Synthesis and NMR-Study of 1-Trimethylsilyl Substituted Silole Anion [Ph4C4Si(SiMe3)]−•[Li]+ and 3-Silolenide 2,5-carbodianions {[Ph4C4Si(n-Bu)2]−2•2[Li]+, [Ph4C4Si(t-Bu)2]−2•2[Li]+} via Silole Dianion [Ph4C4Si]−2•2[Li]+
Abstract
:1. Introduction
2. Results and Discussion
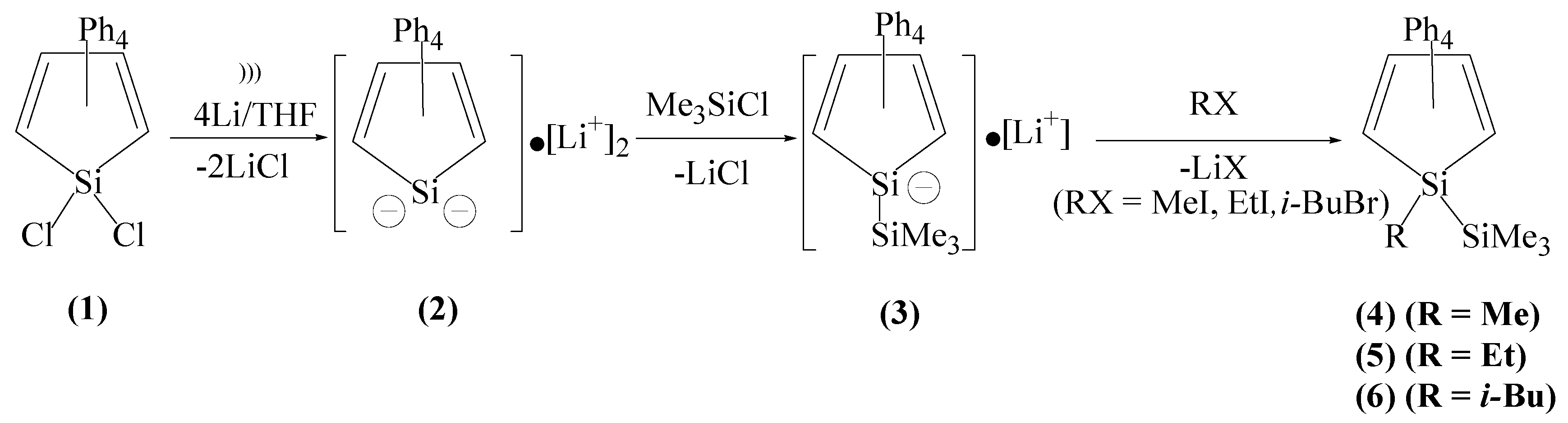
2.1. Preparation of 1-Trimethylsilyl,1-lithio-2,3,4,5-tetraphenyl-1-silacyclopentadienide Anion (3) and Its Reaction with Methyl Iodide, Ethyl Iodide and i-Butyl Bromide
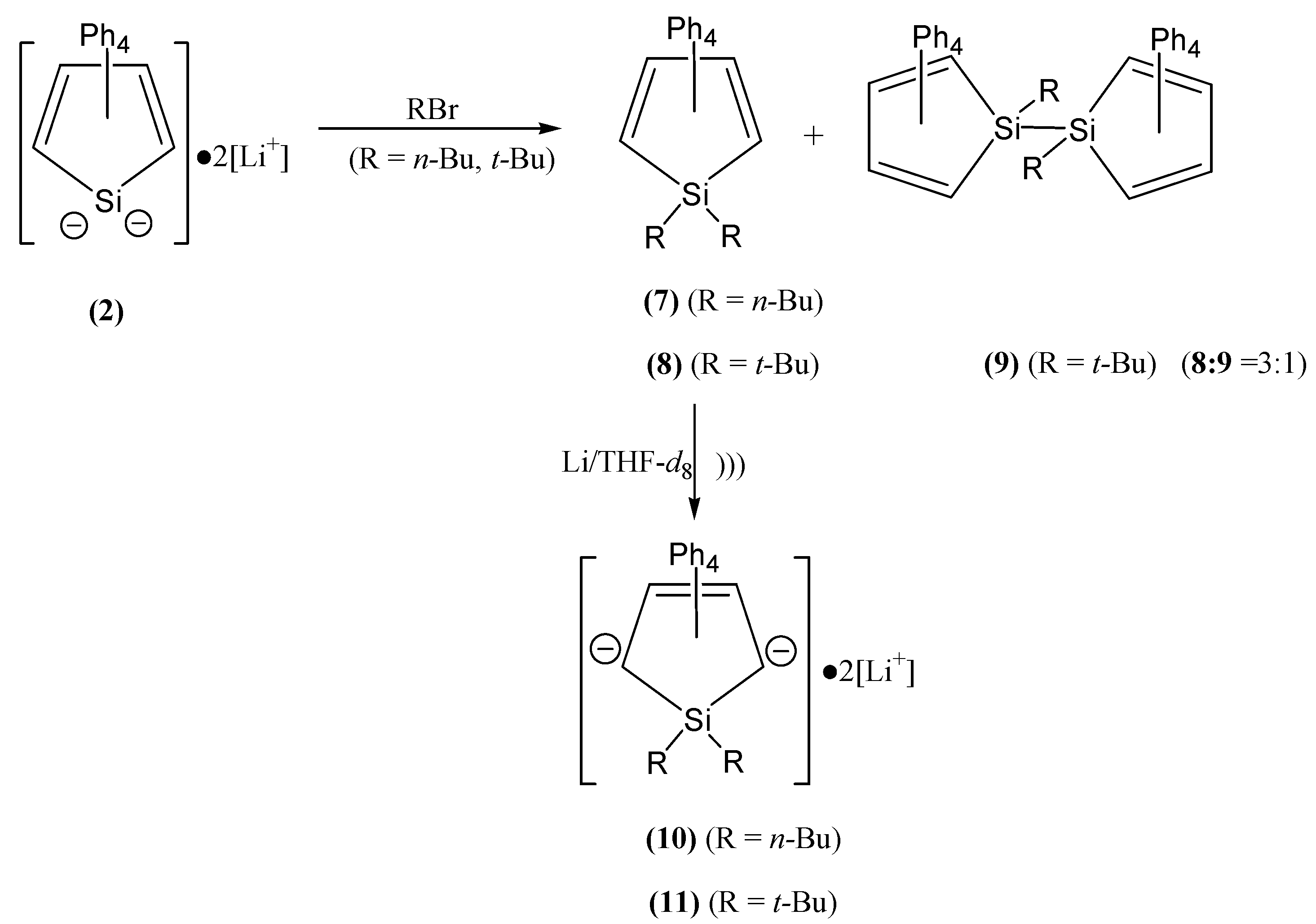
2.2. Synthesis of 1,1-Bis(n-butyl/t-butyl)-2,3,4,5-tetraphenyl-1-silacyclopentadiene and NMR-Study of 3-Silolenide-2,5-carbodianions
2.3. NMR Study of 1-Trimethylsilyl,1-lithio-2,3,4,5-tetraphenyl-1-silacyclopentadienide Anion (3)
| 3-Silenes 2,5-carbanion | [Ph4C4SiMe2]−2•2[Li]+ | [Ph4C4SiMeH]−2•2[Li]+ | [Ph4C4Si( n-Bu)2]−2•2[Li]+ (10) | [Ph4C4Si( t-Bu)2]−2 •2[Li]+ (11) | [Me4C4GePh]−•[Li]+ |
|---|---|---|---|---|---|
| Cα | 77.4 | 76.42 | 73.18 | 78.12 | 138.7 |
| Cβ | 128.5 | 128.82 | 128.06 | 130.34 | 151.5 |
| Sum (Cα + Cβ) | 205.9 | 205.24 | 201.24 | 208.46 | 290.2 |
| Sum (Cβ − Cα) | 51.1 | 52.40 | 73.18 | 78.12 | 12.8 |
| Ph | Ph | Ph | Ph | Ph | |
| Ci | 150.6, 147.3 | 150.33, 147.82 | 151.51, 147.68 | 152.59, 147.36 | 159.6 |
| Co | 123.3, 125.8 | 132.47, 126.62 | 132.88, 126.61 | 132.75, 127.75 | 136.4 |
| Cm | 126.5, 132.4 | 123.05, 125.98 | 123.58, 126.61 | 125.77, 125.93 | 127.3 |
| Cp | 107.8, 120.5 | 107.65, 120.53 | 108.49, 120.80 | 110.87, 120.25 | 124.3 |
| Sum (Ci − Cp)/2 | 69.6/2 = 34.8 | 69.97/2=35.00 | 69.90/2 = 34.95 | 68.83/2 = 34.42 | 35.3a |
| 29Si-Ring | − | -34.14 | -0.27 | 13.69 | − |
| CH3, tert-C | − | 2.58 | 14.70, 18.88, 27.34, 29.08 | 31.5, 33.3 (brd d) | − |
| Reference | 72 b | 73 b | This Work b | This Work b | 75 b |
| [Ph4C4Si]−2•2[Li]+ (2) | [Ph4C4Ge]−2•2[Li]+ | [Ph4C4Si( t-Bu)]−•[Li]+ | [Ph4C4SiSiMe3]−•[Li]+ (3) | |
|---|---|---|---|---|
| Ring carbons | 151.22, 129.71 a | 165.57, 129.92 a | 155.76, 139.51 | 159.67, 139.30 |
| Ph | Ph | Ph | Ph | |
| Ci | 151.67, 145.83 | 152.17, 146.30 | 149.29, 144.72 | 148.81, 145.72 |
| Co | 129.97, 133.43 | 129.92, 133.49 | 130.50, 132.56 | 129.81, 132.85 |
| Cm | 126.38, 126.38 | 126.38, 126.38 | 126.40, 126.51 | 126.30, 126.46 |
| Cp | 119.48, 121.83 | 119.29, 121.91 | 121.38, 123.34 | 120.86, 122.86 |
| Sum(Ci − Cp)/2 | 56.19/2 = 28.10 | 57.27/2 = 28.64 | 49.29/2 = 24.65 | 40.81/2 = 20.41 |
