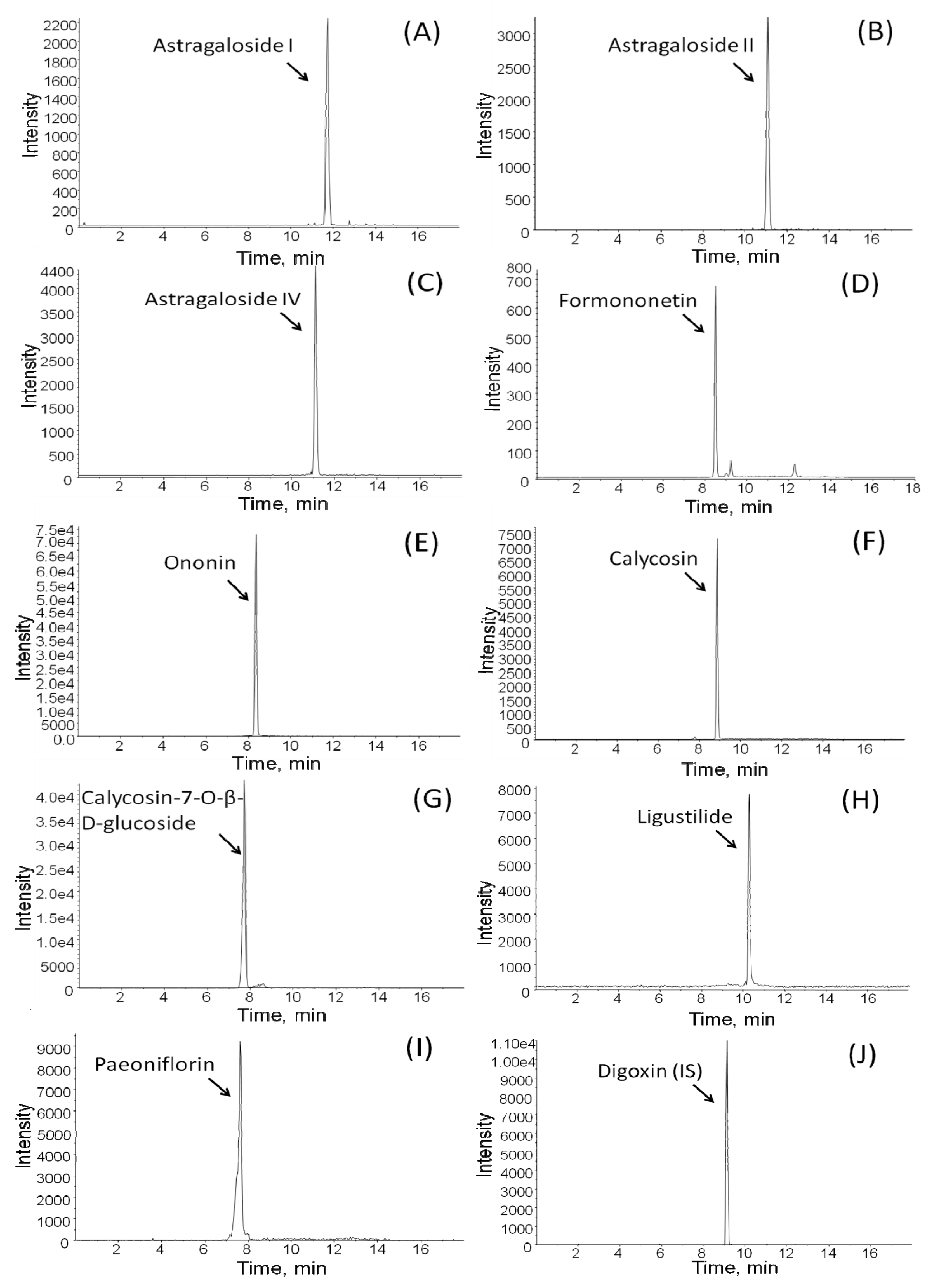
2.2. Validation of the HPLC Procedure
| Compounds | Regression equation | Correlation coefficient | Linear range (ng/mL) | Low limits of quantification (ng/mL) | Limits of determination (ng/mL) |
|---|---|---|---|---|---|
| Astragaloside I | y = 558.86x − 734.12 | 0.997 | 0.5–500 | 0.5 | 0.17 |
| Astragaloside II | y = 916.57x + 4154.8 | 0.999 | 0.5–500 | 0.5 | 0.17 |
| Astragaloside IV | y = 1050.1x + 5612.8 | 0.998 | 0.5–500 | 0.5 | 0.17 |
| Formononetin | y = 219.53x − 1076.2 | 0.997 | 5–1000 | 5 | 1.65 |
| Ononin | y = 24939x + 233299 | 0.999 | 5–1000 | 5 | 1.65 |
| Calycosin | y = 2917.2x − 5405 | 0.998 | 1–500 | 1 | 0.33 |
| Calycosin-7-O-β- d-glucoside | y = 10233x − 12150 | 0.998 | 10–1000 | 10 | 3.3 |
| Ligustilide | y = 7634.8x + 17949 | 0.997 | 5–500 | 5 | 1.65 |
| Paeoniflorin | y = 6093.8x − 30867 | 0.998 | 10–1000 | 10 | 3.3 |
| Nominal concentration (ng/mL) | Intra-day | Inter-day | ||||
|---|---|---|---|---|---|---|
| Observed concentration (ng/mL) | Precision (%) | Accuracy (%) | Observed concentration (ng/mL) | Precision (%) | Accuracy (%) | |
| Astragaloside I | ||||||
| 10 | 11.3 ± 0.1 | 0.6 | 5.2 | 9.4 ± 0.32 | 3.1 | 1.4 |
| 100 | 105 ± 2.7 | 4.3 | 3.9 | 101 ± 6.9 | 4.2 | −5.3 |
| 1000 | 1007 ± 6.8 | 7.4 | −7.8 | 1032 ± 9.1 | 11.6 | 8.8 |
| Astragaloside II | ||||||
| 10 | 12.3 ± 0.3 | 4.2 | 4.2 | 0.5 ± 1.8 | 1.5 | 2.3 |
| 100 | 99.8 ± 3.4 | 5.5 | 5.8 | 101 ± 3.1 | 5.2 | 7.2 |
| 1000 | 1002 ± 11.2 | 9.0 | 11.5 | 101 ± 5.4 | 6.8 | 5.9 |
| Astragaloside IV | ||||||
| 10 | 11.1 ± 0.8 | 4.8 | 1.8 | 9.8 ± 0.7 | 4.4 | 3.2 |
| 100 | 102 ± 2.3 | 4.2 | 3.2 | 103 ± 4.3 | 3.6 | 5.4 |
| 1000 | 1008 ± 6.4 | 7.2 | 4.8 | 1011 ± 5.2 | 4.8 | 6.6 |
| Formononetin | ||||||
| 10 | 11.3 ± 2.3 | 6.2 | 3.4 | 9.78 ± 5.2 | 3.6 | 2.8 |
| 100 | 104 ± 5.8 | 5.8 | 5.6 | 96.7 ± 5.5 | 3.5 | −1.2 |
| 1000 | 992 ± 12.2 | 3.8 | 2.6 | 1004 ± 18.2 | 7.2 | 3.4 |
| Ononin | ||||||
