Synthesis and Quantitative Structure-Property Relationships of Side Chain-Modified Hyodeoxycholic Acid Derivatives
Abstract
:1. Introduction

| Entry | Compound | Estimated CMC (mM) | Determined CMC (mM) | CHI |
|---|---|---|---|---|
| 1 | CDCA | 8.0 | 7.0 | 0.87 |
| 2 | CA | 12.0 | 13.0 | 0.83 |
| 3 | HDCA (1) | 15.0 | 15.0 | 0.81 |
| 4 | 3 | 24.2 | 24.0 | 0.76 |
| 5 | 4 | 18.1 | 17.6 | 0.79 |
| 6 | 5 | 10.2 | - | 0.85 |
| 7 | 6 | 8.7 | 7.8 | 0.86 |
| 8 | 7 | 7.5 | - | 0.88 |
| 9 | 8 | 6.8 | - | 0.89 |
| 10 | 9 | 6.3 | 6.4 | 0.90 |
2. Results and Discussion
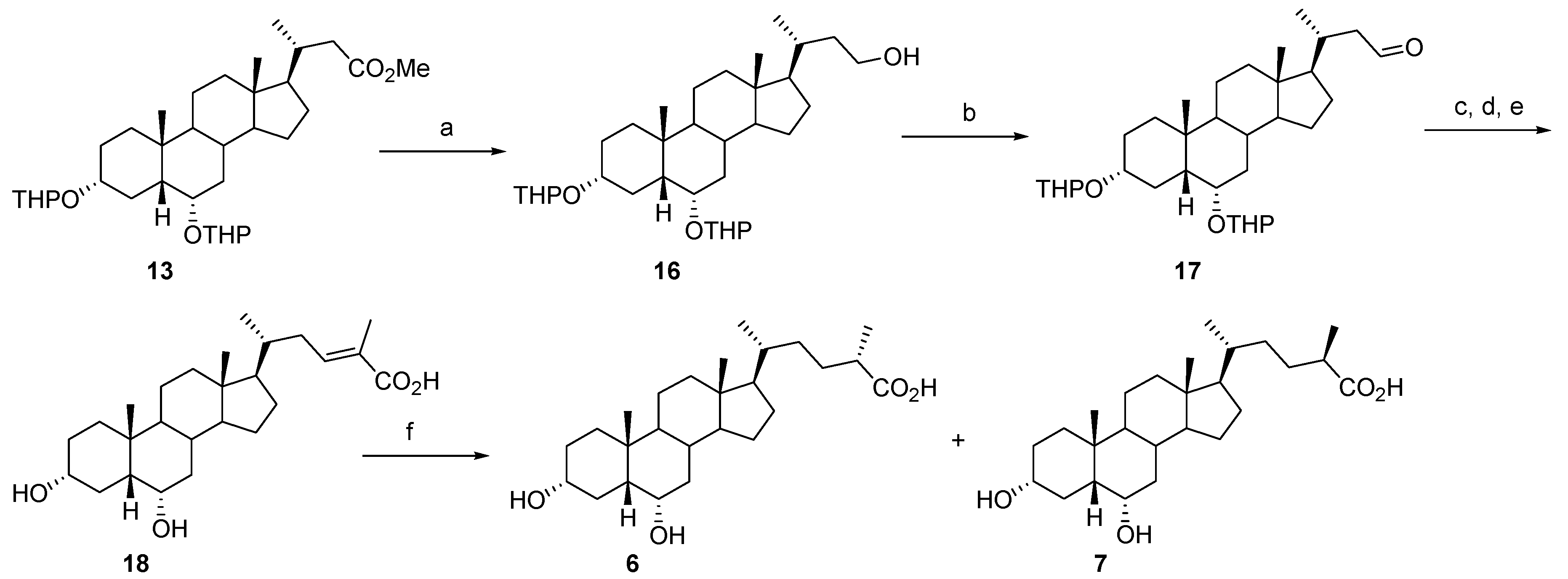
2.1. Synthesis

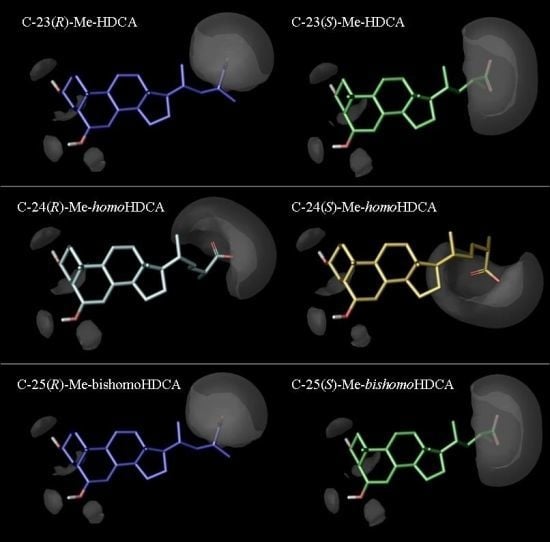
2.2. Chromatographic CMC Estimation
2.3. Discussion

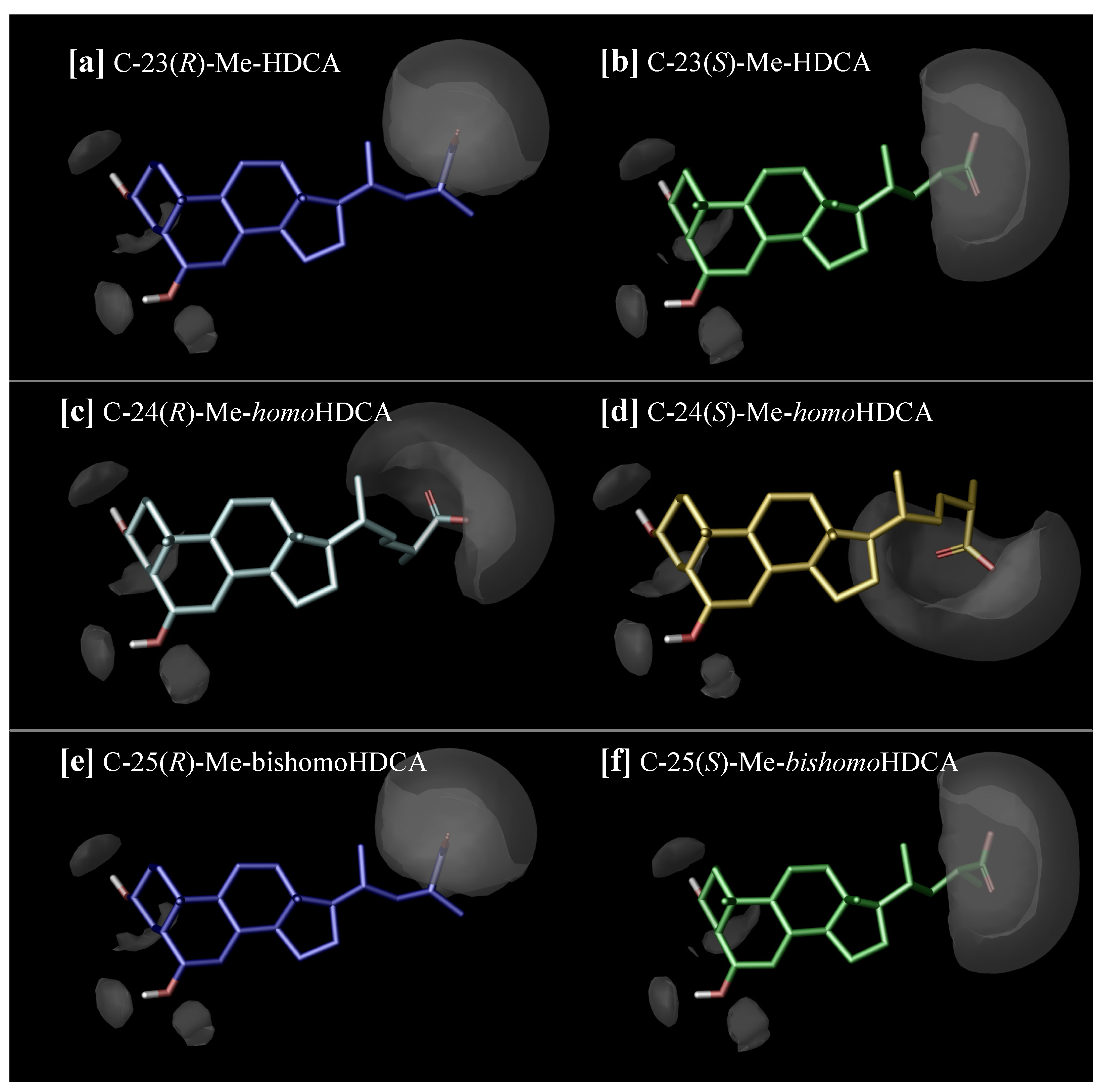
3. Experimental
3.1. General Methods
3.2. Synthesis of Compounds 3–7
3.3. CMC Determination
3.4. Molecular Modelling
4. Conclusions
Conflicts of Interest
References
- Li, T.; Chiang, J.Y.L. Nuclear receptors in bile acid metabolism. Drug Metab. Rev. 2013, 45, 145–155. [Google Scholar] [CrossRef]
- Lefebvre, P.; Cariou, B.; Lien, F.; Kuipers, F.; Staels, B. Role of bile acids and bile acid receptors in metabolic regulation. Physiol. Rev. 2009, 89, 147–191. [Google Scholar] [CrossRef]
- Thomas, C.; Pellicciari, R.; Pruzanski, M.; Auwerx, J.; Schoonjans, K. Targeting bile-acid signalling for metabolic diseases. Nat. Rev. Drug Discov. 2008, 7, 678–693. [Google Scholar] [CrossRef]
- Hoffman, A.F.; Hagey, L.R. Bile acids: Chemistry, Pathochemistry, Biology, Pathobiology, and Therapeutics. Cell. Mol. Life Sci. 2008, 65, 2461–2483. [Google Scholar] [CrossRef]
- Makishima, M.; Okamoto, A.Y.; Repa, J.J.; Tu, H.; Learned, R.M.; Luk, A.; Hull, M.V.; Lusting, K.D.; Mangelsdorf, D.J.; Shan, B. Identification of a nuclear receptor for bile acids. Science 1999, 284, 1362–1365. [Google Scholar] [CrossRef]
- Parks, D.J.; Blanchard, S.G.; Bledsoe, R.K.; Chandra, G.; Consler, T.G.; Kliewer, S.A.; Stimmel, J.B.; Willson, T.M.; Zavacki, A.M.; Moore, D.D.; et al. Bile acids: Natural ligands for an orphan nuclear receptor. Science 1999, 284, 1365–1368. [Google Scholar] [CrossRef]
