Salicortin-Derivatives from Salix pseudo-lasiogyne Twigs Inhibit Adipogenesis in 3T3-L1 Cells via Modulation of C/EBPα and SREBP1c Dependent Pathway
Abstract
:1. Introduction
2. Results and Discussion
2.1. Results
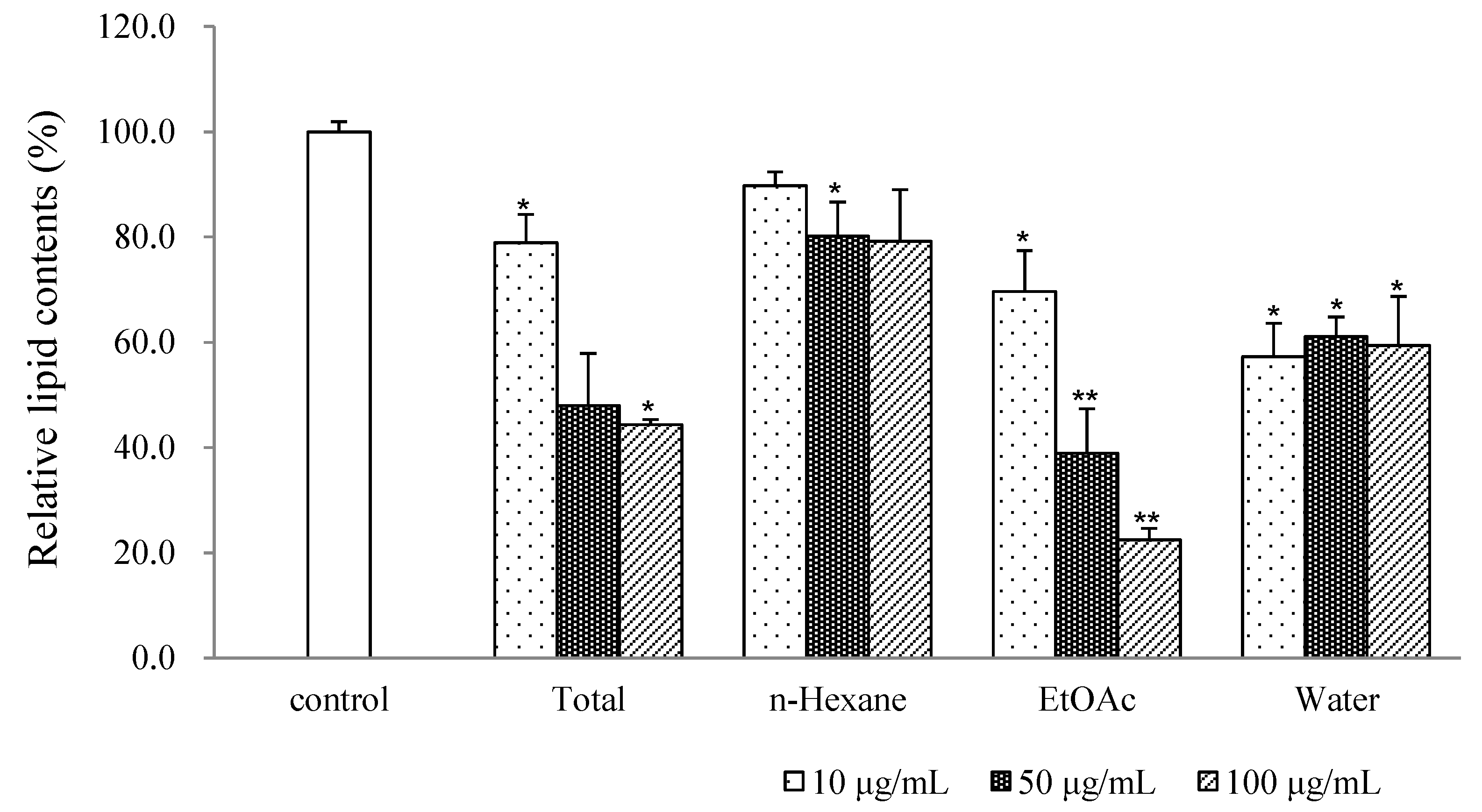
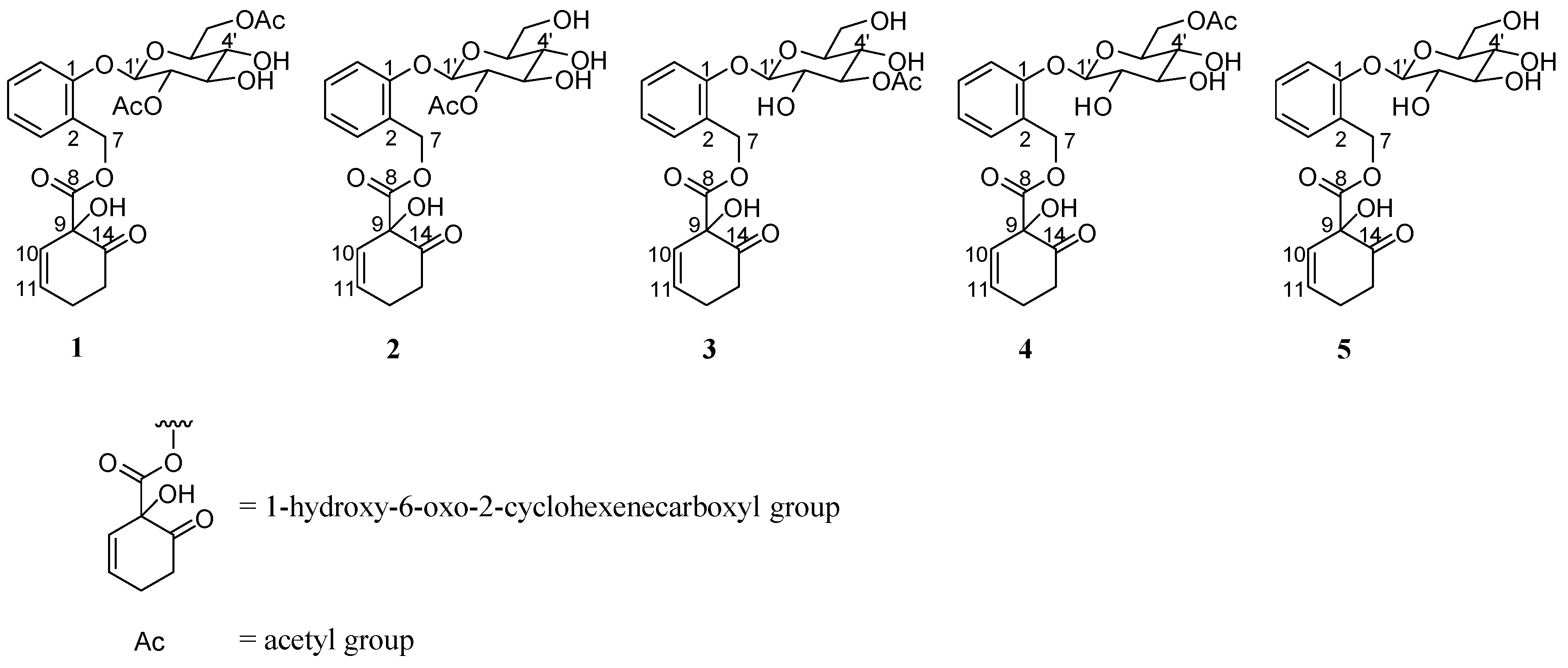
| Compound | IC50 (μM) |
|---|---|
| 1 | 11.6 |
| 2 | 24.6 |
| 3 | 25.0 |
| 4 | 27.1 |
| 5 | 53.5 |
| EGCG | 112.0 |
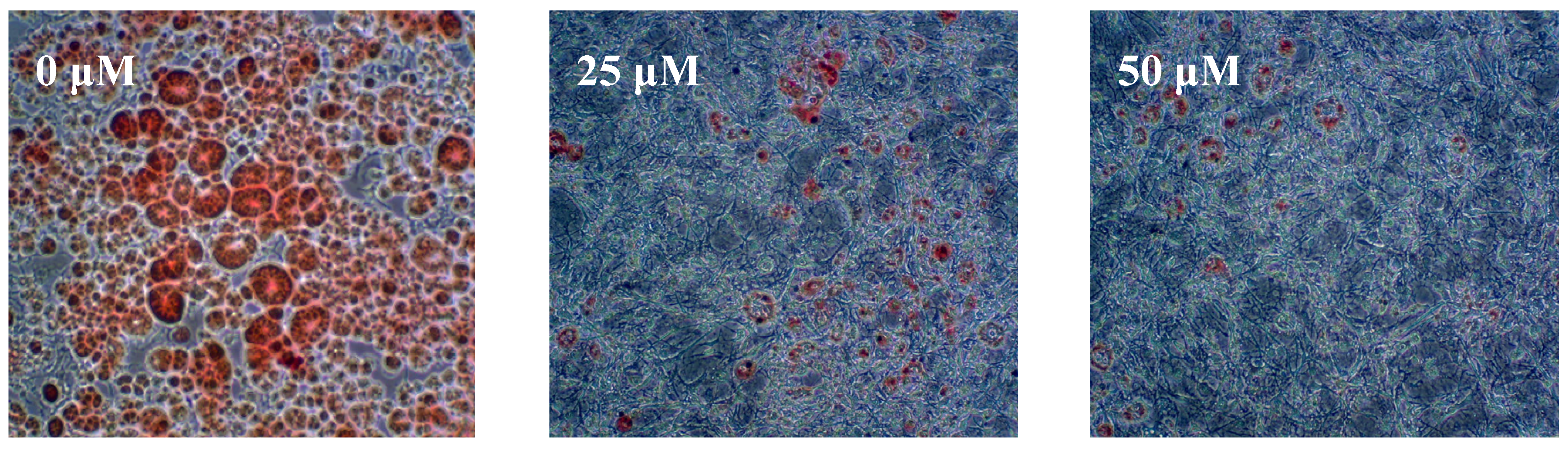
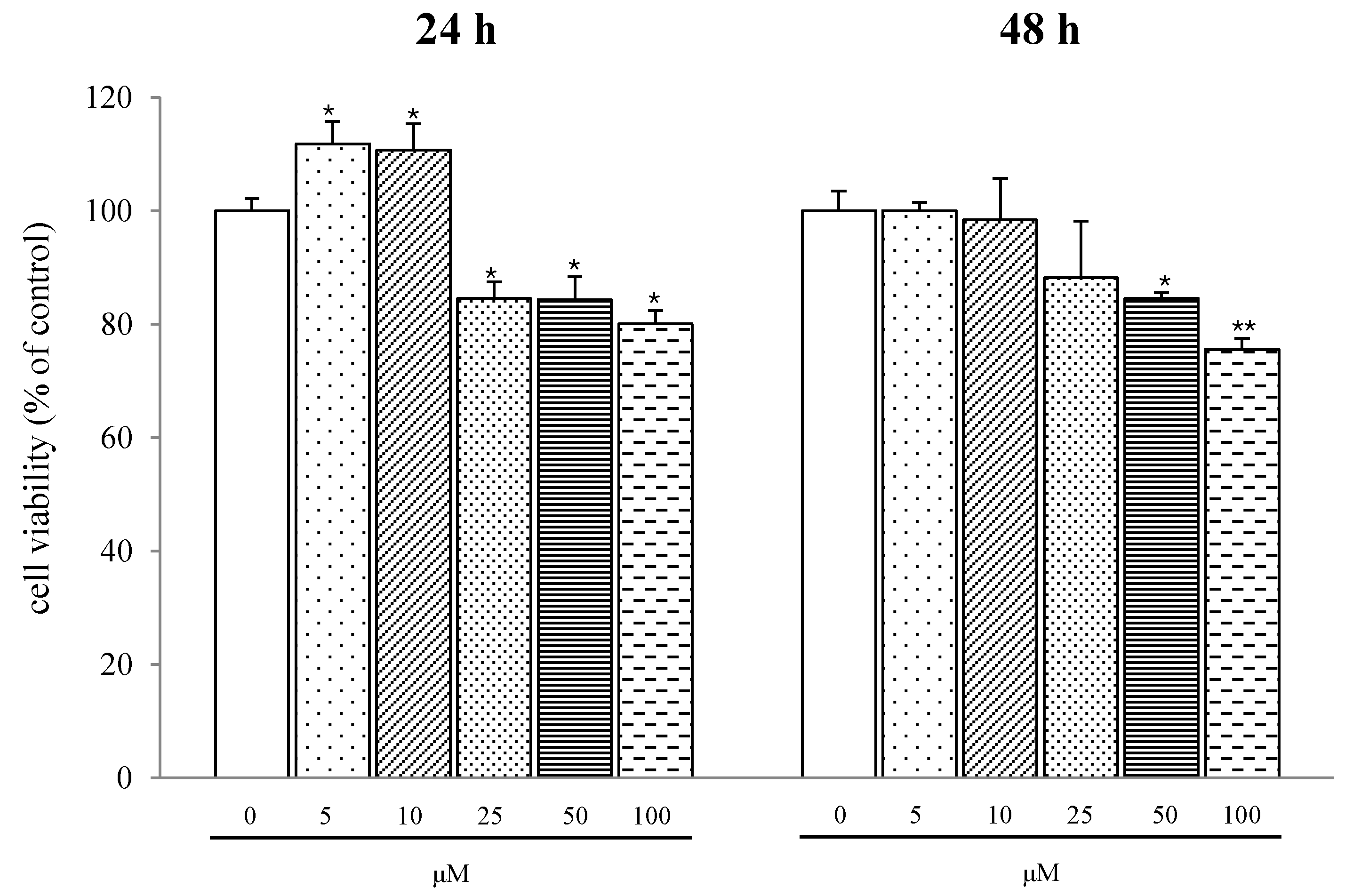

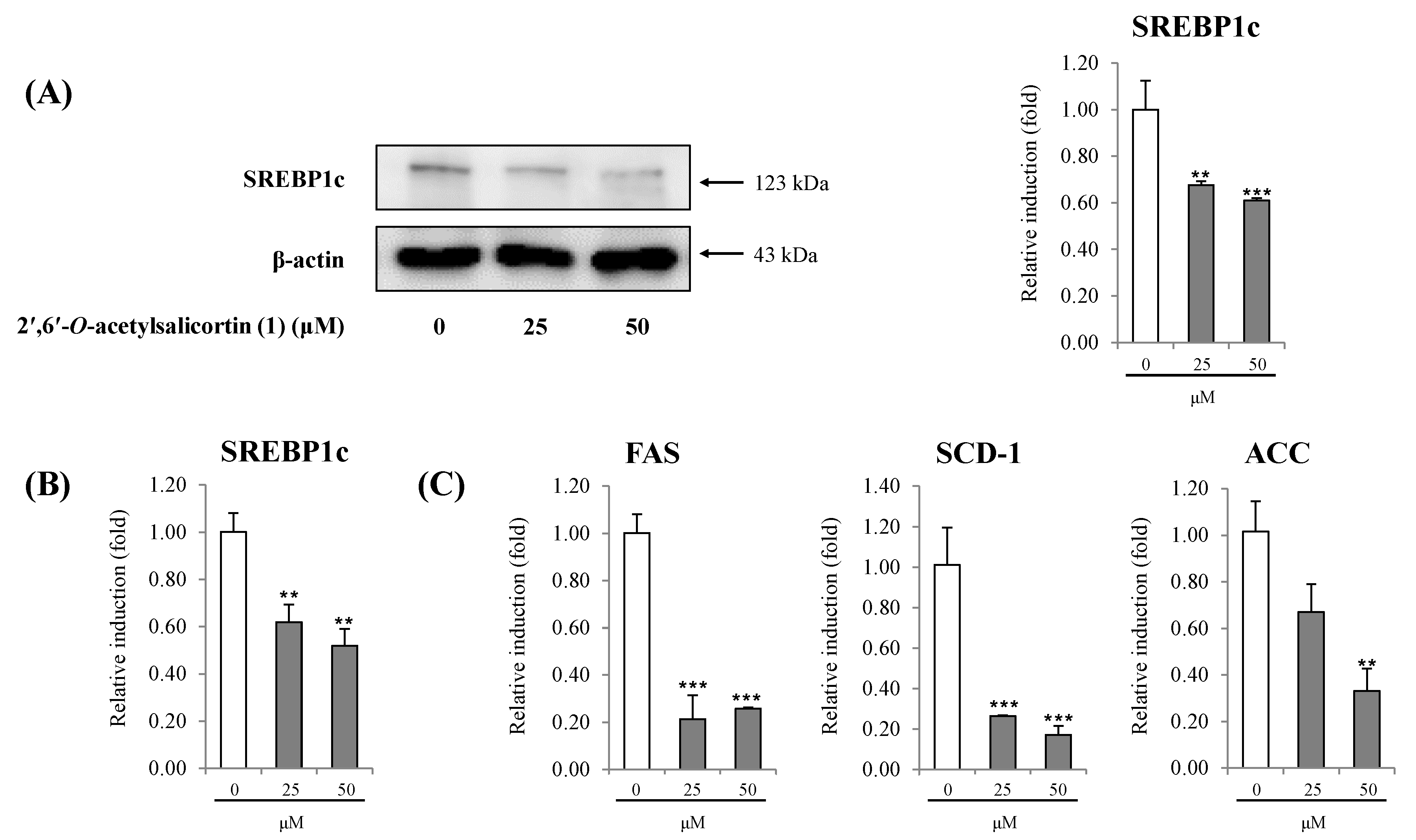
2.2. Discussion
3. Experimental
3.1. Plant Materials
3.2. Extraction and Isolation
3.3. Cell Culture
3.4. Oil Red O staining
3.5. Measurement of Cell Proliferation
3.6. Western Blot Analysis
3.7. Real-Time RT-PCR
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Abate, N. Obesity and cardiovascular disease. Pathogenetic role of the metabolic syndrome and therapeutic implications. J. Diabetes Complicat. 2000, 14, 154–174. [Google Scholar] [CrossRef]
- Kopelman, P.G. Obesity as a medical problem. Nature 2000, 404, 635–643. [Google Scholar]
- Wadden, T.A. Treatment of obesity by moderate and severe caloric restriction. Results of clinical research trials. Ann. Intern. Med. 1993, 119, 688–693. [Google Scholar] [CrossRef]
