Isolation and Identification of Two Novel Attractant Compounds from Chinese Cockroach (Eupolyphaga sinensis Walker) by Combination of HSCCC, NMR and CD Techniques
Abstract
:1. Introduction
2. Results and Discussion
2.1. HSCCC of the New Attractants
| Solvent system(v/v/v/v) | KUP/LP a | α | |
|---|---|---|---|
| KA b | KB c | ||
| n-hexane-ethyl acetate-methanol-water(1:1:1:1) | 1.61 | 3.13 | 1.94 |
| n-hexane-ethyl acetate-methanol-water(1.5:1:1.5:1) | 0.68 | 1.26 | 1.85 |
| n-hexane-ethyl acetate-methanol-water(2:1:2:1) | 0.33 | 0.57 | 1.73 |
| petroleum ether-ethylacetate-methanol-water(1:1:1:1) | 0.89 | 1.75 | 1.97 |
| petroleum ether-ethylacetate-methanol-water(1.8:1:1.8:1) | 0.17 | 0.40 | 2.35 |
| petroleum ether-ethylacetate-methanol-water(1.5:1:1.5:1) | 0.25 | 0.55 | 2.20 |
| petroleum ether-ethylacetate-methanol-water(2:1:2:1) | 0.13 | 0.29 | 2.23 |

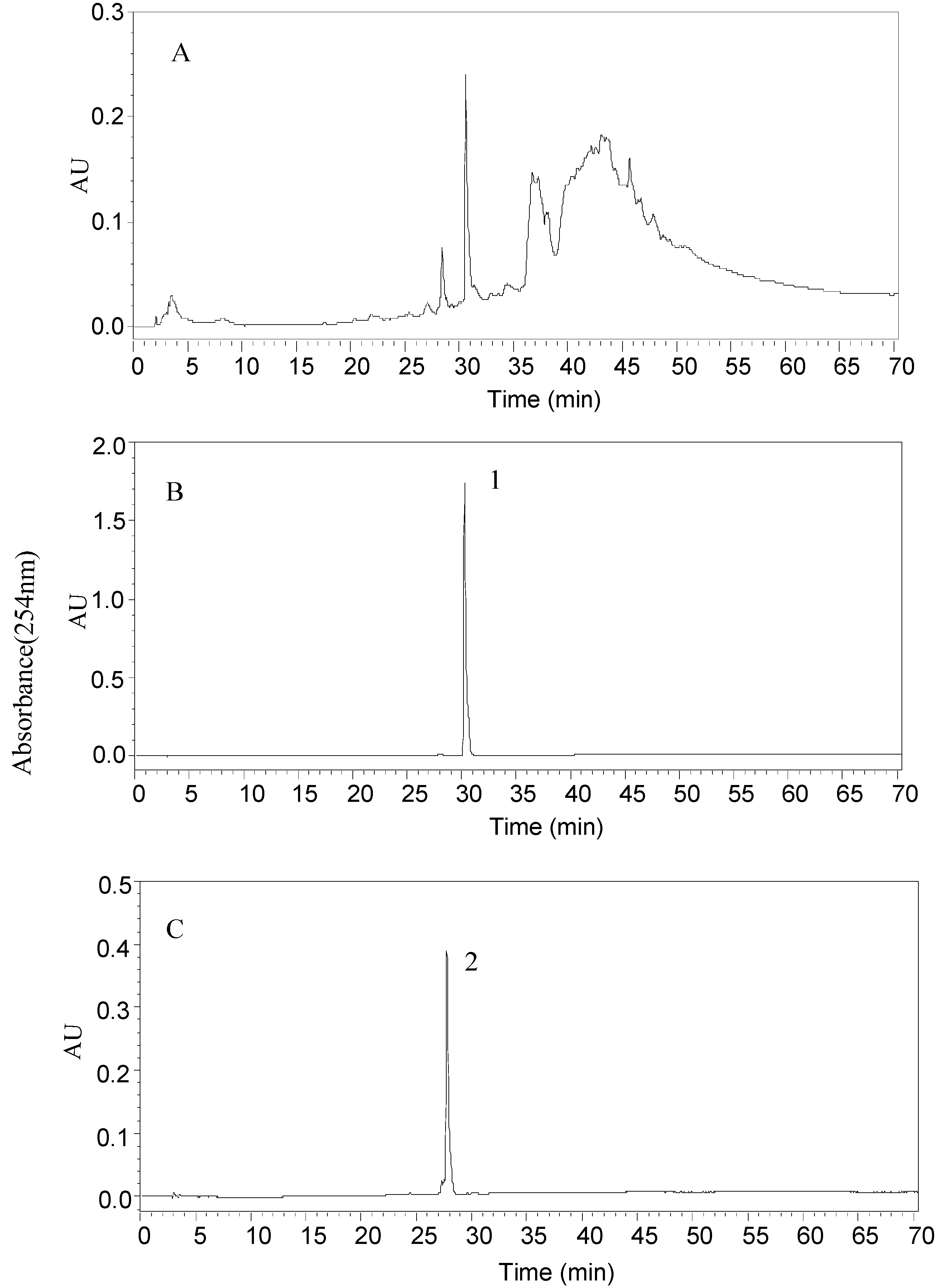
2.2. HPLC Analyses of Fractions
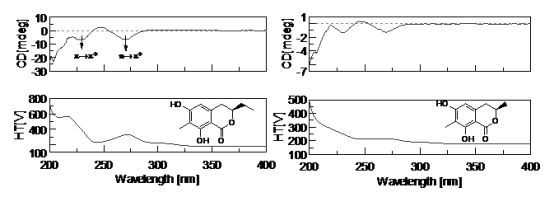
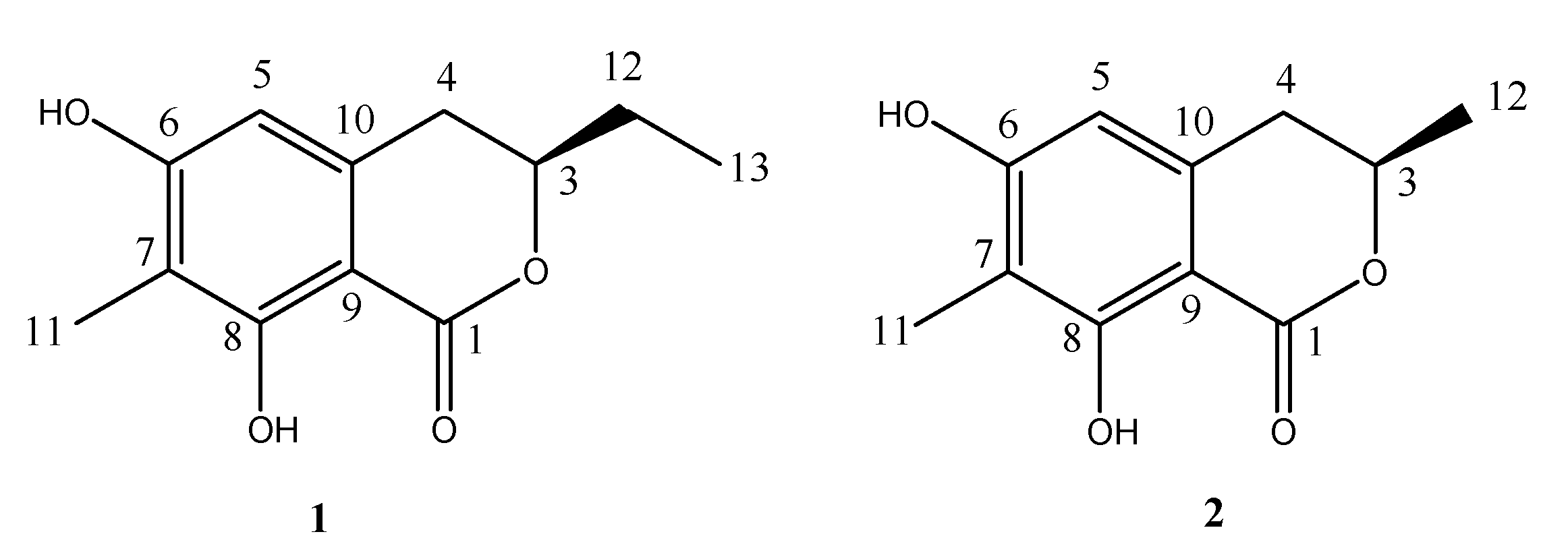
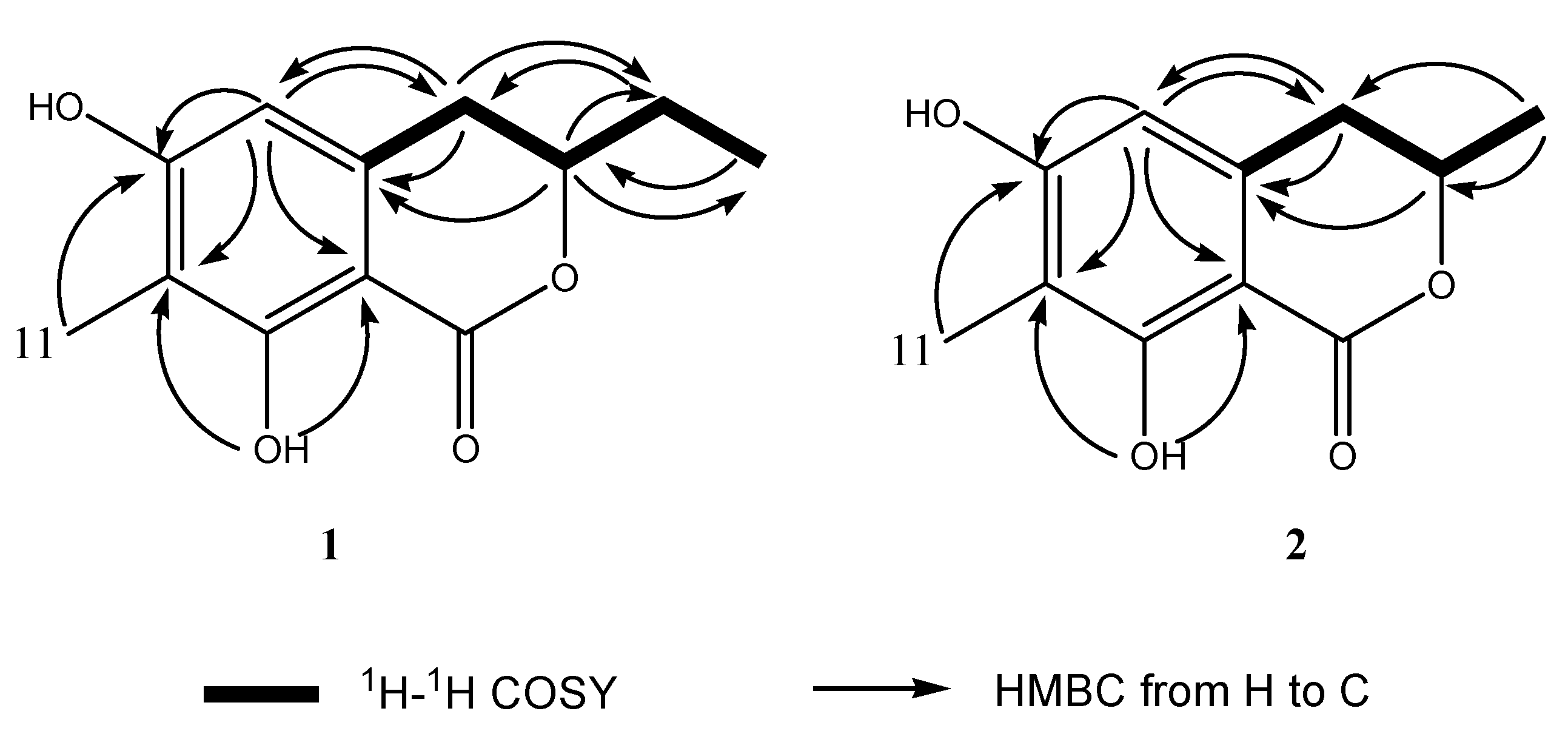
2.3. Structural Elucidation of the New Attractants
| Position | 1 | 2 | |||
|---|---|---|---|---|---|
| δCa,b | δHc mult. (J in Hz) | δCa,b | δHc mult. (J in Hz) | ||
| 1 | 170.8 (s) | 170.4 (s) | |||