- Wang, H.; Chen, J.; Hollister, K.; Sowers, L.C.; Forman, B.M. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol. Cell. 1999, 3, 543–553. [Google Scholar]
- Makishima, M.; Lu, T.T.; Xie, W.; Whitfield, G.K.; Domoto, H.; Evans, R.M.; Haussler, M.R.; Mangelsdorf, D.J. Vitamin D receptor as an intestinal bile acid sensor. Science 2002, 296, 14486–14494. [Google Scholar]
- Goodwin, B.; Gauthier, K.C.; Umetani, M.; Watson, M.A.; Lochansky, M.I.; Collins, J.L.; Leitersdorf, E.; Mangelsdorf, D.J.; Kliewer, S.A.; Repa, J.J. Identification of bile acid precursors as endogenous ligands for the nuclear xenobiotic pregnane X receptor. Proc. Natl. Acad. Sci. 2003, 100, 223–228. [Google Scholar] [CrossRef]
- Maruyama, T.; Miyamoto, Y.; Nakurama, T.; Tamai, Y.; Okada, H.; Sugiyama, E.; Itadani, H.; Tanaka, K. Identification of membrane-type receptor for bile acids (M-BAR). Biochem. Biophys. Res. Commun. 2002, 298, 714–719. [Google Scholar]
- Porez, G.; Prawitt, J.; Gross, B.; Staels, B. Bile acid receptors as targets for the treatment of dyslipidemia and cardiovascular disease. J. Lipid Res. 2012, 53, 1723–1737. [Google Scholar] [CrossRef]
- Pols, T.W.H.; Noriega, L.G.; Nomura, M.; Auwerx, J.; Schoonjans, K. The bile acid membrane receptor TGR5 as an emerging target in metabolism and inflammation. J. Hepatol. 2011, 54, 1263–1272. [Google Scholar] [CrossRef]
- Fiorucci, S.; Mencarelli, A.; Palladino, G.; Cipriani, S. Bile-acid-activated receptors: targeting TGR5 and farnesoid-X-receptor in lipid and glucose disorders. Trends Pharmacol. Sci. 2009, 30, 570–580. [Google Scholar] [CrossRef]
- Sharma, R.; Long, A.; Gilmer, J.F. Advances in bile acid medicinal chemistry. Curr. Med. Chem. 2011, 18, 4029–4052. [Google Scholar] [CrossRef]
- Pellicciari, R.; Gioiello, A.; Sabbatini, P.; Venturoni, F.; Nuti, R.; Colliva, C.; Rizzo, G.; Adorini, L.; Pruzanski, M.; Roda, R.; et al. Avicholic acid: a lead compound from birds on the route to potent TGR5 modulators. ACS Med. Chem. Lett. 2012, 3, 273–277. [Google Scholar] [CrossRef]
