In Vitro Delivery and Controlled Release of Doxorubicin for Targeting Osteosarcoma Bone Cancer
Abstract
:1. Introduction
2. Results and Discussion
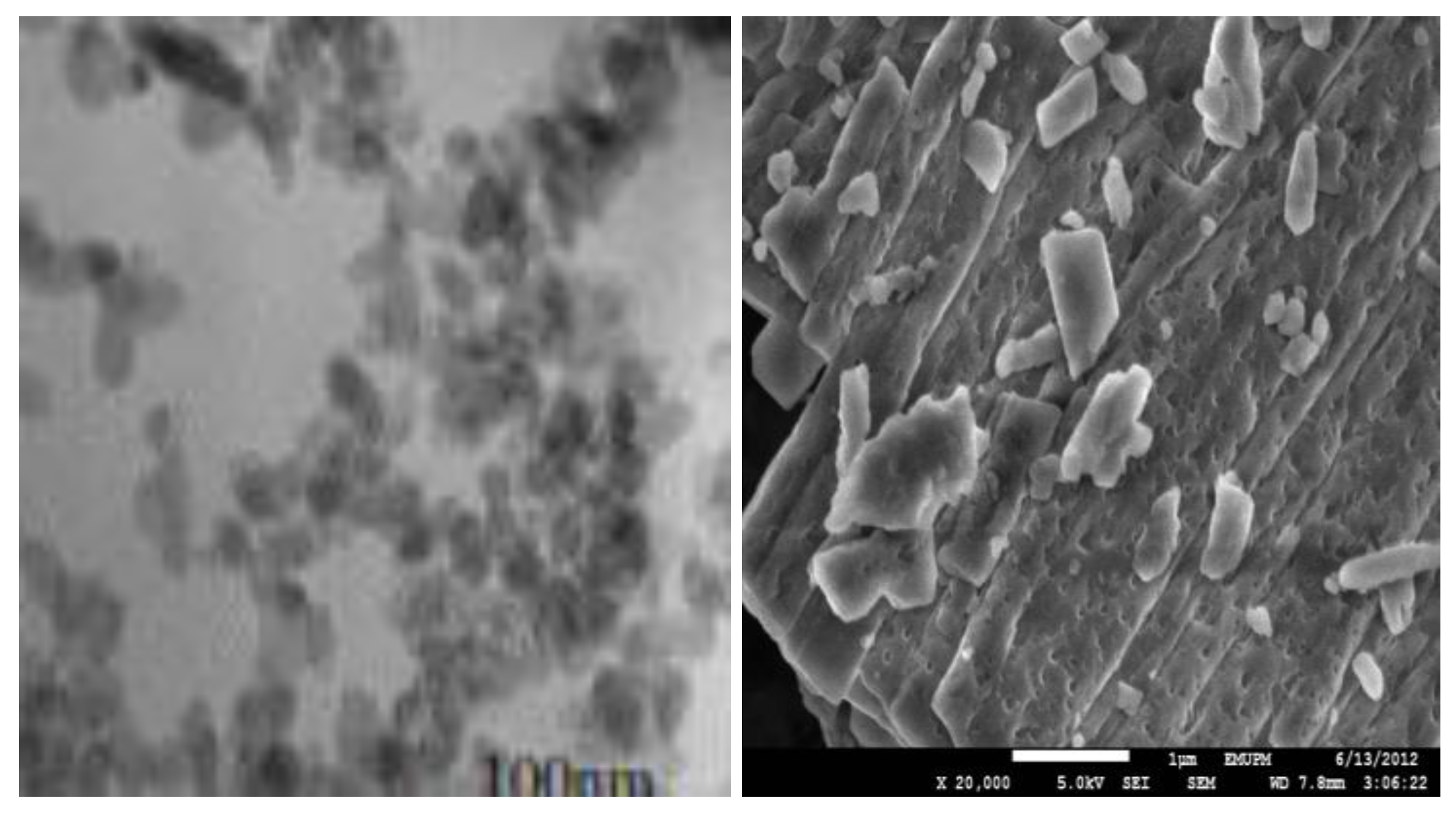
2.1. Transmission Electron Microscope (TEM) and Field Emission Scanning Electron Microscope (FESEM) Remains the Most Important Instrument Used in Nanoparticles Characterisation, Microscopy is The Only Method in Which the Individual Particle Size, and Morphology Are Directly Observed and Measured
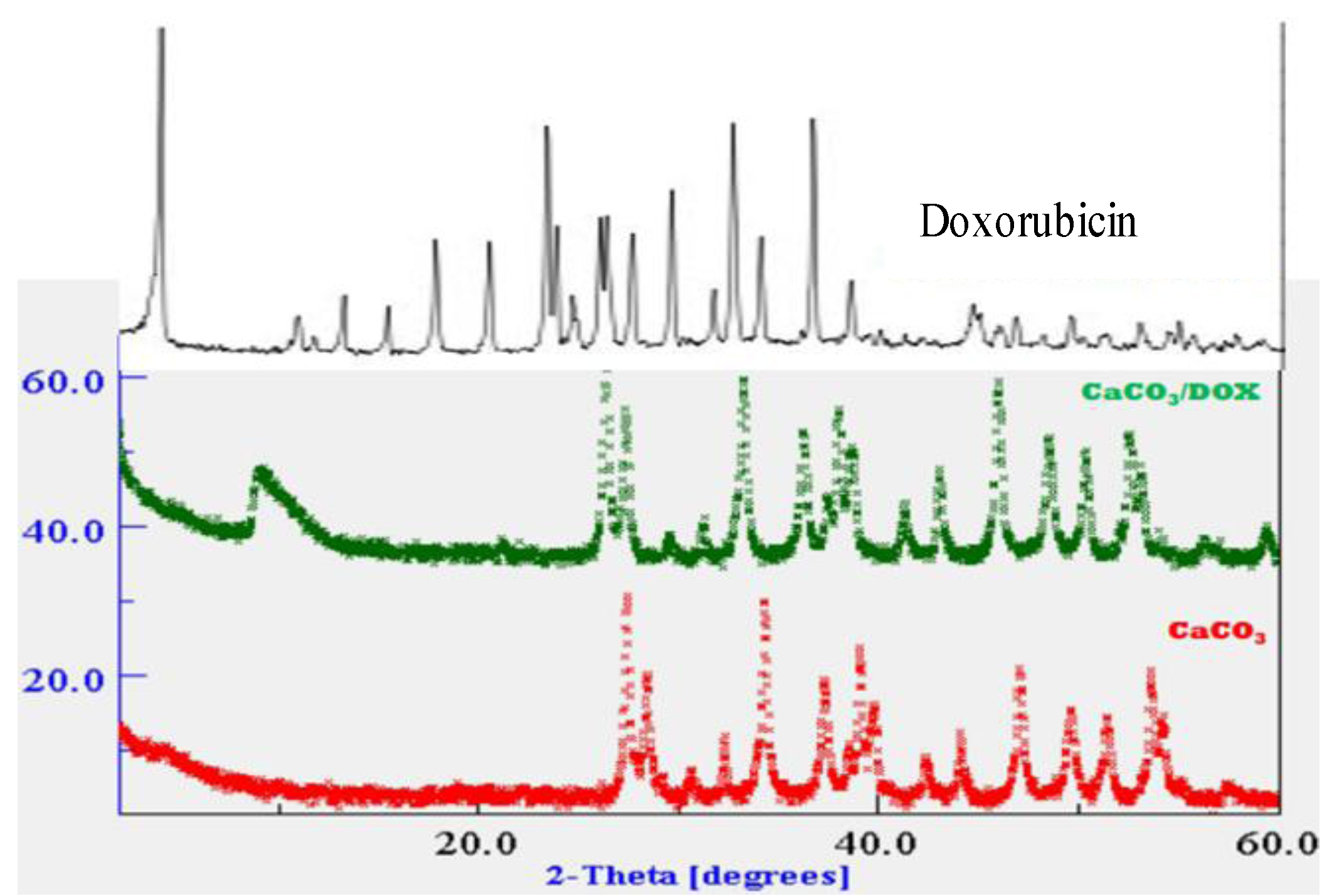
2.2. X-Ray Diffraction (XRD)
2.3. Drug Loading and Encapsulation Efficiency