| 3 | 80.7 (d) | 4.43 (dddd, J = 10.5, 6.5, 5.0, 4.0) | 75.7 (d) | 4.63 (m) | |
| 4 | 32.4 (t) | 2.80 (m) | 34.5 (t) | 2.83 (m) | |
| 5 | 106.0 (d) | 6.26 (s) | 105.7 (d) | 6.22 (s) | |
| 6 | 160.7 (s) | 160.3 (s) | |||
| 7 | 109.9 (s) | 109.8 (s) | |||
| 8 | 162.1 (s) | 11.40 (OH) | 162.2 (s) | 11.46 (OH) | |
| 9 | 101.2 (s) | 101.3 (s) | |||
| 10 | 138.1 (s) | 138.0 (s) | |||
| 11 | 7.5 (q) | 2.12 (s) | 7.4 (q) | 2.13 (s) | |
| 12 | 27.8 (t) | 1.77 (m), 1.87 (m) | 20.7 (q) | 1.51 (d, J = 6.5) | |
| 13 | 9.3 (q) | 1.06 (t, J = 7.5) | |||
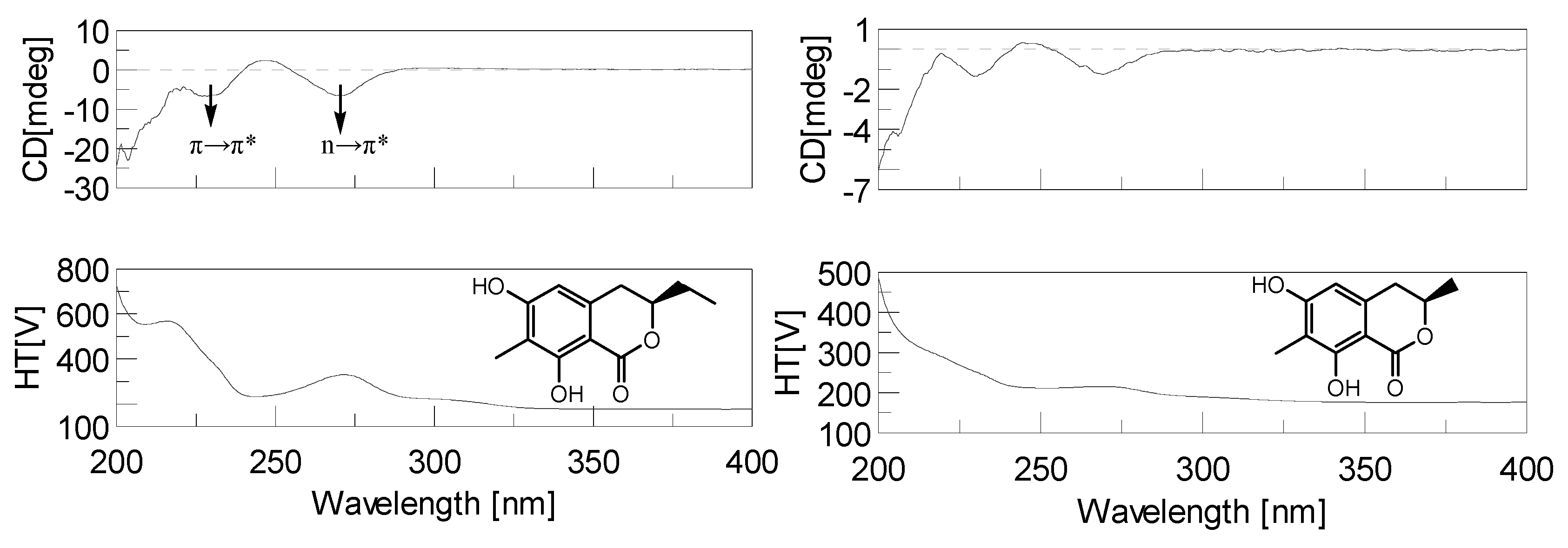
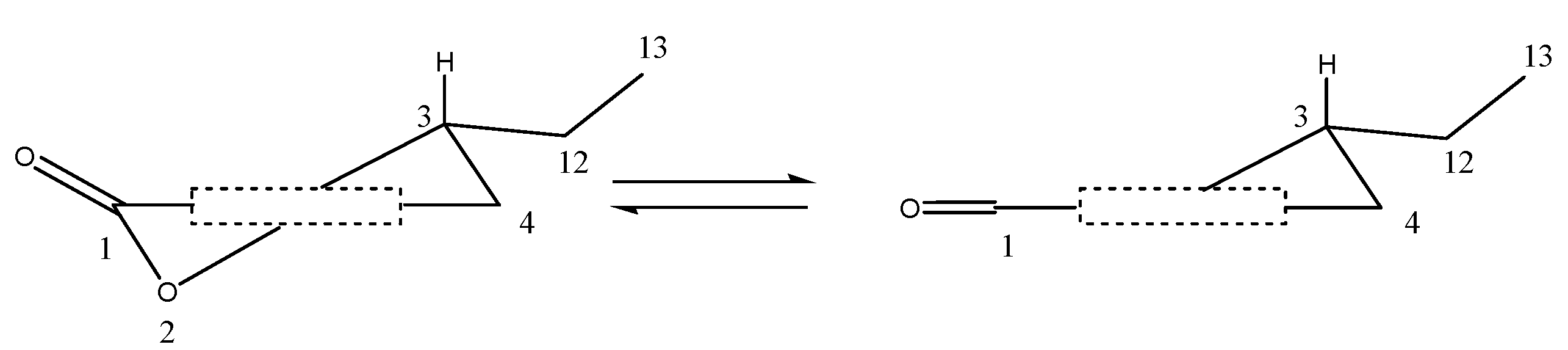
2.4. Attracting Activity
3. Experimental
3.1. Apparatus
3.2. Reagents
3.3. Insect Material
3.4. Preparation of Crude Sample
3.5. Preparation of the Two-phase Solvent Systems and Sample Solution
3.6. HSCCC Separation Procedure
3.7. HPLC Analysis and Identification of HSCCC Peak Fractions
3.8. Attracting Activity Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Conflicts of Interest
References
- Lin, C.-Y.; Sun, J.S.; Sheu, S.-Y.; Lin, F.-H.; Wang, Y.-J.; Chen, L.T. The effect of Chinese Medicine on bone cell activities. Am. J. Chin. Med. 2002, 30, 271–285. [Google Scholar] [CrossRef]