| CH3, tert-C | − | − | 32.78(CH3), 23.58(tert-C) | -0.23 [Si(CH3)3] |
| 29Si-Ring | 68.54 | − | 25.10 | −13.22 |
| Refenence | 17 b | 18 b | 38 b | This Work b |
| Silole Anion | [Me4C4SiSiMe3]−•[M]+ | [Et4C4SiSiMe3]−• [M]+ | [Et4C4Si]−2•2[M]+ | [Ph4C4SiSiMe3]−•[M]+ (3) | ||||
|---|---|---|---|---|---|---|---|---|
| M | Li | K | Li | K | Li | Li | ||
| 29Si-Ring | −45.38 | −43.96 | −42.70 | −41.52 | −53.12 c | −47.38 | 24.96 | −13.22 |
| 29Si-Ring with crown ether | − | 12-CE-4 | − | 18-CE-6 | 12-CE-4 | − | − | − |
| 29Si-SiMe3 | −12.47 | −11.68 | −12.44 | −11.00 | −14.27 | −14.22 | − | −15.54 |
| Reference | 22 a | 22 b | 22 a | 22 b | 22 c | 22 c | 51 c | This work c |
3. Experimental
General Procedures
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Elschenbroich, C.; Salzer, A. Oranometallics-A Concise Introduction, 2nd ed.; VCH: Weinheim, Germany, 1992; pp. 315–343. [Google Scholar]
- Harder, S. Recent developments in cyclopentadienyl-alkalimetal chemistry. Cord. Chem. Rev. 1998, 176, 17–66. [Google Scholar] [CrossRef]
- Tongni, A.; Haterman, R. Metallocenes. Synthesis, Reactivity, Applications; VCH: Weinheim, Germany, 1998. [Google Scholar]
- Barton, T.J. Carbacyclic Silanes. In Comprehensive Organometallic Chemistry; Wilkinson, G., Stone, F.G.A., Abel, E.W., Eds.; Pergamon Press: Oxford, UK, 1982; pp. 205, 250–261. [Google Scholar]
- Dubac, J.; Laporterie, A.; Manuel, G. Group 14 metalloles. 1. Synthesis, organic chemistry, and physicochemical data. Chem. Rev. 1990, 90, 215–263. [Google Scholar] [CrossRef]
- Colomer, E.; Corriu, R.J.P.; Lheureux, M. Group 14 metalloles. 2. Ionic species and coordination compounds. Chem. Rev. 1990, 90, 265–282. [Google Scholar] [CrossRef]
- Dubac, J.; Guerin, C.; Meunier, P. The Chemistry of Organosilicon Compounds; Patai, S., Rappoport, Z., Eds.; Willey-Interscience: Chichester, UK, 1998; Volume 2, pp. 1009–1010. [Google Scholar]
- Gordon, M.S.; Boudjouk, P.; Anwari, F. Are the silacyclopentadienyl anion and the silacyclopropenyl cation aromatic? J. Am. Chem. Soc. 1983, 105, 4972–4976. [Google Scholar] [CrossRef]
- Damewood, J.R. Pyramidal inversion and electron delocalization in the silacyclopentadienyl anion. J. Org. Chem. 1986, 51, 5028–5029. [Google Scholar] [CrossRef]
- Goldfuss, B.; Schleyer, P.R. The Siloyl anion C4H4SiH− is aromatic and the lithium silolide C4H4SiHLi even more so. Organometallics 1995, 14, 1553–1555. [Google Scholar] [CrossRef]