| Samples | Weight of nanocrystals (mg) | Weight of drug (mg) | Loading content (%) | Encapsulation efficiency (%) |
|---|---|---|---|---|
| CaCO3 (1) | 50 | 1 | 4.5 | 97 |
| CaCO3 (2) | 50 | 2 | 8.9 | 86 |
| CaCO3 (3) | 50 | 3 | 11.7 | 75 |
2.4. Elemental Analysis of CaCO3/Dox
| Samples | % C | % H | % N | N/H |
|---|---|---|---|---|
| CaCO3/Dox | 13.128 | 0.5954 | 0.5447 | 1.09 |
| Doxorubicin | 53.91 | 5.21 | 2.22 | 2.34 |
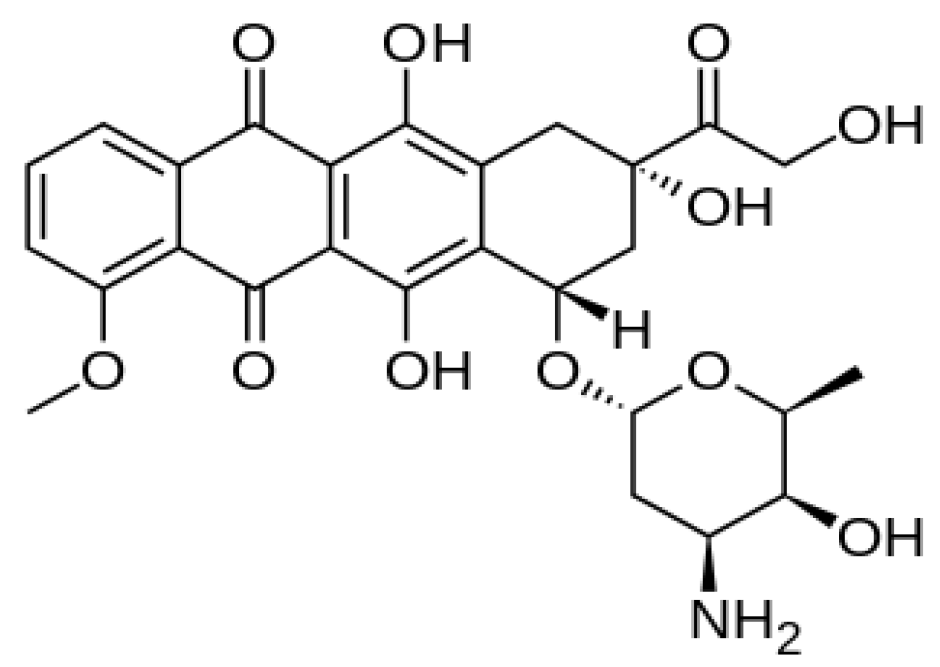
2.5. Doxorubicin Release Profile

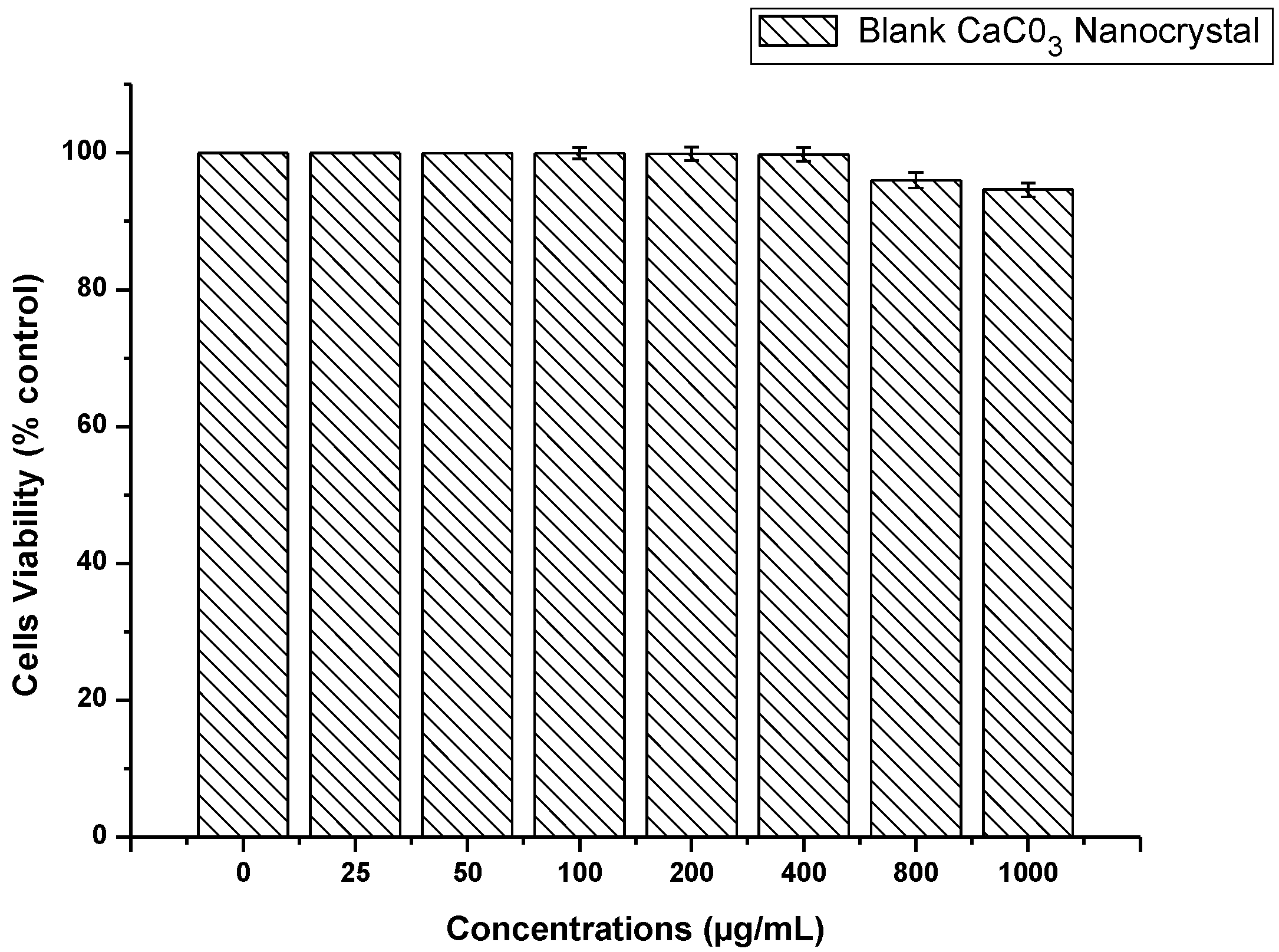
2.6. CaCO3 Nanocrystal Biocompatibility Assay
2.7. Doxorubicin and CaCO3/Dox MTT Cytotoxicity Assay