- Gioiello, A.; Macchiarulo, A.; Carotti, A.; Filipponi, P.; Costantino, G.; Rizzo, G.; Adorini, L.; Pellicciari, R. Extending SAR of bile acids as FXR ligands: Discovery of 23-N-(carbocinnamyloxy)-3α,7α-dihydroxy-6α-ethyl-24-nor-5β-cholan-23-amine. Bioorg. Med. Chem. 2011, 19, 2650–2658. [Google Scholar] [CrossRef]
- Iguchi, Y.; Kihira, K.; Nishimaki-Mogami, T.; Une, M. Structure-activity relationship of bile alcohols as human farnesoid X receptor agonist. Steroids 2010, 75, 95–100. [Google Scholar] [CrossRef]
- Pellicciari, R.; Gioiello, A.; Macchiarulo, A.; Thomas, C.; Rosatelli, E.; Natalini, B.; Sardella, R.; Pruzanski, M.; Roda, A.; Pastorini, E.; et al. Discovery of 6α-ethyl-23(S)-methylcholic acid (S-EMCA, INT-777) as a potent and selective agonist for the TGR5 receptor, a novel target for diabesity. J. Med. Chem. 2009, 52, 7958–7961. [Google Scholar] [CrossRef]
- Sato, H.; Macchiarulo, A.; Thomas, C.; Gioiello, A.; Une, M.; Hofmann, A.F.; Saladin, R.; Schoonjans, K.; Pellicciari, R.; Auwerx, J. Novel potent and selective bile acid derivatives as TGR5 agonists: Biological screening, Structure-activity relationships, and molecular modeling studies. J. Med. Chem. 2008, 51, 1831–1841. [Google Scholar] [CrossRef]
- Pellicciari, R.; Sato, H.; Gioiello, A.; Costantino, G.; Macchiarulo, A.; Sadeghpour, B.M.; Giorgi, G.; Schoonjans, K.; Auwerx, J. Nongenomic actions of bile acids. Synthesis and preliminary characterization of 23- and 6,23-alkyl-substituted bile acid derivatives as selective modulators for the G-protein coupled receptor TGR5. J. Med. Chem. 2007, 50, 4265–4268. [Google Scholar] [CrossRef]
- Pellicciari, R.; Gioiello, A.; Costantino, G.; Sadeghpour, B.M.; Rizzo, G.; Meyer, U.; Parks, D.J.; Entrena-Guadix, A.; Fiorucci, S. Back door modulation of the farnesoid X receptor: Design, Synthesis, and Biological evaluation of a series of side chain modified chenodeoxycholic acid derivatives. J. Med. Chem. 2006, 49, 4208–4215. [Google Scholar]