| 10 | 9.5 ± 4.4 | 4.2 | 3.4 | 9.3 ± 1.7 | 5.8 | 1.8 |
| 100 | 106 ± 4.5 | 3.6 | −6.2 | −96.5 ± 7.1 | 4.3 | −2.4 |
| 1000 | 1009 ± 5.3 | 3.8 | 7.4 | 1003 ± 12.7 | 3.4 | 1.6 |
| Calycosin | ||||||
| 10 | 12.2 ± 8.1 | 7.68 | 3.2 | 9.45 ± 1.3 | 6.4 | 5.6 |
| 100 | 104 ± 6.3 | 4.92 | 1.2 | 101 ± 3.5 | 2.5 | 2.2 |
| 1000 | 1011 ± 3.1 | 5.76 | 1.8 | 1016 ± 9.4 | 4.2 | 4.8 |
| Calycosin-7-O-β-d-glucoside | ||||||
| 10 | 11.5 ± 0.3 | 2.1 | 1.8 | 9.86 ± 1.4 | 5.3 | 2.3 |
| 100 | 105 ± 5.4 | 4.3 | −1.3 | 107 ± 7.6 | 4.29 | −7.9 |
| 1000 | 1009 ± 6.9 | 7.6 | 3.6 | 1011 ± 13.3 | 8.5 | 5.3 |
| Ligustilide | ||||||
| 10 | 9.5 ± 1.6 | 6.9 | 0.3 | 10.3 ± 2.2 | 4.9 | 2.7 |
| 100 | 103 ± 2.1 | 5.8 | 2.4 | 9.43 ± 5.7 | 9.2 | 6.3 |
| 1000 | 1007 ± 5.5 | 3.6 | 11.4 | 1008 ± 8.6 | 5.34 | 10.3 |
| Paeoniflorin | ||||||
| 10 | 10.7 ± 0.45 | 1.5 | −1.4 | 11.5 ± 0.3 | 0.9 | 1.8 |
| 100 | 98.3 ± 8.29 | 4.8 | 3.8 | 104 ± 4.2 | 8.6 | −0.3 |
| 1000 | 995 ± 5.78 | 5.7 | 8.3 | 1005 ± 11 | 4.8 | −9.6 |
| Concentration (μg/mL) | Stability (%) | ||
|---|---|---|---|
| Standard solution | BYHWT Extraction | Commercial product A | |
| Astragaloside I | |||
| 1 | 7.7 ± 2.4 | 4.9 ± 3.4 | 5.2 ± 2.1 |
| 10 | 4.5 ± 6.3 | ||
| 100 | 3.9 ± 5.0 | ||
| Astragaloside II | |||
| 1 | 12 ± 5.3 | 8.8 ± 5.6 | 5.2 ± 4.4 |
| 10 | 6.3 ± 8.8 | ||
| 100 | 3.4 ± 7.8 | ||
| Astragaloside IV | |||
| 1 | 9.4 ± 7.4 | 3.4 ± 6.2 | 10.1 ± 3.5 |
| 10 | 5.3 ± 4.6 | ||
| 100 | 4.4 ± 7.2 | ||
| Formononetin | |||
| 10 | 10 ± 4.1 | 5.8 ± 3.1 | 6.2 ± 5.1 |
| 100 | 6.6 ± 2.1 | ||
| 1000 | 2.8 ± 12 | ||
| Ononin | |||
| 1 | 6.2 ± 3.4 | 7.2 ± 4.7 | 11.2 ± 5.1 |
| 10 | 3.0 ± 2.5 | ||
| 100 | 3.1 ± 2.8 | ||
| Calycosin | |||
| 10 | 4.5 ± 6.3 | 5.8 ± 3.8 | 6.3 ± 4.1 |
| 100 | 3.5 ± 2.7 | ||
| 1000 | 4.1 ± 5.3 | ||
| Calycosin-7-O-β-d-glucoside | |||
| 1 | 8.5 ± 10 | 12.6 ± 3.5 | 6.8 ± 5.1 |
| 10 | 5.2 ± 6.6 | ||
| 100 | 4.5 ± 3.5 | ||
| Ligustilide | |||
| 1 | 7.0 ± 4.3 | 4.6 ± 3.7 | 7.5 ± 4.8 |