- Nathan, P.J.; O’Neill, B.V.; Napolitano, A.; Bullmore, E.T. Neuropsychiatric adverse effects of centrally acting antiobesity drugs. CNS Neurosci. Ther. 2011, 17, 490–505. [Google Scholar] [CrossRef]
- Yang, J.Y.; Della-Fera, M.A.; Rayalam, S.; Ambati, S.; Hartzell, D.L.; Park, H.J.; Baile, C.A. Enhanced inhibition of adipogenesis and induction of apoptosis in 3T3-L1 adipocytes with combinations of resveratrol and quercetin. Life Sci. 2008, 82, 1032–1039. [Google Scholar] [CrossRef]
- Rosen, E.D.; Spiegelman, B.M. Adipocytes as regulators of energy balance and glucose homeostasis. Nature 2006, 444, 847–853. [Google Scholar] [CrossRef]
- Rayalam, S.; Della-Fera, M.A.; Baile, C.A. Phytochemicals and regulation of the adipocyte life cycle. J. Nutr. Biochem. 2008, 19, 717–726. [Google Scholar] [CrossRef]
- Du, Q.; Jerz, G.; Shen, L.; Xiu, L.; Winterhalter, P. Isolation and structure determination of a lignan from the bark of Salix alba. Nat. Prod. Res. 2007, 21, 451–454. [Google Scholar] [CrossRef]
- Freischmidt, A.; Jürgenliemk, G.; Kraus, B.; Okpanyi, S.N.; Müller, J.; Kelber, O.; Weiser, D.; Heilmann, J. Contribution of flavonoids and catechol to the reduction of ICAM-1 expression in endothelial cells by a standardised Willow bark extract. Phytomedicine 2012, 19, 245–252. [Google Scholar] [CrossRef]