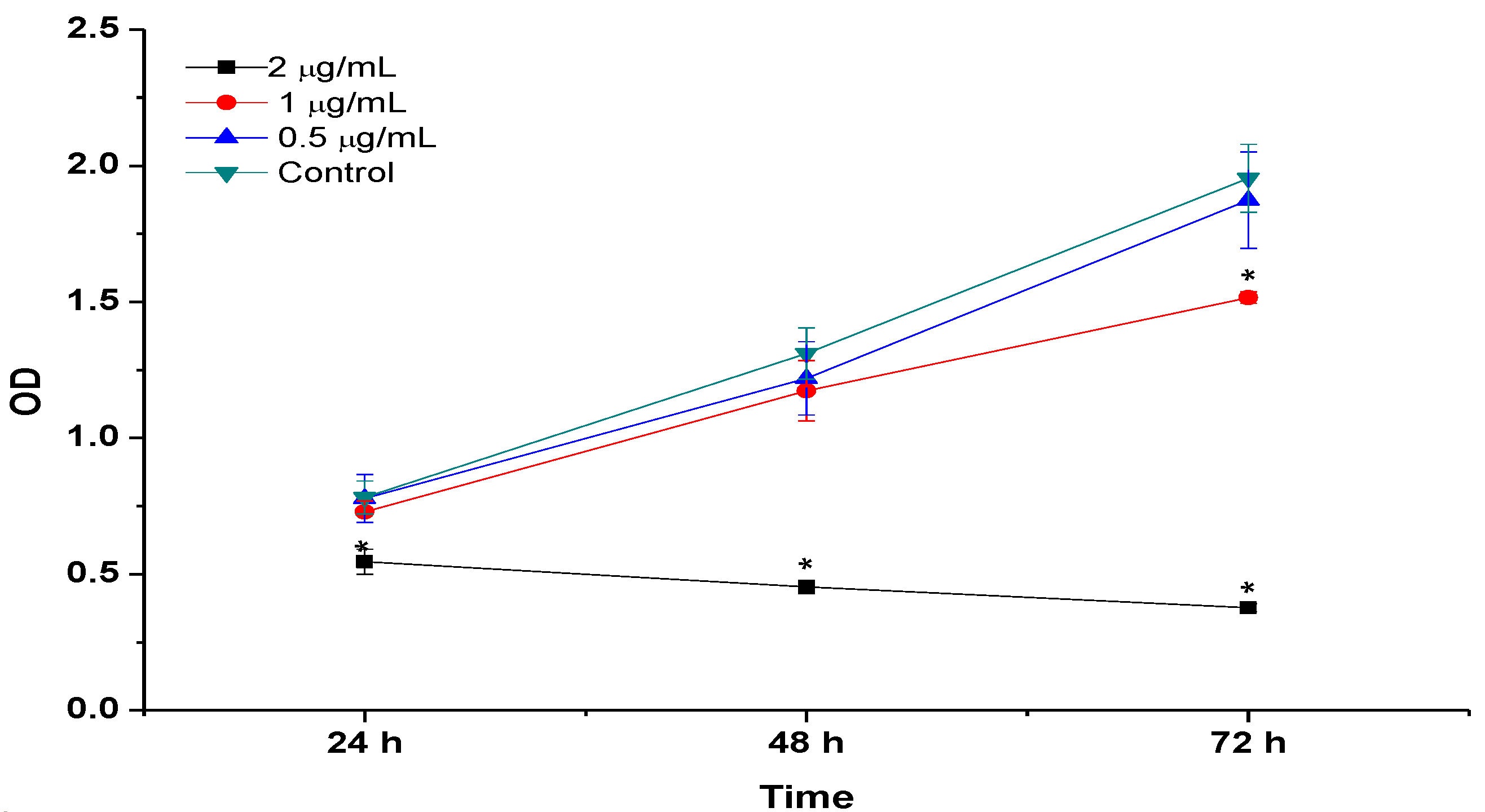
2.8. BrdU Cell Proliferation Assay
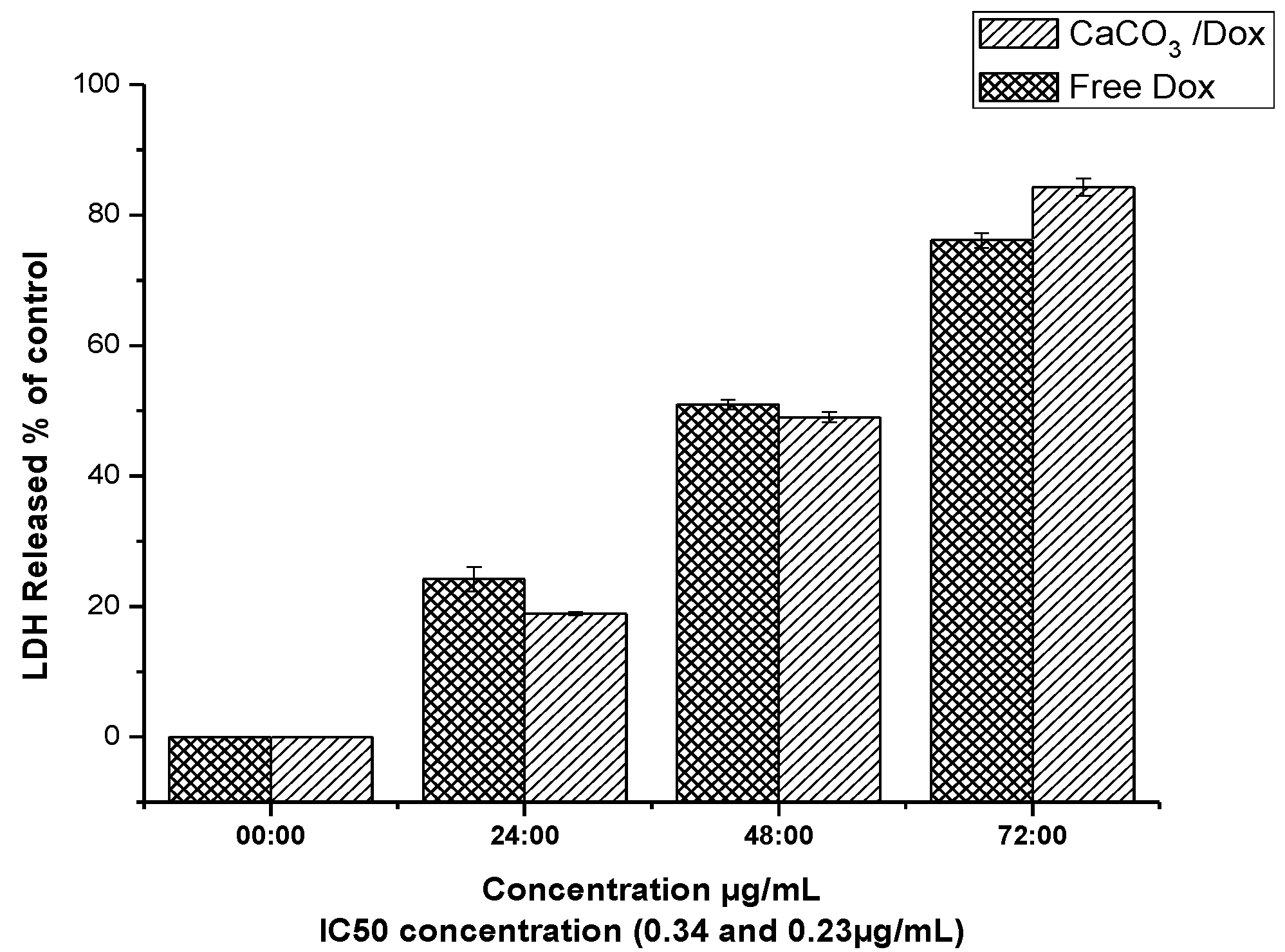
2.9. LDH Release (Membrane Integrity Assay)
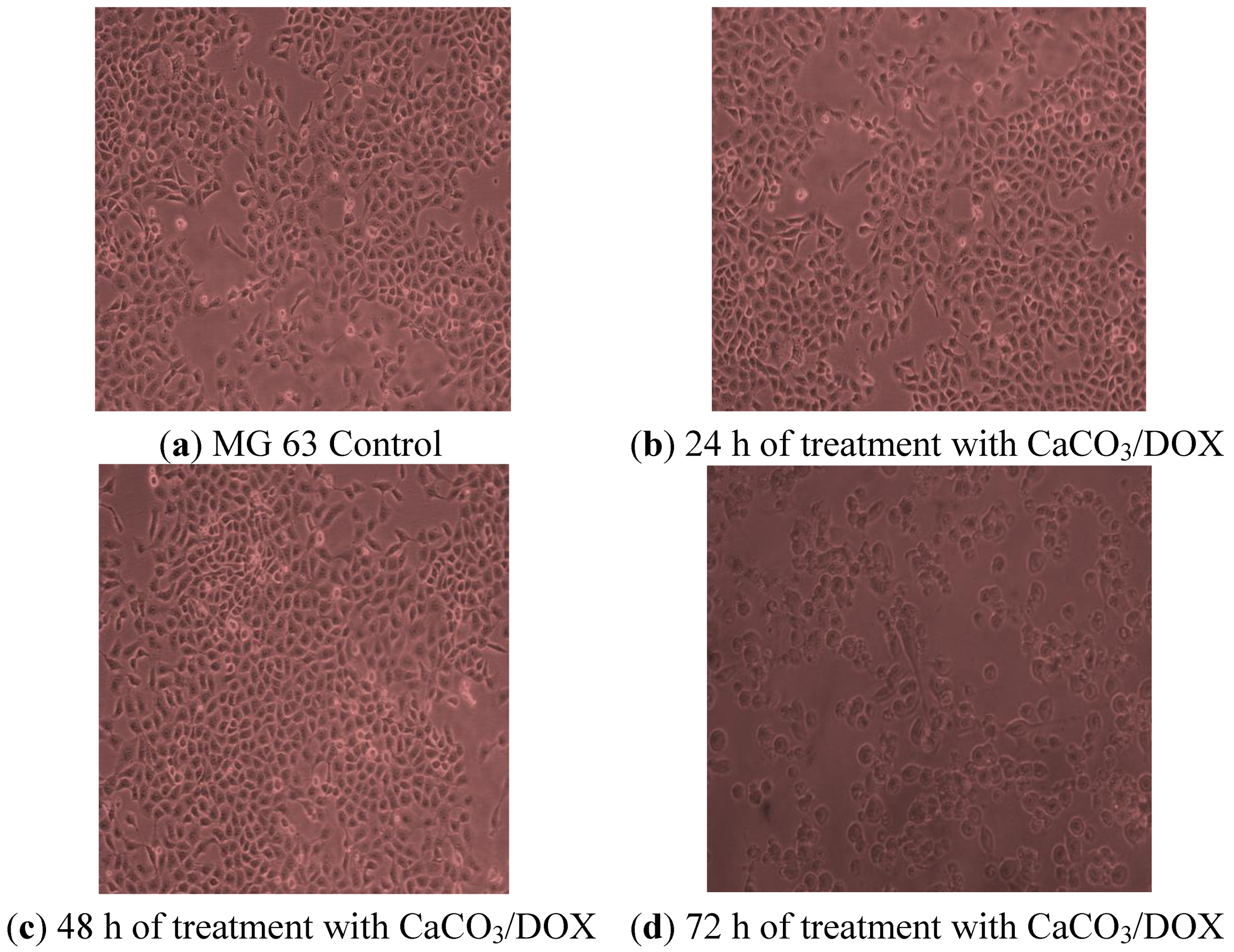
2.10. Morphological Observations
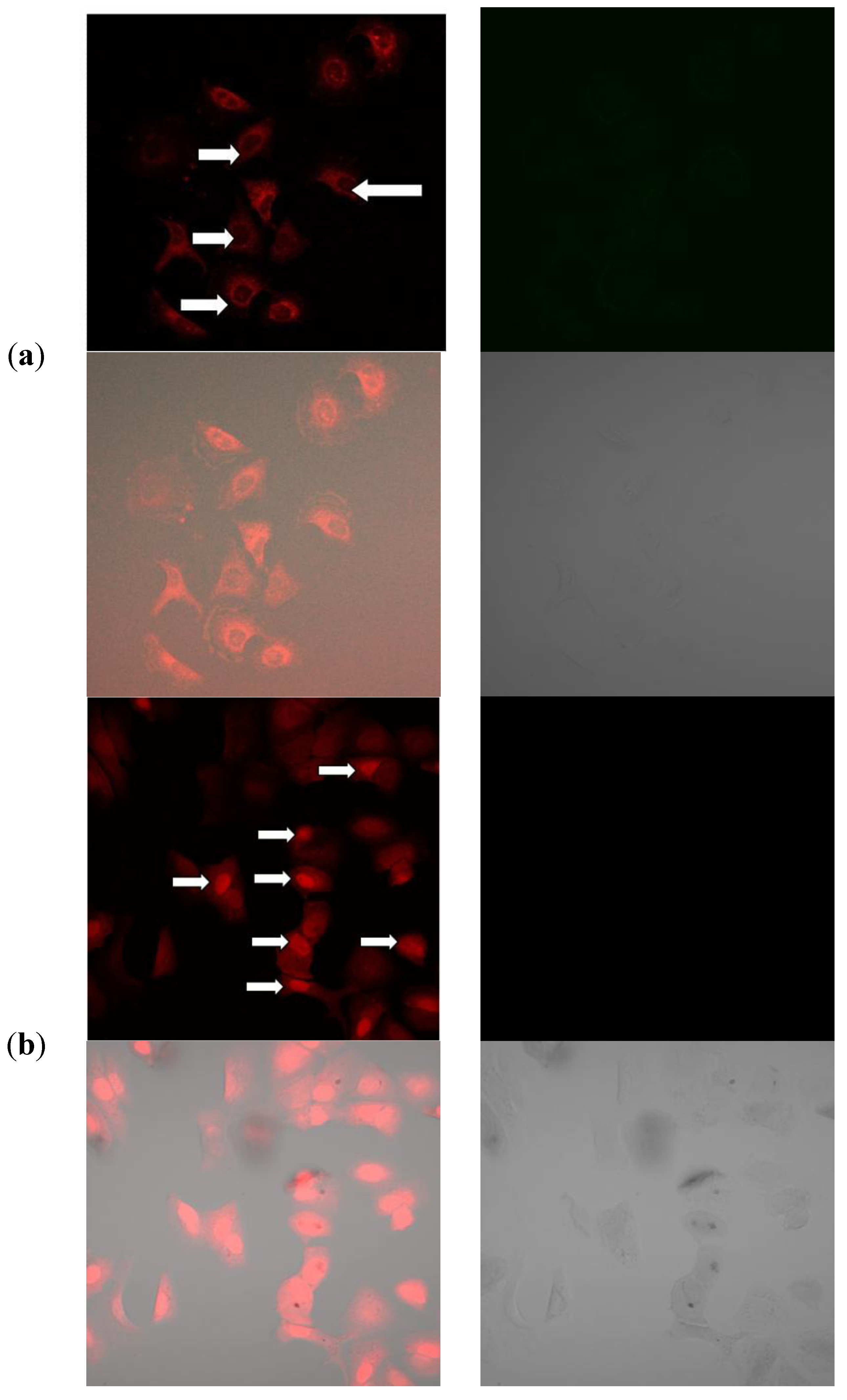
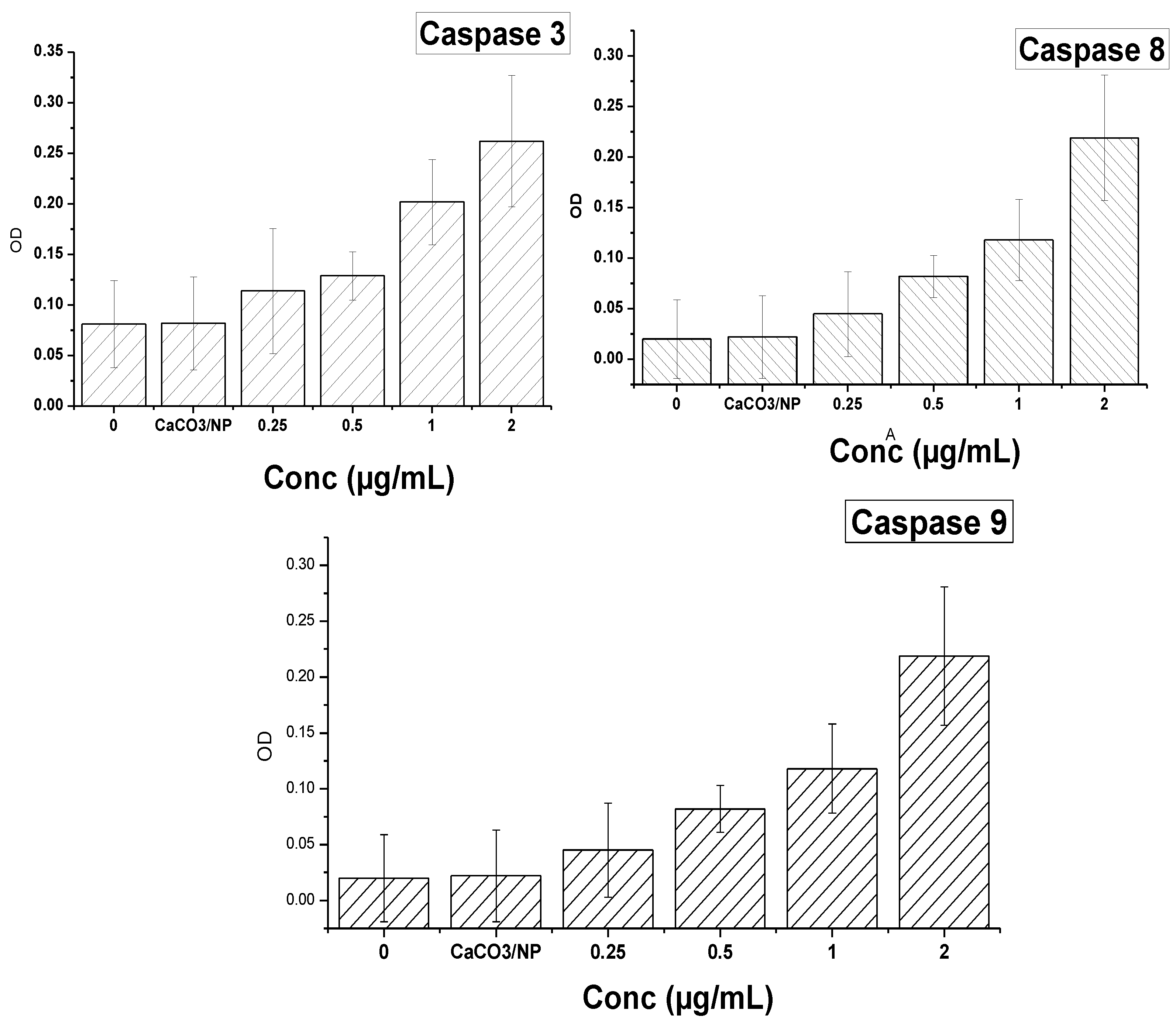
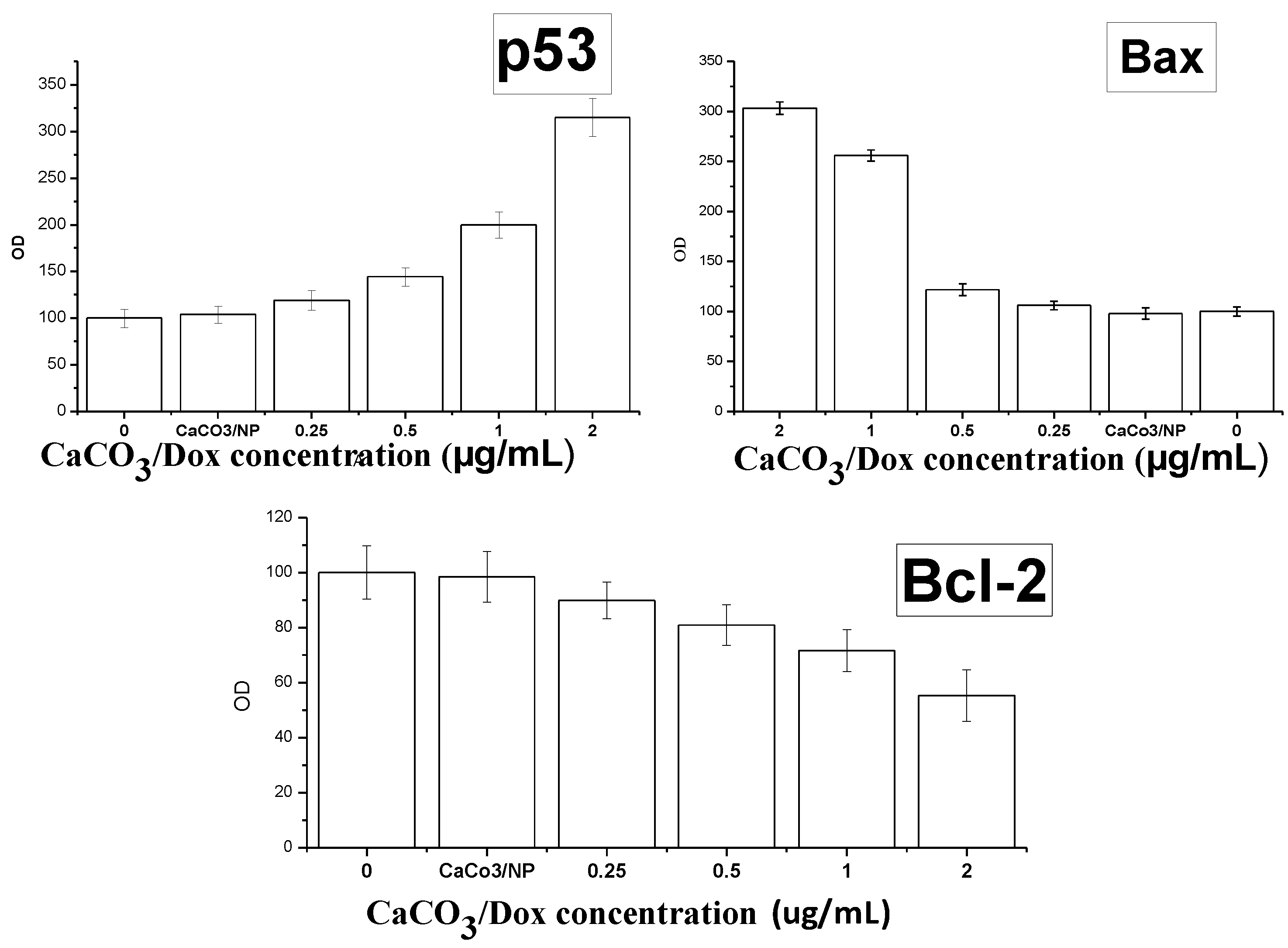
2.11. Mechanism of Cellular Uptake of Doxorubicin
3. Experimental
3.1. Synthesis and Drug Loaded Calcium Carbonate Nanocrystals.
3.2. Characterisation of Calcium Carbonate Nanocrystals
3.3. Determination of Drug Loading Content and Encapsulation Efficiency
3.4. In Vitro Controlled Drug Release Study
3.5. In Vitro Evaluation of Cytotoxicity
3.6. Cells Seeding and Treatment
3.7. LDH Release Membrane Integrity Assays
3.8. BrdU Proliferation Assay
3.9. Morphological Examination
3.10. DOX Cell Uptake and Drug Release Investigations
3.11. Elemental Analysis Determination
3.12. Measurement of Caspase-3 and Caspase-8 and 9 Activities
3.13. Enzyme-Linked Immunosorbent Assay (ELISA)
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Patil, R.; Portilla-Arias, J.; Ding, H.; Konda, B.; Rekechenetskiy, A.; Inoue, S.; Black, K.L.; Holler, E.; Ljubimova, J.Y. Cellular Delivery of Doxorubicin via pH-Controlled Hydrazone Linkage Using Multifunctional Nano Vehicle Based on Poly(ßL-Malic Acid). Int. J. Mol. Sci. 2012, 13, 11681–11693. [Google Scholar]