| 10 | 4.8 ± 5.6 | ||
| 100 | 3.2 ± 5.8 | ||
| Paeoniflorin | |||
| 10 | 6.3 ± 7.8 | 9.3 ± 5.2 | 5.7 ± 2.7 |
| 100 | 4.6 ± 3.4 | ||
| 1000 | 2.7 ± 4.9 | ||
| mg/g ( n = 3) | BYHWT Extraction | A | B | C | D | E | F |
|---|---|---|---|---|---|---|---|
| Astragaloside I | 0.8 | 0.8 | 0.1 | 2.8 | 0.2 | 4.5 | 0.6 |
| Astragaloside II | 1.2 | 2.9 | 2.4 | 5.0 | 1.7 | 8.4 | 6.4 |
| Astragaloside IV | 4.4 | 12.9 | 1.1 | 11.4 | 2.4 | 7.3 | 5.0 |
| Formononetin | 0.0 | 1.9 | 0.7 | 3.5 | 2.6 | 7.3 | 2.8 |
| Ononin | 1.6 | 7.9 | 7.5 | 10.0 | 2.9 | 3.6 | 3.3 |
| Calycosin | 0.1 | 0.8 | 0.5 | 4.9 | 0.2 | 3.0 | 1.8 |
| Calycosin-7-O-β-D-glucoside | 0.4 | 7.1 | 1.1 | 3.9 | 3.4 | 1.4 | 4.4 |
| Ligustilide | 3.0 | 4.0 | 0.7 | 2.5 | 3.1 | 4.0 | 5.0 |
| Paeoniflorin | 1.7 | 0.2 | 0.1 | 2.3 | 4.4 | 0.1 | 6.4 |
2.3. Application to Analysis of BYHWT Commercial Samples
3. Experimental
3.1. Materials and Reagent
3.2. Preparation of Bu-yang-hwan-wu-tang Extract
3.3. Liquid Chromatography-Tandem Mass Spectrometry
3.4. Preparation of Calibration Standards
3.5. Method Validation
3.6. Sample Preparation of BYHWT Commercial Products for Quantitation
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Chinese Pharmacopoeia, 2005 ed.; Chinese Pharmacopeia Commission: Beijing, China, 2005.
- Li, T.F.; Zhang, J.; Gulinuer, M.; Cai, S.Q.; Lu, J.F. Inhibitory effects of BYHWD on ROS generation in stroke rat brain tissues. Acta Biophys. Sin. 2003, 19, 441–447. [Google Scholar]
- Danesh, J.; Wheeler, J.G.; Hirschfield, G.M.; Eda, S.; Eiriksdottir, G.; Rumley, A.; Lowe, G.D.; Pepys, M.B.; Gudnason, V. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N. Engl. J. Med. 2004, 350, 1387–1397. [Google Scholar] [CrossRef]
- Li, X.M.; Bai, X.C.; Qin, L.N.; Huang, H.; Xiao, Z.J.; Gao, T.M. Neuroprotective effects of Buyang Huanwu Decoction on neuronal injury in hippocampus after transient forebrain ischemia in rats. Neurosci. Lett. 2003, 346, 29–32. [Google Scholar] [CrossRef]