- Goldfuss, B.; Schleyer, P.R.; Hampel, F. Aromaticity in Silole Dianions: Structural, energetic, and magnetic aspects. Organometallics 1996, 15, 1755–1757. [Google Scholar] [CrossRef]
- Schleyer, P.R.; Maerker, C.; Dransfeld, A.; Jiao, H.; Hommes, N.J.R.V.E. Nucleus-independentchemical shifts: A simple and efficient aromaticity probe. J. Am. Chem. Soc. 1996, 118, 6317–6318. [Google Scholar]
- Goldfuss, B.; Schleyer, P.R. Aromaticity in group 14 metalloles: Structural, energetic, and magnetic criteria. Organometallics 1997, 16, 1543–1552. [Google Scholar] [CrossRef]
- Schleyer, P.R.; Manoharan, M.; Jiao, H.; Stahl, F. The Acenes: Is there a relationship between aromatic stabilization and reactivity? Org. Lett. 2001, 3, 3643–3646. [Google Scholar] [CrossRef]
- Wrackmeyer, B. Applications of 29Si NMR parameters. Annu. Rep. NMR Spectrosc. 2006, 57, 1–49. [Google Scholar] [CrossRef]
- Joo, W.-C.; Hong, J.-H.; Choi, S.-B.; Son, H.-E. Synthesis and reactivity of 1,1-disodio-2,3,4,5-tetraphenyl-1-silacyclopentadiene. J. Organomet. Chem. 1990, 391, 27–36. [Google Scholar] [CrossRef]
- Hong, J.-H.; Boudjouk, P.; Castellino, S. Synthesis and characterization of two aromatic siliconcontaining dianions: The 2,3,4,5-tetraphenylsilole dianion and the 1,1'-disila-2,2',3,3',4,4',5,5'-octaphenylfulvalene dianion. Organometallics 1994, 13, 3387–3389. [Google Scholar] [CrossRef]
- Hong, J.-H.; Boudjouk, P. Synthesis and characterization of a delocalized germanium-containing dianion: Dilithio-2,3,4,5-tetraphenyl-germole. Bull. Soc. Chim. Fr. 1995, 132, 495–498. [Google Scholar]
- West, R.; Sohn, H.; Bankwitz, U.; Calabrese, J.; Apeloig, T.; Mueller, T. Dilithium derivative of tetraphenylsilole: An η1-η5 dilithium structure. J. Am. Chem. Soc. 1995, 117, 11608–11609. [Google Scholar] [CrossRef]
- West, R.; Sohn, H.; Powell, D.R.; Mueller, T.; Apeloig, Y. Dianion of tetraphenylgermole is aromatic. Angew. Chem. Int. Ed. 1996, 35, 1002–1004. [Google Scholar] [CrossRef]
- Hong, J. H.; Pan, Y.; Boudjouk, P. A Novel Lithocenophane Derivative of a Trisgermole Dianion: [Li(thf)(tmeda)][2,3,4,5-Et4-Ge,Ge-{Li(2,3,4,5-Et4C4Ge)2}C4Ge]. Angew. Chem. Int. Ed. 1996, 35, 186–188. [Google Scholar] [CrossRef]
- Freeman, W.P.; Tilley, T.D.; Yap, G.P.A.; Rheingold, A.L. Siloyl anions and silole dianions: Structure of [K(18-crown-6)+]2[C4Me4Si2−]. Angew. Chem. Int. Ed. 1996, 35, 882–884. [Google Scholar] [CrossRef]