- Minko, T.; Kopečková, P.; Kopeček, J. Efficacy of the chemotherapeutic action of HPMA copolymer-bound doxorubicin in a solid tumor model of ovarian carcinoma. Int. J. Cancer 2000, 86, 108–117. [Google Scholar] [CrossRef]
- Fan, H.; Alekha, K.D. Effect of cross-linking on the in vitro release kinetics of doxorubicin from gelatin implants. Int. J. Pharm. 2001, 213, 103–116. [Google Scholar] [CrossRef]
- Jia, Y.H.; Yuan, M.; Yuan, H.D.; Huang, X.L.; Sui, X.; Cui, X.M.; Tang, F.Q.; Peng, J.; Chen, J.Y.; Lu, S.B.; et al. Co-encapsulation of magnetic Fe3O4 nanoparticles and doxorubicin into biodegradable PLGA nanocarriers for intratumoral drug delivery. Int. J. Nanomed. 2012, 7, 1697–1708. [Google Scholar] [CrossRef]
- Spina, A.; Sorvillo, L.; Maiolo, F.D.; Esposito, A.; D’Auria, R.; Gesto, D. D.; Chiosiet, E.; Naviglio, S. Inorganic phosphate enhances sensitivity of human osteosarcoma U2OS cells to doxorubicin via a p53-dependent pathway. J. Cell. Physiol. 2013, 228, 198–206. [Google Scholar] [CrossRef]
- Min, K.H.; Lee, H.J.; Kim, K.; Kwon, I.C.; Jeong, S.Y.; Lee, S.C. The tumor accumulation and therapeutic efficacy of doxorubicin carried in calcium phosphate-reinforced polymer nanoparticles. Biomaterials 2012, 33, 5788–5797. [Google Scholar] [CrossRef]
- Awang-Hazmi, A.J.; Zuki, A.B.Z.; Nordin, M.M.; Jalila, A.; Norimah, Y. Mineral composition of the cockle (anadara granosa) shells of west coast of peninsular Malaysia and its potential as biomaterial for use in bone repair. J. Anim. Vet. Adv. 2007, 6, 591–594. [Google Scholar]