- Wang, H.W.; Liou, K.T.; Wang, Y.H.; Lu, C.K.; Lin, Y.L.; Lee, I.J.; Huang, S.T.; Tsai, Y.H.; Cheng, Y.C.; Lin, H.J.; et al. Deciphering the neuroprotective mechanisms of Bu-yang Huan-wu decoction by an integrative neurofunctional and genomic approach in ischemic stroke mice. J. Ethnopharmacol. 2011, 138, 22–33. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, W.R.; Lin, R.; Zhang, J.Y.; Ji, Q.L.; Lin, Q.Q.; Yang, L.N. Buyang Huanwu decoction ameliorates coronary heart disease with Qi deficiency and blood stasis syndrome by reducing CRP and CD40 in rats. J. Ethnopharmacol. 2011, 130, 98–102. [Google Scholar]
- Wang, W.R.; Lin, R.; Zhang, H.; Lin, Q.Q.; Yang, L.N.; Zhang, K.F.; Ren, F. The effects of Buyang Huanwu Decoction on hemorheological disorders and energy metabolism in rats with coronary heart disease. J. Ethnopharmacol. 2011, 137, 214–220. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, W.; Li, H.; Chen, B.Y.; Zhang, G.M.; Tang, Y.H.; He, F.Y.; Deng, C.Q. Inhibition of aortic intimal hyperplasia and cell cycle protein and extracellular matrix protein expressions by BuYang HuanWu Decoction. J. Ethnopharmacol. 2009, 125, 423–435. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, W.; Li, H.; Zhang, G.M.; Chen, B.Y.; Tang, Y.H.; Deng, C.Q. Effects of Buyang Huanwu Decoction and its alkaloids and glycosides on aortic intimal hyperplasia and expression of proliferating cell nuclear antigen in rats with aortic intimal injuries. J. Chin. Integr. Med. 2008, 6, 836–842. [Google Scholar] [CrossRef]
- Rios, J.L.; Waterman, P.G. Review of the pharmacology and toxicology of astragalus. Phytother. Res. 1997, 11, 411–418. [Google Scholar] [CrossRef]
- State Administration of Traditional Chinese Medicine, Chinese Materia Medica; Shanghai Science and Technology Publishing House: Shanghai, China, 1999; pp. Volume 3, pp. 521–528; Volume 4, pp. 341–356; Volume 4, pp. 75–80; Volume 5, pp. 976–984.