- Wang, W.; Wang, J.; Zhao, D.; Liu, H.; Zhou, W.; Chen, K. Comparison of Spatholobus. suberectus Dum, Euonymus alatus (Thunb.) Sieb. and Eupolyphaga. sinensis Walker on regulation of plasma lipid. Chin. J. Chin. Mater. Med. 1991, 16, 299–310. [Google Scholar]
- Tillman, J.A.; Seybold, S.J.; Jurenka, R.A.; Blomquist, G.J. Insect pheromones–an overview of biosynthesis and endocrine regulation. Insect Biochem. Molec. Biol. 1999, 29, 481–514. [Google Scholar] [CrossRef]
- Barth, R.H. The mating behavior of Periplaneta americana (Linnaeus) and Blatta orientalis Linnaeus (Blattaria, Blattinae), with Notes on 3 additional species of Periplaneta and Interspecific action of female sex pheromones. Z. Tierpsychol. 1970, 27, 223–230. [Google Scholar]
- Appel, A.G.; Smith, L.M., II. Biology and management of the smokybrown cockroach. Annu. Rev. Entomol. 2002, 47, 33–55. [Google Scholar] [CrossRef]
- Takahashi, S.; Watanabe, K.; Saito, S.; Nomura, Y. Isolation and biological activity of the sex pheromone of the smoky brown cockroach, Periplaneta. fuliginosa Serville (Dictyoptera: Blattidae). Appl. Entomol. Zool. 1995, 30, 357–360. [Google Scholar]
- Simon, D.; Barth, R.H. Sexual behavior in the cockroach genera Periplaneta. and Blatta. IV. Interspecific interactions. Z. Tierpsychol. 1977, 45, 85–103. [Google Scholar]