- Freeman, W.P.; Tilley, T.D.; Liable-Sands, L.M.; Rheingold, A.L. Synthesis and study of cyclic π-systems containing silicon and germanium. The question of aromaticity in cyclopentaidenyl analogues. J. Am. Chem. Soc. 1996, 118, 10457–10468. [Google Scholar] [CrossRef]
- Dysard, J.M.; Tilley, T.D. η5-Silolyl and η5-Germoyl complexes of d0 hafnium. Structural characterization of an η5-Silolyl complex. J. Am. Chem. Soc. 1998, 120, 8245–8246. [Google Scholar] [CrossRef]
- Choi, S.-B.; Boudjouk, P.; Hong, J.-H. Unique Bis-η5/η1bonding in a dianionic germole. synthesis and structural characterization of the dilithium salt of the 2,3,4,5-tetraethyl germole dianion. J. Am. Chem. Soc. 1999, 18, 2919–2921. [Google Scholar]
- Dysard, J.M.; Tilley, T.D. Hafnium-rhodium and hafnium-iridium heterobimetallic complexes featuring the bridging germole dianion ligand [GeC4Me4]2−. Organometallics 2000, 19, 2671–2675. [Google Scholar] [CrossRef]
- Dysard, J.M.; Tilley, T.D. Synthesis and reactivity of η5-Silolyl, η5-Germoyl, and η5-Germoyl dianion complexes of zirconium and hafnium. J. Am. Chem. Soc. 2000, 122, 3097–3105. [Google Scholar] [CrossRef]
- Saito, M.; Haga, M.; Yoshioka, M. Formation of the first monoanion and dianion of stannole. Chem. Commun. 2002, 1002–1003. [Google Scholar] [CrossRef]
- Saito, M.; Haga, M.; Yoshioka, M.; Ishimura, K.; Nagase, S. The Aromaticity of the stannole dianion. Angew. Chem. Int. Ed. 2005, 44, 6553–6556. [Google Scholar] [CrossRef]
- Saito, M.; Kuwabara, T.; Kambayashi, C.; Yoshioka, M.; Ishimura, K.; Nagase, S. Synthesis, structure, and reaction of tetraethyldilithiostannole. Chem. Lett. 2010, 39, 700–701. [Google Scholar] [CrossRef]
- Saito, M.; Sakaguchi, M.; Tajima, T.; Ishimura, K.; Nagase, S.; Hada, M. Dilithioplumbole: A lead-bearing aromatic cyclopentadienyl analog. Science 2010, 328, 339–342. [Google Scholar] [CrossRef]
- Lee, V.Y.; Sekiguchi, A.; Ichinohe, M.; Fukaya, N. Stable aromatic compounds containing heavier group 14 elements. J. Organomet. Chem. 2000, 611, 228–235. [Google Scholar] [CrossRef]
- Hissler, M.; Dyer, P.W.; Reau, R. Linear organic π-conjugated systems featuring the heavy group 14 and 15 elements. Coord. Chem. Rev. 2003, 244, 1–44. [Google Scholar] [CrossRef]
- Grützmacher, H. Five-membered aromatic and antianromatic rings with gallium, germanium, and bismuth centers: A short march through the periodic table. Angw. Chem. Int. Ed. 2005, 34, 295–298. [Google Scholar]
