Palladium and Organocatalysis: An Excellent Recipe for Asymmetric Synthesis
Abstract
:1. Introduction
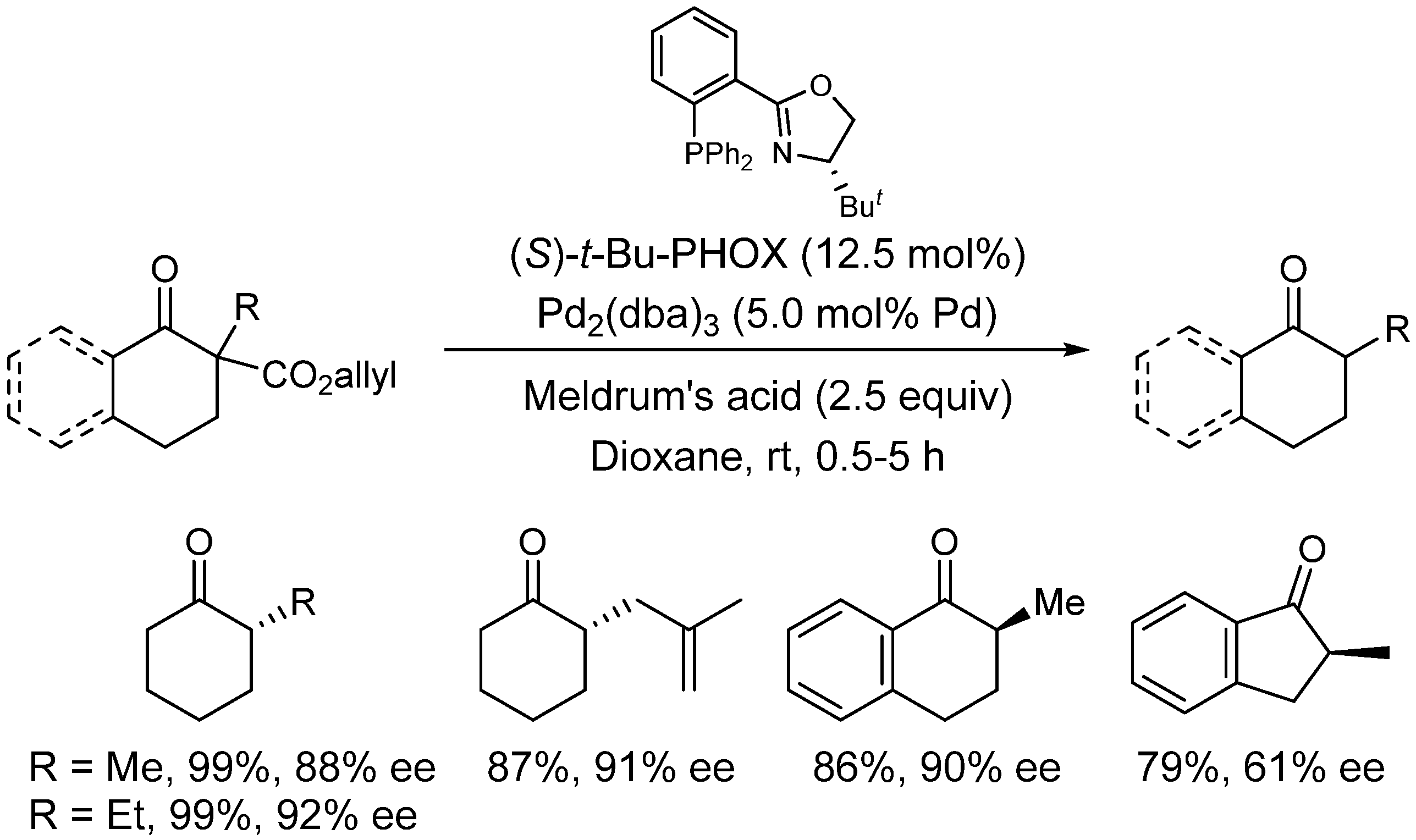
2. Asymmetric α-Allylic Alkylation
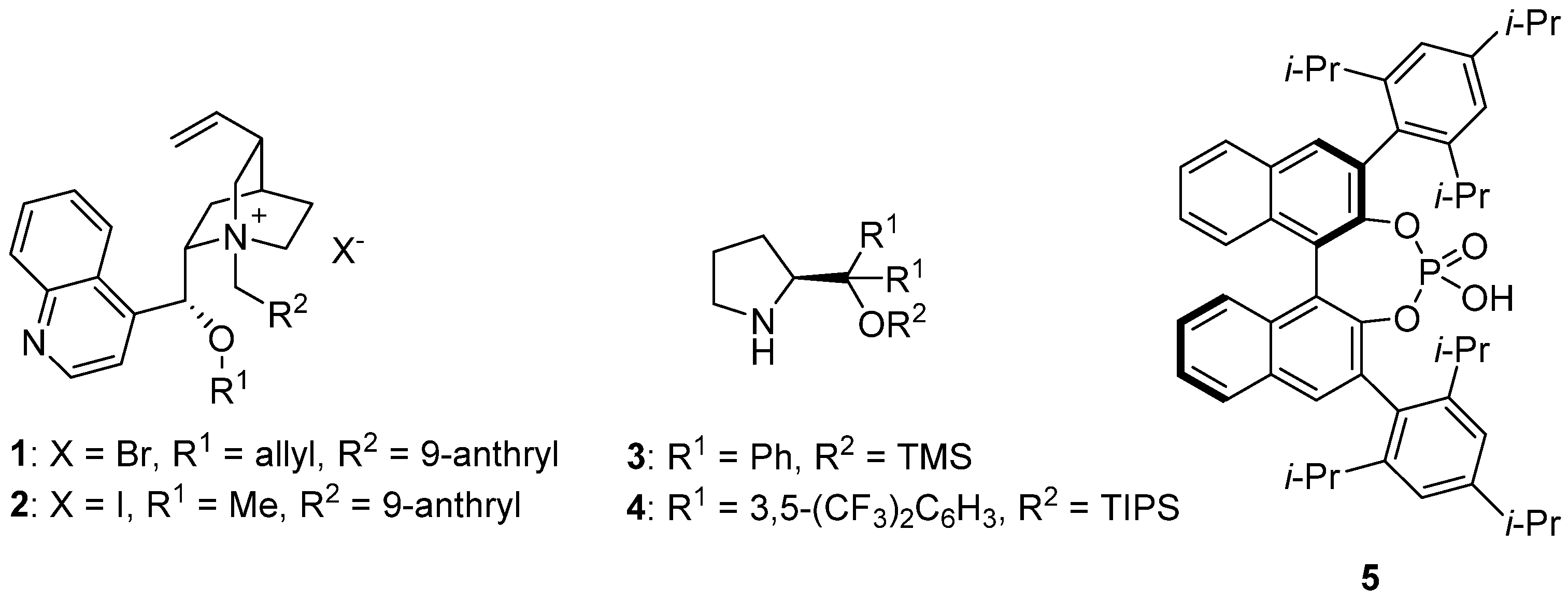
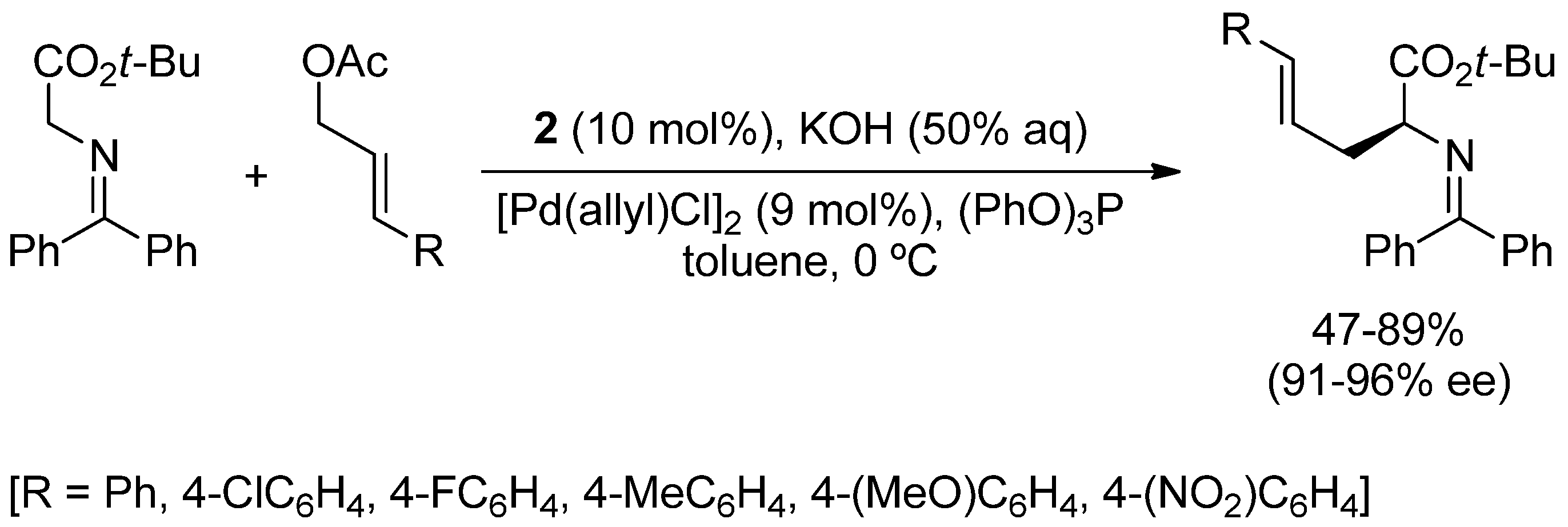
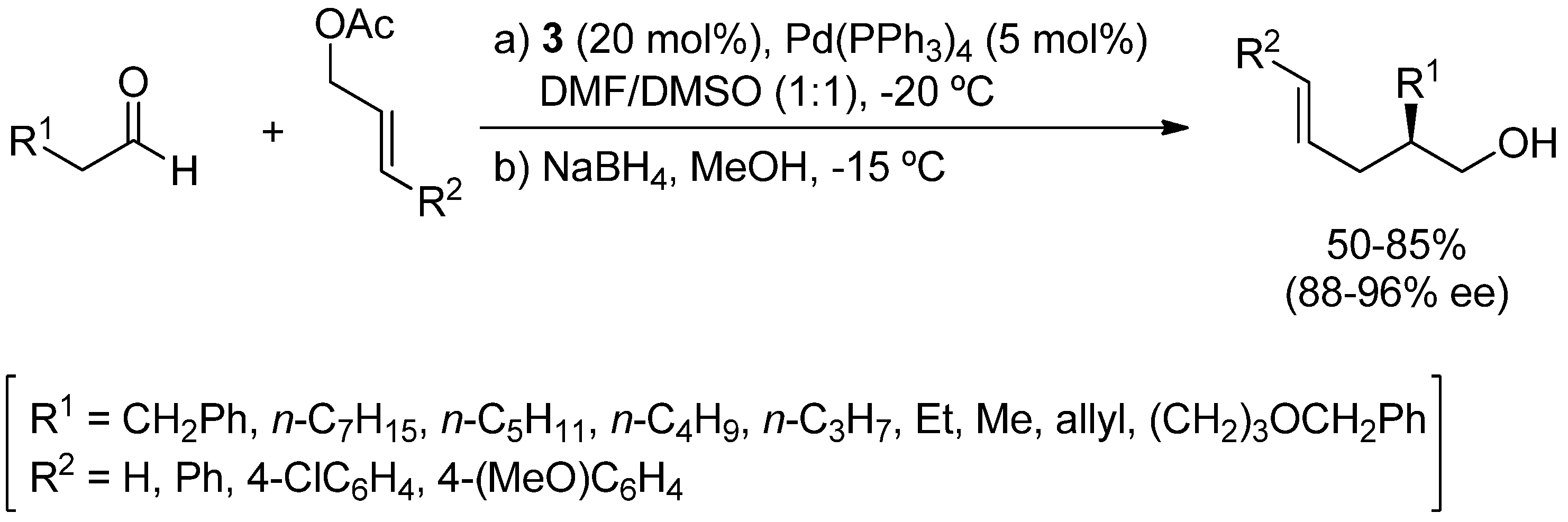
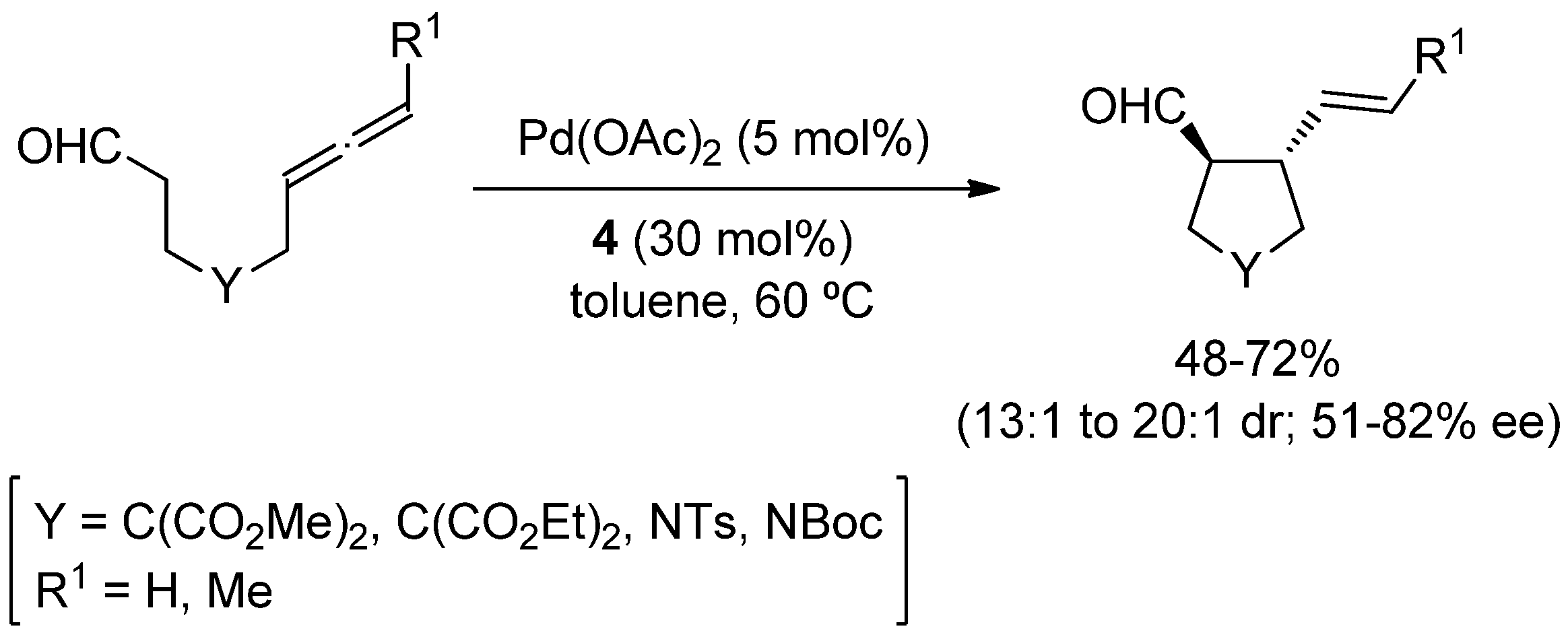
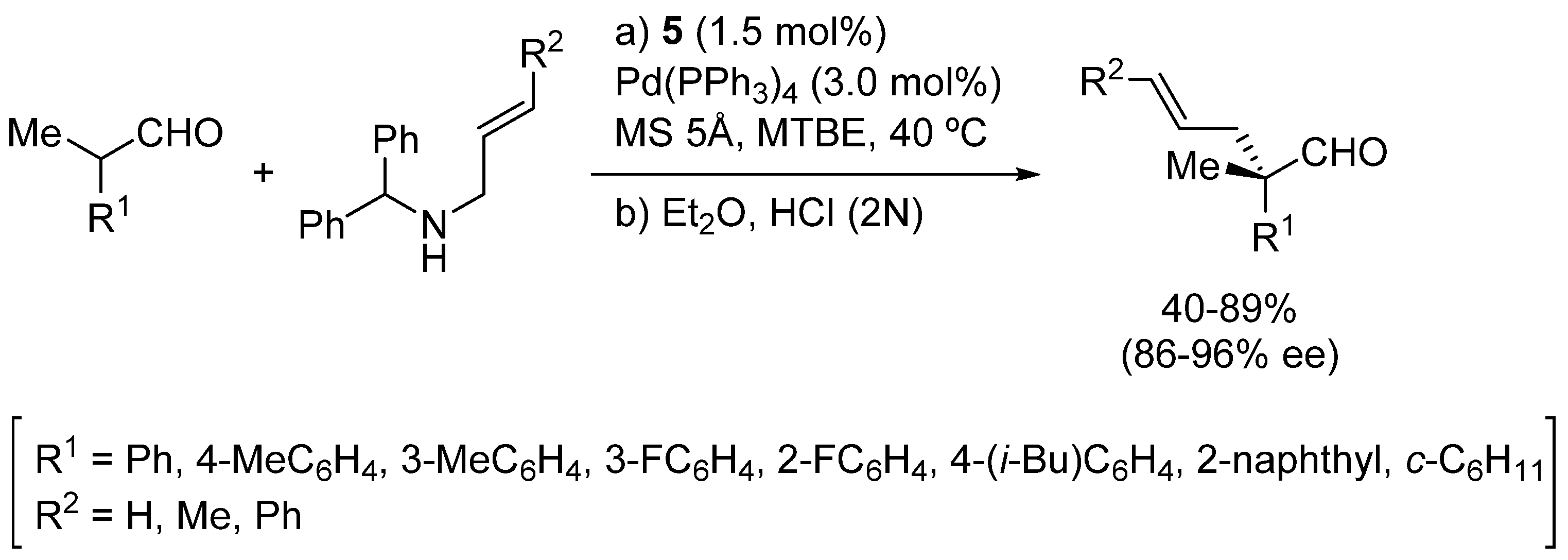
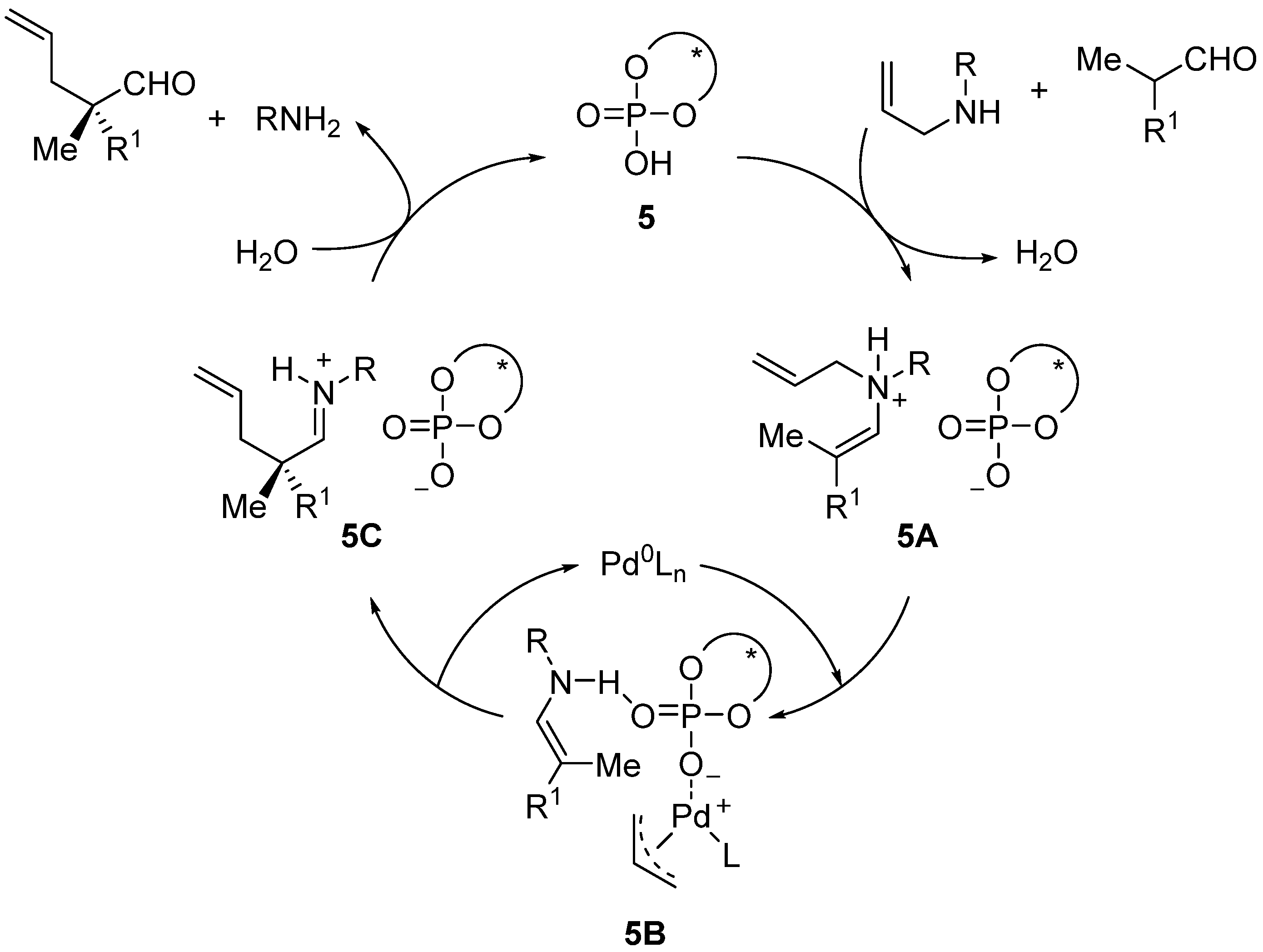
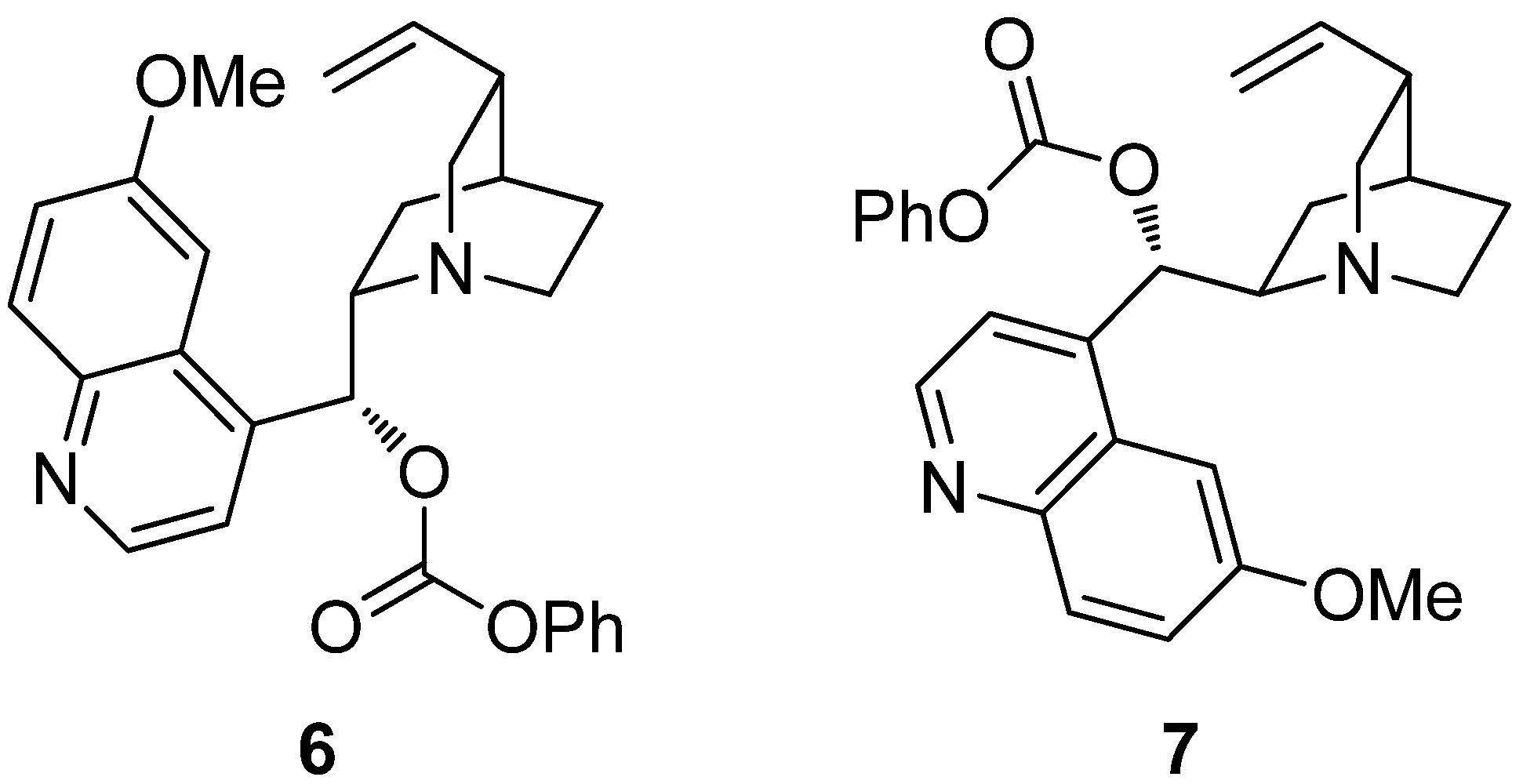
3. Asymmetric α-Fluorination
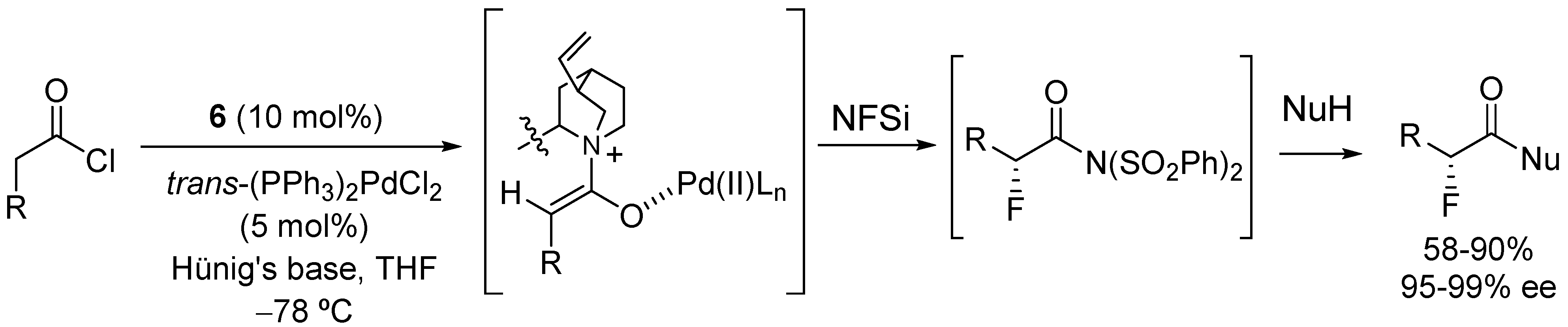



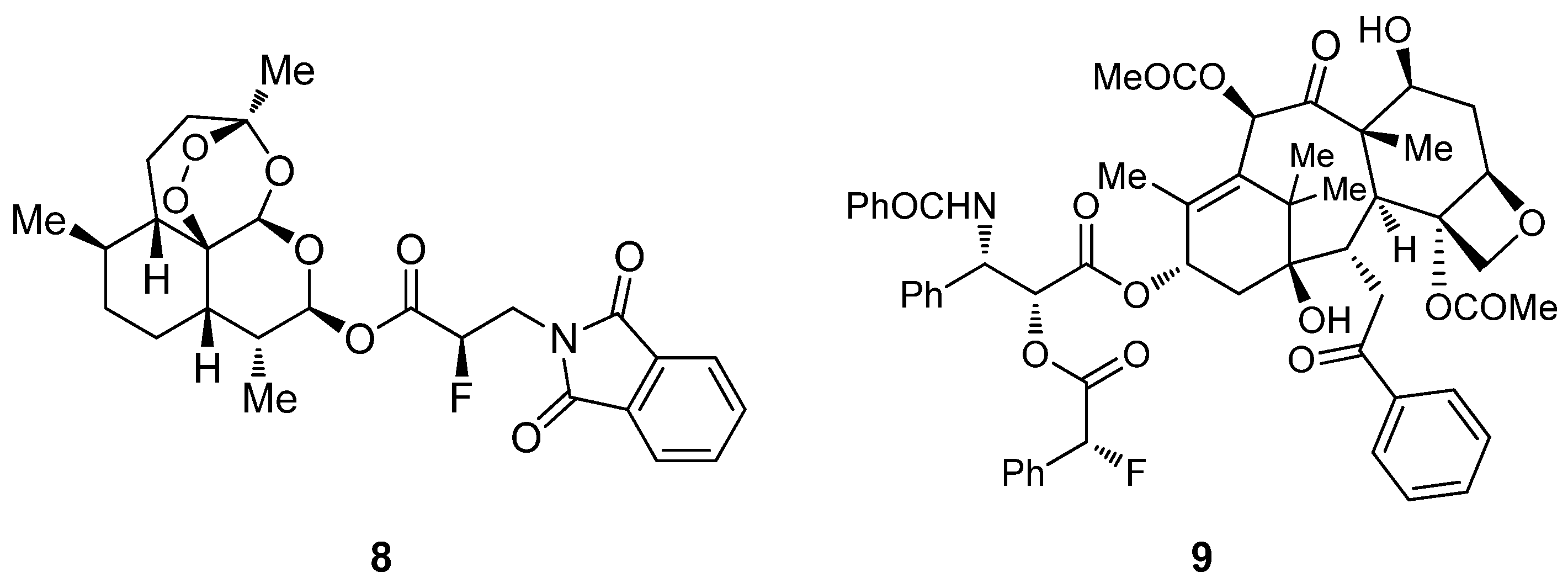
4. Enantioselective Decarboxylative Protonation
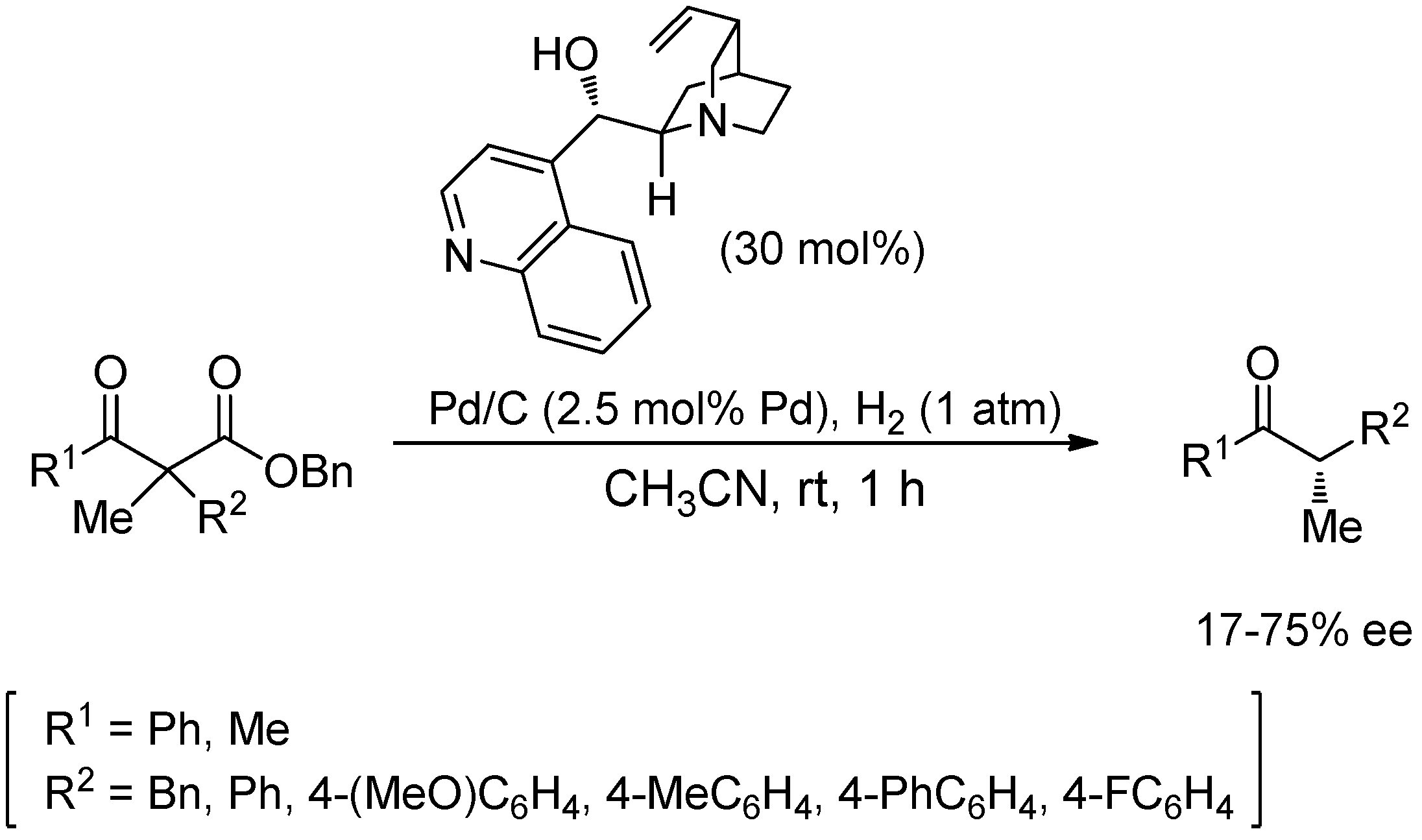
5. Cascade Iminium/Enamine-Palladium Cooperative Catalysis




6. Conclusions
Acknowledgments
Conflicts of Interest
References
- Bertelsen, S.; Jørgensen, K.A. Organocatalysis—After the gold rush. Chem. Soc. Rev. 2009, 38, 2178–2189. [Google Scholar] [CrossRef]