- Islam, K.N.; Zuki, A.B.Z.; Wahid, H.; Ali, M.E.; Hussein, M.Z.B.; Noordin, M.M.; Loqman, M.Y.; Wahid, H.; Hakim, M.A.; Hamid, S.B.A. Facile synthesis of calcium carbonate nanoparticles from cockle shells. J. Nanomater. 2012, 2012, 2. [Google Scholar]
- Kamba, A.S.; Ismail, M.; Ibrahim, T.A.T.; Zakaria, Z.A.B. Synthesis and characterisation of calcium carbonate aragonite nanocrystals from cockle shell powder (anadara granosa). J. Nanomater. 2013, 2013, 398357:1–398357:9. [Google Scholar]
- Naruphontjirakul, P.; Viravaidya-Pasuwat, K. Development of doxorubicin—Core shell o-succinyl chitosan graft pluronic®127 copolymer nanoparticles to treat human cancer. Int. J. Biosci. Biochem. Bioinforma. 2011, 1, 131–136. [Google Scholar]
- Pharmacopoeia commission of the Peoples Republic of China. Pharmacopoeia of the Peoples Republic of China; Chemistry Industry Press: Beijing, China, 2000. [Google Scholar]
- Wang, J.; Chen, S.; Zong, J. Y.; Zhao, D.; Li, F.; Zhuo, R. X.; Cheng, S. X. Calcium carbonate/carboxymethyl chitosan hybrid microspheres and nanospheres for drug delivery. J. Phys. Chem. C 2010, 114, 18940–18945. [Google Scholar]
- Zhao, Q.H.; Han, B.S.; Wang, Z.H.; Gao, C.Y.; Peng, C.H.; Shen, J.C. Hollow chitosan-alginate multilayer microcapsules as drug delivery vehicle: Doxorubicin loading and in vitro and in vivo studies. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 63–74. [Google Scholar] [CrossRef]