- Li, X.; Liu, Z.; Zhang, X.F.; Wang, L.J.; Zheng, Y.N.; Yuan, C.C.; Sun, G.Z. Isolation and characterization of phenolic compounds from the leaves of Salix matsudana. Molecules 2008, 13, 1530–1537. [Google Scholar] [CrossRef]
- Alam, M.S.; Kaur, G.; Jabbar, Z.; Javed, K.; Athar, M. Evaluation of antioxidant activity of Salix caprea flowers. Phytother. Res. 2006, 20, 479–483. [Google Scholar] [CrossRef]
- Sultana, S.; Saleem, M. Salix caprea inhibits skin carcinogenesis in murine skin: Inhibition of oxidative stress, Ornithine decarboxylase activity and DNA synthesis. J. Ethnopharmacol. 2004, 91, 267–276. [Google Scholar] [CrossRef]
- Han, L.K.; Sumiyoshi, M.; Zhang, J.; Liu, M.X.; Zhang, X.F.; Zheng, Y.N.; Okuda, H.; Kimura, Y. Anti-obesity action of Salix matsudana leaves (Part 1). Anti-obesity action by polyphenols of Salix matsudana in high fat-diet treated rodent animals. Phytother. Res. 2003, 17, 1188–1194. [Google Scholar] [CrossRef]
- Yang, H.; Lee, S.H.; Sung, S.H.; Kim, J.; Kim, Y.C. Neuroprotective compounds from Salix pseudo-lasiogyne twigs and their anti-amnesic effects on scopolamine-induced memory deficit in mice. Planta Med. 2013, 79, 78–82. [Google Scholar]