- Pellicciari, R.; Costantino, G.; Camaioni, E.; Sadeghpour, B.M.; Entrena, A.; Willson, T.M.; Fiorucci, S.; Clerici, C.; Gioiello, A. Bile acid derivatives as ligands of the farnesoid X receptor. Synthesis, Evaluation, and Structure-activity relationship of a series of body and side chain modified analogues of chenodeoxycholic acid. J. Med. Chem. 2004, 47, 4559–4569. [Google Scholar] [CrossRef]
- Fujino, T.; Une, M.; Imanaka, T.; Inoue, K.; Nishimaki-Mogami, T. Structure-activity relationship of bile acids and bile acid analogs in regard to FXR activation. J. Lipid Res. 2004, 45, 132–138. [Google Scholar]
- Hofmann, A.F.; Roda, A. Physicochemical properties of bile acids and their relationship to biological properties: an overview of the problem. J. Lipid Res. 1984, 25, 1477–1489. [Google Scholar]
- Gurantz, D.; Hofmann, A.F. Influence of bile acid structure on bile flow and biliary lipid secretion in the hamster. Am. J. Physiol-Gastrointest. L. 1984, 247, G736–G748. [Google Scholar]
- Carey, M.C.; Montet, J.C.; Phillips, M.C.; Armstrong, M.J.; Mazer, N.A. Thermodynamic and molecular basis for dissimilar cholesterol-solubilizing capacities by micellar solutions of bile salts: cases of sodium chenodeoxycholate and sodium ursodeoxycholate and their glycine and taurine conjugates. Biochemistry 1981, 20, 3637–3648. [Google Scholar] [CrossRef]
- Gioiello, A.; Sabbatini, P.; Rosatelli, E.; Macchiarulo, A.; Pellicciari, R. Divergent and stereoselective synthesis of dafachronic acids. Tetrahedron 2011, 67, 1924–1929. [Google Scholar] [CrossRef]
- Kim, D.; Han, G.H.; Kim, K. Stereoselective ester enolate alkylation and hydroxylation at C-22 of a steroid side chain. Tetrahedron Lett. 1989, 30, 1579–1580. [Google Scholar] [CrossRef]
- Carey, M.C.; Small, D.M. Micelle formation by bile salts. Physical-chemical and thermodynamic considerations. Arch. Intern. Med. 1972, 130, 506–527. [Google Scholar] [CrossRef]
- Calabresi, M.; Andreozzi, P.; La Mesa, C. Supra-molecular association and polymorphic behaviour in systems containing bile acid salts. Molecules 2007, 12, 1731–1754. [Google Scholar] [CrossRef]