- Persoons, C.J.; Verwiel, P.E.J.; Ritter, F.J.; Nooyen, W.J. Studies on sex pheromone of American cockroach, with emphasis on structure elucidation of periplanone-A. J. Chem. Ecol. 1982, 8, 439–451. [Google Scholar]
- Adams, M.A.; Nakanishi, K.; Still, W.C.; Arnold, E.V.; Clardy, J.; Persoons, C.J. Sex pheromone of the American cockroach: Absolute configuration of periplanone-B. J. Am. Chem. Soc. 1979, 101, 2495–2498. [Google Scholar] [CrossRef]
- Nojima, S.; Schal, C.; Webster, F.X.; Santangelo, R.G.; Roelofs, W.L. Identification of the sex pheromone of the German cockroach, Blattella. germanica. Science 2005, 307, 1104–1106. [Google Scholar] [CrossRef]
- Morgan, E.D. Preparation of small-scale samples from insect for chromatography. Anal. Chim. Acta 1990, 236, 227–235. [Google Scholar] [CrossRef]
- Yamamoto, K.; Kawai, Y.; Hayashi, T.; Ohe, Y.; Hayashi, H.; Toyoda, F.; Kawahara, G.; Iwata, T.; Kikuyama, S. Silefrin, a sodefrin-like pheromone in the abdominal gland of the sword-tailed newt, Cynops. ensicauda. FEBS Lett. 2000, 472, 267–270. [Google Scholar] [CrossRef]
- Felici, A.; Alimenti, C.; Ortenzi, C.; Luporini, P. Purification and initial characterization of two pheromones from the marine Antarctic ciliate, Euplotes. nobilii. Ital. J. Zool. 1999, 66, 355–360. [Google Scholar] [CrossRef]