- Lin, J.; Della-Fera, M.A.; Baile, C.A. Green tea polyphenol epigallocatechin gallate inhibits adipogenesis and induces apoptosis in 3T3-L1 adipocytes. Obes. Res. 2005, 13, 982–990. [Google Scholar] [CrossRef]
- Huang, B.; Yuan, H.D.; Kim, D.Y.; Quan, H.Y.; Chung, S.H. Cinnamaldehyde prevents adipocyte differentiation and adipogenesis via regulation of peroxisome proliferator-activated receptor-γ (PPARγ) and AMP-activated protein kinase (AMPK) pathways. J. Agric. Food. Chem. 2011, 59, 3666–3673. [Google Scholar]
- Paton, C.M.; Ntambi, J.M. Biochemical and physiological function of stearoyl-CoA desaturase. Am. J. Physiol. Endocrinol. MeTable 2009, 297, 28–37. [Google Scholar] [CrossRef]
- Roncari, D.A.; Lau, D.C.; Kindler, S. Exaggerated replication in culture of adipocyte precursors from massively obese persons. Metabolism 1981, 30, 425–427. [Google Scholar] [CrossRef]
- Martineau, L.C.; Muhammad, A.; Saleem, A.; Hervé, J.; Harris, C.S.; Arnason, J.T.; Haddad, P.S. Anti-adipogenic activities of Alnus incana and Populus balsamifera bark extracts, part II: bioassay-guided identification of actives salicortin and oregonin. Planta Med. 2010, 76, 1519–1524. [Google Scholar] [CrossRef]
- Subramanian, B.; Nakeff, A.; Tenney, K.; Crews, P.; Gunatilaka, L.; Valeriote, F. A new paradigm for the development of anticancer agents from natural products. J. Exp. Ther. Oncol. 2006, 5, 195–204. [Google Scholar]