- Beller, M.; Bolm, C. Transition Metals for Organic Synthesis: Building Blocks and Fine Chemicals, 2nd ed; Wiley-VCH: New York, NY, USA, 2004; Volume 1–2. [Google Scholar]
- Jacobsen, E.N.; Pfaltz, A.; Yamamoto, H. Comprehensive Asymmetric Catalysis I-III; Springer: Berlin, Germany, 2000. [Google Scholar]
- Shao, Z.; Zhang, H. Combining transition metal catalysis and organocatalysis: A broad new concept for catalysis. Chem. Soc. Rev. 2009, 38, 2745–2755. [Google Scholar] [CrossRef]
- Zhong, C.; Shi, X. When organocatalysis meets transition-metal catalysis. Eur. J. Org. Chem. 2010, 2010, 2999–3025. [Google Scholar] [CrossRef]
- Zhou, J. Recent advances in multicatalyst promoted asymmetric tandem reactions. Chem. Asian J. 2010, 5, 422–434. [Google Scholar] [CrossRef]
- Du, Z.; Shao, Z. Combining transition metal catalysis and organocatalysis—An update. Chem. Soc. Rev. 2013, 42, 1337–1378. [Google Scholar] [CrossRef]
- Diederich, F.; Stang, P.J. Metal-Catalyzed Cross-Coupling Reactions; Wiley-VCH: New York, NY, USA, 1998. [Google Scholar]
- Negishi, E. Handbook of Organopalladium Chemistry for Organic Synthesis; Wiley-Interscience: New York, NY, USA, 2002. [Google Scholar]
- Miyaura, N. Cross-Coupling Reactions. A Practical Guide; Springer: Berlin, Germany, 2002. [Google Scholar]
- Tsuji, J. Palladium Reagents and Catalysts: Innovations in Organic Synthesis; Wiley: New York, NY, USA, 2004. [Google Scholar]
- Diederich, F.; de Meijere, A. Metal-Catalyzed Cross-Coupling Reactions, 2nd ed; Wiley-VCH: New York, NY, USA, 2004. [Google Scholar]
- Otera, J. Modern Carbonyl Chemistry; Wiley-VCH: New York, NY, USA, 2000. [Google Scholar]
- Trost, B.M.; Crawley, M.L. Asymmetric transition-metal-catalyzed allylic alkylations: Applications in total synthesis. Chem. Rev. 2003, 103, 2921–2943. [Google Scholar] [CrossRef]