- Djozan, D.; Baheri, T.; Farshbaf, R.; Azhari, S. Investigation of solid-phase microextraction efficiency using pencil lead fiber for in vitro and in vivo sampling of defensive volatiles from insect's scent gland. Anal. Chim. Acta 2005, 554, 197–201. [Google Scholar] [CrossRef]
- He, S.; Lu, Y.B.; Jiang, L.Y.; Wu, B.; Zhang, F.Y.; Pan, Y.J. Preparative isolation and purification of antioxidative stilbene oligomers from Vitis. chunganeniss using high-speed counter-current chromatography in stepwise elution mode. J. Sep. Sci. 2009, 32, 2339–2345. [Google Scholar] [CrossRef]
- He, S.; Lu, Y.B.; Wu, B.; Pan, Y.J. Isolation and purification of antioxidative isomeric polyphenols from the roots of Parthenocissus. laetevirens by counter-current chromatography. J. Chromatogr. A 2007, 115, 1175–1179. [Google Scholar]
- Mandava, N.B.; Ito, Y. Countercurrent Chromatography: Theory and Practice; Marcel Dekker: New York, NY, USA, 1988. [Google Scholar]
- Ito, Y. Golden rules and pitfalls in selecting optimum conditions for high-speed counter-current chromatography. J. Chromatogr. A 2005, 1065, 145–168. [Google Scholar]