- Mahdi, J.G.; Mahdi, A.J.; Mahdi, A.J.; Bowen, I.D. The historical analysis of aspirin discovery, its relation to the willow tree and antiproliferative and anticancer potential. Cell. Prolif. 2006, 39, 147–155. [Google Scholar] [CrossRef]
- Lee, M.; Lee, H.H.; Lee, J.K.; Ye, S.K.; Kim, S.H.; Sung, S.H. Anti-adipogenic activity of compounds isolated from Idesia polycarpa on 3T3-L1 cells. Bioorg. Med. Chem. Lett. 2013, 23, 3170–3174. [Google Scholar] [CrossRef]
- Farmer, S.R. Transcriptional control of adipocyte formation. Cell Metab. 2006, 4, 263–273. [Google Scholar] [CrossRef]
- Rosen, E.D. The transcriptional basis of adipocyte development. Prostaglandins Leukot. Essent. Fatty Acids 2005, 73, 31–34. [Google Scholar] [CrossRef]
- Tang, Q.Q.; Lane, M.D. Activation and centromeric localization of CCAAT/enhancer-binding proteins during the mitotic clonal expansion of adipocyte differentiation. Genes Dev. 1999, 13, 2231–2241. [Google Scholar] [CrossRef]
- Cao, Z.; Umek, R.M.; McKnight, S.L. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev. 1991, 5, 1538–1552. [Google Scholar] [CrossRef]