- Cui, W.; Cui, Y.; Zhao, J.; Li, J.B. Fabrication of tumor necrosis factor-related apoptosis inducing ligand (TRAIL)/ALG modified CaCO3 as drug carriers with the function of tumor selective recognition. J. Mater. Chem. B 2013, 1, 1326–1332. [Google Scholar]
- Barua, S.; Yoo, J.W.; Kolhar, P.; Wakankar, A.; Gokarn, Y.R.; Mitragotri, S. Particle shape enhances specificity of antibody-displaying nanoparticles. Proc. Natl. Acad. Sci. USA 2013, 110, 10753–10758. [Google Scholar]
- Gillies, E.R. pH-responsive copolymer assemblies for controlled release of doxorubicin. Bioconjug. Chem. 2005, 16, 361–368. [Google Scholar]
- Chittasupho, C.; Xie, S.X.; Baoum, A.; Yakovleva, T.; Siahaan, T.J.; Berkland, C.J. ICAM-1 targeting of doxorubicin-loaded PLGA nanoparticles to lung epithelial cells. Eur. J. Pharm. Sci. 2009, 37, 141–150. [Google Scholar]
- Larsen, A.K.; Escargueil, A.E.; Skladanowski, A. Resistance mechanisms associated with altered intracellular distribution of anticancer agents. Pharmacol. Ther. 2000, 85, 217–229. [Google Scholar]
- Mohan, P.; Rapoport, N. Doxorubicin as a molecular nanotheranostic agent: Effect of doxorubicin encapsulation in micelles or nanoemulsions on the ultrasound-mediated intracellular delivery and nuclear trafficking. Mol. Pharm. 2010, 7, 1959–1973. [Google Scholar] [CrossRef]
- Manaspon, C.; Viravaidya-Pasuwat, K.; Pimpha, N. Preparation of folate-conjugated pluronic F127/chitosan core-shell nanoparticles encapsulating doxorubicin for breast cancer treatment. J. Nanomater. 2012, 2012, 593878. [Google Scholar]