- Roda, A.; Hoffman, A.F.; Mysels, K.J. The influence of bile salt structure on self-association in aqueous solutions. J. Biol. Chem. 1983, 258, 6362–6370. [Google Scholar]
- Matsuoka, K.; Moroi, Y. Micelle formation of sodium deoxycholate and sodium ursodeoxycholate (part 1). Biochim. Biophys. Acta 2002, 1580, 189–199. [Google Scholar] [CrossRef]
- Li, G.; McGrown, L.B. Model for bile salt micellization and solubilization from studies of a “polydisperse” array of fluorescent probes and molecular modelling. J. Phys. Chem. 1994, 98, 13711–13719. [Google Scholar]
- Coello, A.; Meijide, F.; Núñez, A.R.; Tato, J.V. Aggregation behaviour of bile salts in aqueous solution. J. Pharm. Sci. 1996, 85, 9–15. [Google Scholar] [CrossRef]
- Nagarajan, R. On interpreting fluorescence measurements: what does thermodynamics have to say about change in micellar aggregation number versus change in size distribution induced by increasing concentration of the surfactant in solution? Langmuir 1994, 10, 2028–2034. [Google Scholar] [CrossRef]
- Mazer, N.A.; Carey, M.C.; Kwasnick, R.K.; Benedek, G.B. Quasielastic light scattering studies of aqueous biliary lipid systems. Size, Shape, and Thermodynamics of bile salt micelles. Biochemistry 1979, 18, 3064–3075. [Google Scholar] [CrossRef]
- Reis, S.; Moutinho, C.G.; Matos, C.; de Castro, B.; Gameiro, P.; Lima, F.C. Noninvasive methods to determine the critical micelle concentration of some bile acid salts. Anal. Biochem. 2004, 334, 117–126. [Google Scholar] [CrossRef]
- Natalini, B.; Sardella, R.; Gioiello, A.; Rosatelli, E.; Ianni, F.; Camaioni, E.; Pellicciari, R. Fast chromatographic determination of the bile salt critical micellar concentration. Anal. Bioanal. Chem. 2011, 401, 267–274. [Google Scholar] [CrossRef]
- Gordon, G.S.; Moses, A.C.; Silver, R.D.; Flier, J.S.; Carey, M.C. Nasal absorption of insulin: enhancement by hydrophobic bile salts. Proc. Natl. Acad. Sci. 1985, 82, 7419–7423. [Google Scholar]
- Aldini, R.; Roda, A.; Montagnani, M.; Cerrè, C.; Pellicciari, R.; Roda, E. Relationship between structure and intestinal absorption of bile acids with a steroid or side-chain modification. Steroids 1996, 61, 590–597. [Google Scholar] [CrossRef]