- Tang, Q.Q.; Otto, T.C.; Lane, M.D. CCAAT/enhancerbinding protein beta is required for mitotic clonal expansion during adipogenesis. Proc. Natl. Acad. Sci. USA 2003, 100, 850–855. [Google Scholar] [CrossRef]
- Horton, J.D.; Goldstein, J.L.; Brown, M.S. SREBPs: transcriptional mediators of lipid homeostasis. Cold Spring Harb. Symp. Quant. Biol. 2002, 67, 491–498. [Google Scholar] [CrossRef]
- Rosen, E.D.; Walkey, C.J.; Puigserver, P.; Spiegelman, B.M. Transcriptional regulation of adipogenesis. Genes Dev. 2000, 14, 1293–1307. [Google Scholar]
- Chen, S.; Li, Z.; Li, W.; Shan, Z.; Zhu, W. Resveratrol inhibits cell differentiation in 3T3-L1 adipocytes via activation of AMPK. Can. J. Physiol. Pharmacol. 2011, 89, 793–799. [Google Scholar]
- Yao-Borengasser, A.; Rassouli, N.; Varma, V.; Bodles, A.M.; Rasouli, N.; Unal, R.; Phanavanh, B.; Ranganathan, G.; McGehee, R.E., Jr.; Kern, P.A. Stearoyl-coenzyme A desaturase 1 gene expression increases after pioglitazone treatment and is associated with peroxisomal proliferator-activated receptor-gamma responsiveness. J. Clin. Endocrinol. MeTable 2008, 93, 4431–4439. [Google Scholar] [CrossRef]
- Kim, E.; Lee, J.H.; Ntambi, J.M.; Hyun, C.K. Inhibition of stearoyl-CoA desaturase1 activates AMPK and exhibits beneficial lipid metabolic effects in vitro. Eur. J. Pharmacol. 2011, 672, 38–44. [Google Scholar] [CrossRef]