- Saito, M.; Yoshioka, M. The anions and dianions of group 14 metalloles. Coord. Chem. Rev. 2005, 249, 765–780. [Google Scholar] [CrossRef]
- Lee, V.Y.; Sekiguchi, A. Aromaticity of group 14 organometallics: Experimental aspects. Angw. Chem. Int. Ed. 2007, 46, 6596–6620. [Google Scholar] [CrossRef]
- Saito, M. Challenge to expand the concept of aromaticity to tin- and lead-containing carbocyclic compounds: Synthesis, structures and reactions of dilithiostannoles and dilithioplumbole. Coord. Chem. Rev. 2012, 256, 627–636. [Google Scholar] [CrossRef]
- Hong, J.-H.; Boudjouk, P. A stable aromatic species containing silicon. Synthesis and characterization of the 1-tert-Butyl-2,3,4,5-tetraphenyl-1-silacyclopentadienide anion. J. Am. Chem. Soc. 1993, 115, 5883–5884. [Google Scholar] [CrossRef]
- Sohn, H.; Powel, R.; West, R.; Hong, J.-H.; Joo, W.-C. Dimerization of the silole anion [C4Ph4SiMe]− to a tricyclic diallylic dianion. Organometallics 1997, 16, 2770–2772. [Google Scholar] [CrossRef]
- Hong, J.-H. Dissociation of the disilatricyclic diallylic dianion [(C4Ph4SiMe)2]−2 to the silole anion [MeSiC4Ph4]− by halide ion coordination or halide ion nucleophilic substitution at the silicon atom. Molecules 2011, 16, 8451–8462. [Google Scholar] [CrossRef]
- Lee, V.Y.; Kato, R.; Ichinohe, M.; Sekiguchi, A. The heavy analogue of CpLi: Lithium 1,2-disila-3-germacyclopentadienide, a 6π-electron aromatic system. J. Am. Chem. Soc. 2005, 127, 13142–13143. [Google Scholar] [CrossRef]
- Yasuda, H.; Lee, V.Y.; Sekiguchi, A. Si3C2-Rings: From a nonconjugated trisilacyclopentadiene to an aromatic trisilacyclopentadienide and cyclic disilenide. J. Am. Chem. Soc. 2009, 131, 6352–6353. [Google Scholar] [CrossRef]
- Wang, W.; Yao, S.; Wüllen, C.V.; Driess, M. A cyclopentadienide analogues containing divalent germanium and a heavy cyclobutadiene-like dianion with an unusual Ge4 core. J. Am. Chem. Soc. 2008, 130, 9640–9641. [Google Scholar] [CrossRef]
- Lee, V.Y.; Kato, R.; Sekiguchi, A.; Krapp, A.; Frenking, G. Heavy ferrocene: A sandwich complex containing Si and Ge atoms. J. Am. Chem. Soc. 2007, 129, 10340–10341. [Google Scholar] [CrossRef]
- Yasuda, H.; Lee, V.Y.; Sekiguchi, A. η5-1,2,3-Trisilacyclopentadienyl—A ligand for transition metal complexes: Rhodium half-sandwich and ruthenium sandwich. J. Am. Chem. Soc. 2009, 131, 9902–9903. [Google Scholar] [CrossRef]