- Liang, P.; Zhao, D.; Wang, C.Q.; Zong, J.Y.; Zhuo, R.X.; Cheng, S.X. Facile preparation of heparin/CaCO3/CaP hybrid nano-carriers with controllable size for anticancer drug delivery. Colloids Surf. B Biointerfaces 2013, 102, 783–788. [Google Scholar]
- Upadhyay, K.K.; Bhatt, A. N.; Mishra, A. K.; Dwarakanath, B.S.; Jain, S.; Schatz, C.; Meins, J.F.L.; Farooque, A.; Chandraiah, G.; Misra, A.; et al. The intracellular drug delivery and anti tumor activity of doxorubicin loaded poly(g-benzyl L-glutamate)-b-hyaluronan polymersomes. Biomaterials 2010, 31, 2882–2892. [Google Scholar]
- Shuai, X.T.; Ai, H.; Nasongkla, N.; Kim, S.; Gao, J.M. Micellar carriers based on block copolymers of poly(epsilon-caprolactone) and poly(ethylene glycol) for doxorubicin delivery. J. Control. Release 2004, 98, 415–426. [Google Scholar] [CrossRef]
- Zheng, C; Qiu, L; Yao, X.; Zhu, K. Novel micelles from graft polyphosphazenes as potential anti-cancer drug delivery systems: Drug encapsulation and in vitro evaluation. Int. J. Pharm. 2009, 373, 133–140. [Google Scholar]
- Zhao, D.; Liu, C.J.; Zhuo, R.X.; Cheng, S.X. Alginate/CaCO3 hybrid nanoparticles for effcient co-delivery of antitumor gene and drug. Mol. Pharm. 2012, 9, 2887–2893. [Google Scholar]
- Selim, M.E.; Hendi, A.A. Gold nanoparticles induce apoptosis in MCF-7 human breast cancer cells. Asian Pac. J. Cancer Prev. 2012, 13, 1617–1620. [Google Scholar]