- Zhang, C.; Wang, X.H.; Zhong, M.F.; Liu, R.H.; Li, H.L.; Zhang, W.D.; Chen, H. Mechanisms underlying vasorelaxant action of astragaloside IV in isolated rat aortic rings. Clin. Exp. Pharmacol. Physiol. 2007, 34, 387–392. [Google Scholar] [CrossRef]
- Xiao, H.B.; Krucker, M.; Albert, K.; Liang, X.M. Determination and identification of isoflavonoids in Radix astragali by matrix solid-phase dispersion extraction and high-performance liquid chromatography with photodiode array and mass spectrometric detection. J. Chromatogr. A 2004, 1032, 117–124. [Google Scholar]
- Wagner, H.; Bauer, R.; Melchart, D.; Xiao, P.G.; Staudinger, A. Radix Astragali—Huang Qi. Chromatogr. Fingerpr. Anal. Herb. Med. 2011, 1024, 83–98. [Google Scholar]
- Qi, L.W.; Yu, Q.T.; Li, P.; Li, S.L.; Wang, Y.X.; Sheng, L.H.; Yi, L. Quality evaluation of Radix Astragali through a simultaneous determination of six major active isoflavonoids and four main saponins by high-performance liquid chromatography coupled with diode array and evaporative light scattering detectors. J. Chromatogr. A 2011, 1024, 162–169. [Google Scholar]
- Yan, R.; Ko, N.L.; Li, S.L.; Tam, Y.K.; Lin, G. Pharmacokinetics and metabolism of ligustilide, a major bioactive component in Rhizoma Chuanxiong, in the rat. Drug Metab. Dispos. 2008, 36, 400–408. [Google Scholar] [CrossRef]
- Lu, Q.; Qiu, T.Q.; Yang, H. Ligustilide inhibits vascular smooth muscle cells proliferation. Eur. J. Pharmacol. 2006, 542, 136–140. [Google Scholar] [CrossRef]
- Li, C.R.; Zhou, Z.; Zhu, D.; Sun, Y.N.; Dai, J.M.; Wang, S.Q. Protective effect of paeoniflorin on irradiation-induced cell damage involved in modulation of reactive oxygen species and the mitogen-activated protein kinases. Int. J. Biochem. Cell Biol. 2007, 39, 426–438. [Google Scholar] [CrossRef]
- Ji, B.; Geng, P.; Liu, J.G.; Shi, D.Z.; Wang, Y.Y. Effects of active components extracted from Qixue Bingzhi Recipe on proliferation of vascular smooth muscle cells and expressions of platelet-derived growth factor and its receptor genes. J. Chin. Integr. Med. 2006, 4, 30–34. [Google Scholar] [CrossRef]
- Yang, D.Z.; An, Y.Q.; Jiang, X.L.; Tang, D.Q.; Gao, Y.Y.; Zhao, H.T.; Wu, X.W. Development of a novel method combining HPLC fingerprint and multi-ingredients quantitative analysis for quality evaluation of traditional Chinese medicine preparation. Talanta 2011, 85, 885–890. [Google Scholar] [CrossRef]
- Shaw, L.H.; Lin, L.C.; Tsai, T.H. HPLC–MS/MS analysis of a traditional Chinese medical formulation of Bu-Yang-Huan-Wu-Tang and its pharmacokinetics after oral administration to rats. PLoS One 2012, 7, e43848. [Google Scholar] [CrossRef]
- Lurie, I.S.; Toske, S.G. Applicability of ultra-performance liquid chromatography-tandem mass spectrometry for heroin profiling. J. Chromatogr. A 2008, 1188, 322–326. [Google Scholar] [CrossRef]