- Freeman, W.P.; Tilley, T.D. Stable Silacyclopentadienyl complexes of ruthenium: (η5-C5Me5)Ru[η5-C5Me5SiSi(SiMe3)3] and X-ray structure of its protonated form. J. Am. Chem. Soc. 1994, 116, 8428–8429. [Google Scholar] [CrossRef]
- Freeman, W.P.; Dysard, J.M.; Tilley, T.D.; Rheingold, A.L. Synthesis and reactivity of η5-germacyclopentadienyl complexes of iron. Organometallics 2002, 21, 1734–1738. [Google Scholar] [CrossRef]
- Saito, M.; kuwabara, T.; Ishimura, K.; Nagase, S. Synthesis and structures of lithium salts of stannole anions. Bull. Chem. Soc. Jpn. 2010, 83, 825–827. [Google Scholar] [CrossRef]
- Braye, E.H.; Hübel, W.; Caplier, I. New unsaturated heterocyclic systems. I. J. Am. Chem. Soc. 1961, 83, 4406–4413. [Google Scholar] [CrossRef]
- Bankwitz, U.; Sohn, H.; Powell, D.R.; West, R. Synthesis, soilid-state structure, and reduction of 1,1-dichloro-2,3,4,5-tetramethylsilole. J. Organomet. Chem. 1995, 499, C7–C9. [Google Scholar] [CrossRef]
- Hong, J.H. Synthesis and NMR-study of the 2,3,4,5-tetraethylsilole dianion [SiC4Et4]2−•2[Li]+. Molecules 2011, 16, 8033–8040. [Google Scholar] [CrossRef]
- Tamao, K.; Yamaguchi, S.; Shiro, M. Oligosiloles: First synthesis based on a novel endo-endo modeintramolecular reductive cyclization of diethynylsilanes. J. Am. Chem. Soc. 1994, 116, 11715–11722. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Jin, R.-Z.; Tamao, K.; Shiro, M. Synthesis of a series of 1,1-difunctionalized siloles. Organometallics 1997, 16, 2230–2232. [Google Scholar] [CrossRef]
- Fagan, P.J.; Nugent, W.A.; Calabrese, J.C. Metallacycle transfer from zirconium to main group element: A versatile synthesis of heterocycles. J. Am. Chem. Soc. 1994, 116, 1880–1889. [Google Scholar] [CrossRef]
- Wrackmeyer, B. Metallacyclopentadienes and related heterocycles via 1,1-organoboration of alkyn-1-ylmetal compounds. Heteroat. Chem. 2006, 17, 188–208. [Google Scholar] [CrossRef]
- Wrackmeyer, B.; Tok, O.L. Comprehensive Heterocyclic Chemistry III; Katritzky, A.R., Ramsden, C.A., Scriven, E.F.V., Taylor, R.J.K., Eds.; Elsevier: Oxford, UK, 2008; Chapter 3.17; pp. 1181–1223. [Google Scholar]
- Khan, E.; Bayer, S.; Kempe, R.; Wrackmeyer, B. Synthesis and molecular structure of silole derivatives bearing functional groups on silicon: 1,1-organoboration of dialkynylsilanes. Eur. J. Inorg. Chem. 2009, 4416–4424. [Google Scholar]