- Lanvin, O.; Gouilleux, F.; Mullié, C.; Fuentes, V.; Bissac, E.; Dantin, F.; Mazière, J.C.; Régnier, A.; Lassoued, K.; Gouilleux-Gruart, V. Interleukin-7 induces apoptosis of 697 pre-B cells expressing dominant negative forms of STAT5 evidence for caspase-dependent and-independent mechanisms. Oncogene 2004, 23, 3040–3047. [Google Scholar]
- Youle, R.J.; Strasser, A. The BCL-2 protein family: Opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol. 2008, 9, 47–59. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kamba, S.A.; Ismail, M.; Hussein-Al-Ali, S.H.; Ibrahim, T.A.T.; Zakaria, Z.A.B. In Vitro Delivery and Controlled Release of Doxorubicin for Targeting Osteosarcoma Bone Cancer. Molecules 2013, 18, 10580-10598. https://doi.org/10.3390/molecules180910580
Kamba SA, Ismail M, Hussein-Al-Ali SH, Ibrahim TAT, Zakaria ZAB. In Vitro Delivery and Controlled Release of Doxorubicin for Targeting Osteosarcoma Bone Cancer. Molecules. 2013; 18(9):10580-10598. https://doi.org/10.3390/molecules180910580
Chicago/Turabian StyleKamba, Shafiu Abdullahi, Maznah Ismail, Samer Hasan Hussein-Al-Ali, Tengku Azmi Tengku Ibrahim, and Zuki Abu Bakar Zakaria. 2013. "In Vitro Delivery and Controlled Release of Doxorubicin for Targeting Osteosarcoma Bone Cancer" Molecules 18, no. 9: 10580-10598. https://doi.org/10.3390/molecules180910580
APA StyleKamba, S. A., Ismail, M., Hussein-Al-Ali, S. H., Ibrahim, T. A. T., & Zakaria, Z. A. B. (2013). In Vitro Delivery and Controlled Release of Doxorubicin for Targeting Osteosarcoma Bone Cancer. Molecules, 18(9), 10580-10598. https://doi.org/10.3390/molecules180910580