- Diéguez, M.; Pàmies, O. Biaryl phosphites: New efficient adaptative ligands for Pd-catalyzed asymmetric allylic substitution reactions. Acc. Chem. Res. 2010, 43, 312–322. [Google Scholar] [CrossRef]
- Guerrero-Rios, I.; Rosas-Hernández, A.; Martin, E. Recent advances in the application of chiral phosphine ligands in Pd-catalysed asymmetric allylic alkylation. Molecules 2011, 16, 970–1010. [Google Scholar] [CrossRef]
- Chen, G.; Deng, Y.; Gong, L.; Mi, A.; Cui, X.; Jiang, Y.; Choi, M.C.K.; Chan, A.S.C. Palladium-catalyzed allylic alkylation of tert-butyl(depenylmethylene)-glycinate with simple allyl esters under chiral phase transfer conditions. Tetrahedron Asymmetry 2001, 12, 1567–1571. [Google Scholar] [CrossRef]
- Nakoji, M.; Kanayama, T.; Okino, T.; Takemoto, Y. Chiral phosphine-free Pd-mediated asymmetric allylation of prochiral enolate with a chiral phase-transfer catalyst. Org. Lett. 2001, 3, 3329–3331. [Google Scholar]
- Nakoji, M.; Kanayama, T.; Okino, T.; Takemoto, Y. Pd-catalyzed asymmetric allylic alkylation of glycine imino ester using a chiral phase-transfer catalyst. J. Org. Chem. 2002, 67, 7418–7423. [Google Scholar] [CrossRef]
- Ibrahem, I.; Córdova, A. Direct catalytic intermolecular α-allylic alkylation of aldehydes by combination of transition-metal and organocatalysis. Angew. Chem. Int. Ed. 2006, 45, 1952–1956. [Google Scholar] [CrossRef]
- Afewerki, S.; Ibrahem, I.; Rydfjord, J.; Breistein, P.; Córdova, A. Direct regiospecific and highly enantioselective intermolecular α-allylic alkylation of aldehydes by a combination of transition-metal and chiral amine catalysts. Chem. Eur. J. 2012, 18, 2972–2977. [Google Scholar] [CrossRef]