- Dierker, G.; Ugolotti, J.; Kehr, G.; Fröhlich, R.; Erker, G. Reaction of bis(alkynyl)silanes with tris(pentafluorophenyl)borane: Synthesis of bulky silole derivatives by means of 1,1-carboboration under mild reaction conditions. Adv. Synth. Catal. 2009, 351, 1080–1088. [Google Scholar] [CrossRef]
- Ishigawa, M.; Tabohashi, T.; Sugisawa, H.; Nishimura, K.; Kumada, M. Chemistry of siloles. the reaction of siloles with organolithium reagents. J. Orgamomet. Chem. 1983, 205, 109–119. [Google Scholar]
- Ishigawa, M.; Tabohashi, T.; Ohashi, H.; Kumada, M.; Iyoda, J. Chemistry of siloles. 1-methyldibenzosilacyclopentadienide anion. Orgamometallics 1983, 2, 351–352. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Jin, R.-Z.; Tamao, K. Silicon-catenated silole oligomers: Oligo(1,1-silole)s. Organometallics 1997, 16, 2486–2488. [Google Scholar] [CrossRef]
- Sohn, H.; Merritt, J.; Powell, D.R.; West, R. A new spirocyclic system: Synthesis of a silaspirotropylidene. Organometallics 1997, 16, 5133–5134. [Google Scholar] [CrossRef]
- Sanji, T.; Sakai, T.; Kabuto, C.; Sakurai, H. Silole-incorporated polysilanes. J. Am. Chem. Soc. 1998, 120, 4552–4553. [Google Scholar] [CrossRef]
- Kanno, K.; Ichinohe, M.; Kabuto, C.; Kira, M. Synthesis and structure of a series of oligo [1,1-(2,3,4,5-tetramethylsilole)]s. Chem. Lett. 1998, 27, 99–100. [Google Scholar]
- Yamaguchi, S.; Jin, R.-Z.; Tamao, K. Silole polymer and cyclic hexamer catenating through the ring silicones. J. Am. Chem. Soc. 1999, 121, 2937–3938. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Jin, R.-Z.; Tamao, K. New type of polysilanes: Poly(1,1-silole)s. J. Organomet. Chem. 2000, 611, 5–11. [Google Scholar]
- Saito, M.; Haga, M.; Yoshioka, M. Synthesis of stannole anion by alkylation of stannole dianion. Chem. Lett. 2003, 32, 912–913. [Google Scholar] [CrossRef]
- Haga, R.; Saito, M.; Yoshioka, M. Synthesis and reactions of stannole anions. Eur. J. Inorg. Chem. 2007, 1297–1306. [Google Scholar] [CrossRef]
- Haga, R.; Saito, M.; Yoshioka, M. Reversible redox behavior between stannole dianion and bistannole-1,2-dianion. J. Am. Chem. Soc. 2006, 128, 4934–4935. [Google Scholar] [CrossRef]
- Haga, R.; Saito, M.; Yoshioka, M. Stepwise oxidation of the stannole dianion. Chem. Eur. J. 2008, 14, 4068–4073. [Google Scholar] [CrossRef]
- Schäfer, A.; Weidenbruch, M.; Pohl, S. Siliciumverbindungen mit starken intramolekularen sterischen wechselwirkungen: XIX. Simultane bildung und reaktionen von di-t-butylsilandiyl und tetra-t-butyldisilen. J. Organomet. Chem. 1985, 282, 305–313. [Google Scholar] [CrossRef]