- Jung, D.; Abu-Elheiga, L.; Ayuzawa, R.; Gu, Z.; Shirakawa, T.; Fujiki, Y.; Nakatsuji, N.; Wakil, S.J.; Uesugi, M. Mislocalization and inhibition of acetyl-CoA carboxylase 1 by a synthetic small molecule. Biochem. J. 2012, 448, 409–416. [Google Scholar] [CrossRef]
- Abu-Elheiga, L.; Matzuk, M.M.; Kordari, P.; Oh, W.; Shaikenov, T.; Gu, Z.; Wakil, S.J. Mutant mice lacking acetyl-CoA carboxylase 1 are embryonically lethal. Proc. Natl. Acad. Sci. USA 2005, 102, 12011–12016. [Google Scholar]
- Smith, S. The animal fatty acid synthase: one gene, one polypeptide, seven enzymes. FASEB J. 1994, 8, 1248–1259. [Google Scholar]
- Lee, M.; Song, J.Y.; Chin, Y.W.; Sung, S.H. Anti-adipogenic diarylheptanoids from Alnus hirsuta f. sibirica on 3T3-L1 cells. Bioorg. Med. Chem. Lett. 2013, 23, 2069–2073. [Google Scholar]
- Popivanova, B.K.; Kitamura, K.; Wu, Y.; Kondo, T.; Kagaya, T.; Kaneko, S.; Oshima, M.; Fujii, C.; Mukaida, N. Blocking TNF-alpha in mice reduces colorectal carcinogenesis associated with chronic colitis. J. Clin. Invest. 2008, 118, 560–570. [Google Scholar]