- Yeh, H-Z.; Schteingart, C.D.; Hagey, L.R.; Ton-Nu, H.T.; Bolder, U.; Gavrilkina, M.A.; Steinbach, J.H.; Hofmann, A.F. Effect of side chain length on biotransformation, Hepatic transport, and Choleretic properties of chenodeoxycholyl homologues in the rodent: Studies with dinorchenodeoxycholic acid, Norchenodeoxycholic acid, and Chenodeoxycholic acid. Hepatology 1997, 26, 374–385. [Google Scholar] [CrossRef]
- Pellicciari, R.; Natalini, B.; Cecchetti, S.; Porter, B.; Roda, A.; Grigolo, B.; Balducci, R. Bile acids with a cyclopropyl-containing side chain. 3. Separation, Identification, and Properties of all four stereoisomers of 3α, 7β-dihydroxy-22,23-methylene-5β-cholan-24-oic acid. J. Med. Chem. 1988, 31, 730–736. [Google Scholar] [CrossRef]
- Dauben, W.G.; Gerdes, J.M.; Bunce, R.A. Organic reactions at high pressure. Preparation of Wittig phosphonium salts at ambient temperature. J. Org. Chem. 1984, 49, 4293–4295. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 1, 3-4, 6 and 10 are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sabbatini, P.; Filipponi, P.; Sardella, R.; Natalini, B.; Nuti, R.; Macchiarulo, A.; Pellicciari, R.; Gioiello, A. Synthesis and Quantitative Structure-Property Relationships of Side Chain-Modified Hyodeoxycholic Acid Derivatives. Molecules 2013, 18, 10497-10513. https://doi.org/10.3390/molecules180910497
Sabbatini P, Filipponi P, Sardella R, Natalini B, Nuti R, Macchiarulo A, Pellicciari R, Gioiello A. Synthesis and Quantitative Structure-Property Relationships of Side Chain-Modified Hyodeoxycholic Acid Derivatives. Molecules. 2013; 18(9):10497-10513. https://doi.org/10.3390/molecules180910497
Chicago/Turabian StyleSabbatini, Paola, Paolo Filipponi, Roccaldo Sardella, Benedetto Natalini, Roberto Nuti, Antonio Macchiarulo, Roberto Pellicciari, and Antimo Gioiello. 2013. "Synthesis and Quantitative Structure-Property Relationships of Side Chain-Modified Hyodeoxycholic Acid Derivatives" Molecules 18, no. 9: 10497-10513. https://doi.org/10.3390/molecules180910497