- Zu, Y.; Yan, M.; Fu, Y.; Liu, W.; Zhang, L.; Gu, C.; Efferth, T. Determination and quantification of astragalosides in Radix Astragali and its medicinal products using LC-MS. J. Sep. Sci. 2009, 32, 517–525. [Google Scholar] [CrossRef]
- Wen, X.D.; Qi, L.W.; Li, B.; Li, P.; Yi, L.; Wang, Y.Q.; Liu, E.H.; Yang, X.L. Microsomal metabolism of calycosin, formononetin and drug-drug interactions by dynamic microdialysis sampling and HPLC-DAD-MS analysis. J. Pharm. Biomed. Anal. 2009, 50, 100–105. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, H.B.; Xue, X.Y.; Sun, Y.G.; Liang, X.M. Simultaneous characterization of isoflavonoids and astragalosides in two Astragalus species by high-performance liquid chromatography coupled with atmospheric pressure chemical ionization tandem mass spectrometry. J. Sep. Sci. 2007, 30, 2059–2069. [Google Scholar] [CrossRef]
- Tong, L.; Wan, M.; Zhou, D.; Gao, J.; Zhu, Y.; Bi, K. LC-MS/MS determination and pharmacokinetic study of albiflorin and paeoniflorin in rat plasma after oral administration of Radix Paeoniae Alba extract and Tang-Min-Ling-Wan. Biomed. Chromatogr.: BMC 2010, 24, 1324–1331. [Google Scholar] [CrossRef]
- Wang, D.; Song, Y.; Li, S.L.; Bian, Y.Y.; Guan, J.; Li, P. Simultaneous analysis of seven astragalosides in Radix Astragali and related preparations by liquid chromatography coupled with electrospray ionization time-of-flight mass spectrometry. J. Sep. Sci. 2006, 29, 2012–2022. [Google Scholar] [CrossRef]
- Xu, Q.; Ma, X.; Liang, X. Determination of astragalosides in the roots of Astragalus spp. using liquid chromatography tandem atmospheric pressure chemical ionization mass spectrometry. Phytochem. Anal.: PCA 2007, 18, 419–427. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Shaw, L.-H.; Chen, W.-M.; Tsai, T.-H. Identification of Multiple Ingredients for a Traditional Chinese Medicine Preparation (Bu-yang-huan-wu-tang) by Liquid Chromatography Coupled with Tandem Mass Spectrometry. Molecules 2013, 18, 11281-11298. https://doi.org/10.3390/molecules180911281
Shaw L-H, Chen W-M, Tsai T-H. Identification of Multiple Ingredients for a Traditional Chinese Medicine Preparation (Bu-yang-huan-wu-tang) by Liquid Chromatography Coupled with Tandem Mass Spectrometry. Molecules. 2013; 18(9):11281-11298. https://doi.org/10.3390/molecules180911281
Chicago/Turabian StyleShaw, Lee-Hsin, Wei-Ming Chen, and Tung-Hu Tsai. 2013. "Identification of Multiple Ingredients for a Traditional Chinese Medicine Preparation (Bu-yang-huan-wu-tang) by Liquid Chromatography Coupled with Tandem Mass Spectrometry" Molecules 18, no. 9: 11281-11298. https://doi.org/10.3390/molecules180911281