- Cheng, M.J.; Yang, P.H.; Wu, M.D.; Chen, I.S.; Hsieh, M.T.; Chen, Y.L.; Yuan, G.F. Secondary metabolites from the fungus Monascus. purpureus and evaluation of their cytotoxic activity. Helv. Chim. Acta 2011, 94, 1638–1650. [Google Scholar] [CrossRef]
- Perez, A.L.; Hallett, R.H.; Gries, R.; Gries, G.; Oehlschlager, A.C.; Borden, J.H. Pheromone chirality of asian palm weevils, Rhynchophorus. ferrugineus (Oliv.) and R. vulneratus (Panz.) (Coleoptera: Curculionidae). J. Chem. Ecol. 1996, 22, 357–368. [Google Scholar] [CrossRef]
- Antus, S.; Snatzke, G.; Steinke, I. Circulardichroismus, LXXXI. Synthese und Circulardichroismus von Steroiden mit Isochromanon-Chromophor. Liebigs Ann. Chem. 1993, 1983, 2247–2261. [Google Scholar] [CrossRef]
- Dornhege, E.; Snatzke, G. Circulardichroismus—XL: Chiroptische eigenschaften von aminoindanolen und verwandten verbindungen. Tetrahedron 1970, 26, 3059–3067. [Google Scholar] [CrossRef]
- Krohn, K.; Bahramsari, R.; Flörke, U.; Ludewig, K.; Kliche-Spory, C.; Michel, A.; Aust, H.J.; Draeger, S.; Schula, B.; Antus, S. Dihydroisocoumarins from fungi: Isolation, structure elucidation, circular dichroism and biological activity. Phytochemistry 1997, 45, 313–320. [Google Scholar]