- Sample Availability: Samples of the compounds 1–5 are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lee, M.; Lee, S.H.; Kang, J.; Yang, H.; Jeong, E.J.; Kim, H.P.; Kim, Y.C.; Sung, S.H. Salicortin-Derivatives from Salix pseudo-lasiogyne Twigs Inhibit Adipogenesis in 3T3-L1 Cells via Modulation of C/EBPα and SREBP1c Dependent Pathway. Molecules 2013, 18, 10484-10496. https://doi.org/10.3390/molecules180910484
Lee M, Lee SH, Kang J, Yang H, Jeong EJ, Kim HP, Kim YC, Sung SH. Salicortin-Derivatives from Salix pseudo-lasiogyne Twigs Inhibit Adipogenesis in 3T3-L1 Cells via Modulation of C/EBPα and SREBP1c Dependent Pathway. Molecules. 2013; 18(9):10484-10496. https://doi.org/10.3390/molecules180910484
Chicago/Turabian StyleLee, Mina, Sang Hoon Lee, Jimmy Kang, Heejung Yang, Eun Ju Jeong, Hong Pyo Kim, Young Choong Kim, and Sang Hyun Sung. 2013. "Salicortin-Derivatives from Salix pseudo-lasiogyne Twigs Inhibit Adipogenesis in 3T3-L1 Cells via Modulation of C/EBPα and SREBP1c Dependent Pathway" Molecules 18, no. 9: 10484-10496. https://doi.org/10.3390/molecules180910484
APA StyleLee, M., Lee, S. H., Kang, J., Yang, H., Jeong, E. J., Kim, H. P., Kim, Y. C., & Sung, S. H. (2013). Salicortin-Derivatives from Salix pseudo-lasiogyne Twigs Inhibit Adipogenesis in 3T3-L1 Cells via Modulation of C/EBPα and SREBP1c Dependent Pathway. Molecules, 18(9), 10484-10496. https://doi.org/10.3390/molecules180910484