- O’Brien, D.H.; Breeden, D.L. Tetraanion of 1,1-dimethyl-2,3,4,5-tetraphenyl-1-silacyclopentadiene. J. Am. Chem. Soc. 1981, 103, 3237–3239. [Google Scholar] [CrossRef]
- Wakahara, T.; Ando, W. Reaction of hydrosilanes with lithium. Formation of silole anions from 1-methylsilole via carbodianion. Chem. Lett. 1997, 11, 1179–1180. [Google Scholar] [CrossRef]
- Hong, J.-H.; Boudjouk, P. Synthesis and characterization of a novel pentavalent silane: 1-methyl-1,1-dihydrido-2,3,4,5-tetraphenyl-1-silacyclopentadiene silicate, [Ph4C4SiMeH2−][K+]. Organometallics 1995, 14, 574–576. [Google Scholar] [CrossRef]
- Dufour, P.; Dubac, J.; Dartiguenave, M.; Dartiguenave, Y. C-methylated (germacyclopentadienyl) lithium. Organometallics 1990, 9, 3001–3003. [Google Scholar] [CrossRef]
- Tandura, S.N.; Troitskii, N.A.; Kolesnikov, S.P.; Nosov, K.S.; Egorov, M.P. 13C NMR study of 2,3,4,5-tetraphenyl silole dilithium salt. Russ. Chem. Bull. 1999, 48, 214–217. [Google Scholar] [CrossRef]
- Tandura, S.N.; Kolesnikov, S.P.; Nosov, K.S.; Egorov, M.P.; Nefedov, O.M. Charge localization in the dianion of tetraphenylgermole according to 13C NMR data. Main Group Met. Chem. 1999, 22, 9–14. [Google Scholar]
- Jutzi, P.; Karl, A. Synthese und reaktionen einiger 1R,1R’-2,3,4,5-tetraphenyl-1-silacyclopentadiene. J. Organomet. Chem. 1981, 214, 289–302. [Google Scholar] [CrossRef]
- Sample Availability: Not Available.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hong, J.-H. Synthesis and NMR-Study of 1-Trimethylsilyl Substituted Silole Anion [Ph4C4Si(SiMe3)]−•[Li]+ and 3-Silolenide 2,5-carbodianions {[Ph4C4Si(n-Bu)2]−2•2[Li]+, [Ph4C4Si(t-Bu)2]−2•2[Li]+} via Silole Dianion [Ph4C4Si]−2•2[Li]+. Molecules 2013, 18, 10568-10579. https://doi.org/10.3390/molecules180910568
Hong J-H. Synthesis and NMR-Study of 1-Trimethylsilyl Substituted Silole Anion [Ph4C4Si(SiMe3)]−•[Li]+ and 3-Silolenide 2,5-carbodianions {[Ph4C4Si(n-Bu)2]−2•2[Li]+, [Ph4C4Si(t-Bu)2]−2•2[Li]+} via Silole Dianion [Ph4C4Si]−2•2[Li]+. Molecules. 2013; 18(9):10568-10579. https://doi.org/10.3390/molecules180910568
Chicago/Turabian StyleHong, Jang-Hwan. 2013. "Synthesis and NMR-Study of 1-Trimethylsilyl Substituted Silole Anion [Ph4C4Si(SiMe3)]−•[Li]+ and 3-Silolenide 2,5-carbodianions {[Ph4C4Si(n-Bu)2]−2•2[Li]+, [Ph4C4Si(t-Bu)2]−2•2[Li]+} via Silole Dianion [Ph4C4Si]−2•2[Li]+" Molecules 18, no. 9: 10568-10579. https://doi.org/10.3390/molecules180910568