- Dimitriadis, C.; Gill, M.; Harte, M.F. The first stereospecific approach to both enantiomers of mellein. Tetrahedron: Asymmetry 1997, 8, 2153–2158. [Google Scholar] [CrossRef]
- Guss, P.L.; Tumlinson, J.H.; Sonnet, P.E.; Proveaux, A.T. Identification of a female-produced sex pheromone of the western corn rootworm. Chem. Ecol. 1982, 8, 545–556. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 1 and 2 are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jiang, W.; Wu, X.; Wu, B. Isolation and Identification of Two Novel Attractant Compounds from Chinese Cockroach (Eupolyphaga sinensis Walker) by Combination of HSCCC, NMR and CD Techniques. Molecules 2013, 18, 11299-11310. https://doi.org/10.3390/molecules180911299
Jiang W, Wu X, Wu B. Isolation and Identification of Two Novel Attractant Compounds from Chinese Cockroach (Eupolyphaga sinensis Walker) by Combination of HSCCC, NMR and CD Techniques. Molecules. 2013; 18(9):11299-11310. https://doi.org/10.3390/molecules180911299
Chicago/Turabian StyleJiang, Wei, Xiaodan Wu, and Bin Wu. 2013. "Isolation and Identification of Two Novel Attractant Compounds from Chinese Cockroach (Eupolyphaga sinensis Walker) by Combination of HSCCC, NMR and CD Techniques" Molecules 18, no. 9: 11299-11310. https://doi.org/10.3390/molecules180911299