- Bihelovic, F.; Matovic, R.; Vulovic, B.; Saicic, R.N. Organocatalyzed cyclizations of π-allylpalladium complexes: A new method for the construction of five- and six-membered rings. Org. Lett. 2007, 9, 5063–5066. [Google Scholar] [CrossRef]
- Vulovic, B.; Bihelovic, F.; Matovic, R.; Saicic, R.N. Organocatalyzed tsuji—Trost reaction: A new method for the closure of five- and six-membered rings. Tetrahedron 2009, 65, 10485–10494. [Google Scholar] [CrossRef]
- The excess of amine needed to perform this particular cyclization strictly disqualifies this methodology as organocatalytic.
- Li, M.; Datta, S.; Barber, D.M.; Dixon, D.J. Dual amine and palladium catalysis in diastereo- and enantioselective allene carbocyclization reactions. Org. Lett. 2012, 14, 6350–6353. [Google Scholar]
- Mukherjee, S.; List, B. Chiral counteranions in asymmetric transition-Metal catalysis: Highly enantioselective Pd/Brønsted acid-catalyzed direct α-allylation of aldehydes. J. Am. Chem. Soc. 2007, 129, 11336–11337. [Google Scholar] [CrossRef]
- Jiang, G.; List, B. Direct asymmetric α-allylation of aldehydes with simple allylic alcohols enabled by the concerted action of three different catalysts. Angew. Chem. Int. Ed. 2011, 50, 9471–9474. [Google Scholar] [CrossRef]
- Paull, D.H.; Scerba, M.T.; Alden-Danforth, E.; Widger, L.R.; Lectka, T. Catalytic, asymmetric α-fluorination of acid chlorides: Dual metal-ketene enolate activation. J. Am. Chem. Soc. 2008, 130, 17260–17261. [Google Scholar]
- Erb, J.; Alden-Danforth, E.; Kopf, N.; Scerba, M.T.; Lectka, T. Combining asymmetric catalysis with natural product functionalization through enantioselective α-fluorination. J. Org. Chem. 2010, 75, 969–971. [Google Scholar] [CrossRef]
- Erb, J.; Paull, D.H.; Dudding, T.; Belding, L.; Lectka, T. From bifucntional to trifunctional (tricomponent nucleophile-transition metal-lewis acid) catalysis: The catalytic, enantioselective α-fluorination of acid chlorides. J. Am. Chem. Soc. 2011, 133, 7536–7546. [Google Scholar]
- Mohr, J.T.; Hong, A.Y.; Stoltz, B.M. Enantioselective protonation. Nat. Chem. 2009, 1, 359–369. [Google Scholar] [CrossRef]
- Blanchet, J.; Baudoux, J.; Amere, M.; Lasne, M.C.; Rouden, J. Asymmetric malonic and acetoacetic acid syntheses—A century of enantioselective decarboxylative protonations. Eur. J. Org. Chem. 2008, 2008, 5493–5596. [Google Scholar] [CrossRef]
- Claraz, A.; Oudeyer, S.; Levacher, V. Deracemization of α-substituted carbonyl compounds via catalytic enantioselective protonation of their corresponding enolates. Curr. Org. Chem. 2012, 16, 2192–2205. [Google Scholar] [CrossRef]
- Aboulhoda, S.J.; Hénin, F.; Muzart, J.; Thorey, C.; Behnen, W.; Martens, J.; Mehler, T. Production of optically active ketones by a palladium-induced cascade reaction from racemic β-ketoesters. Tetrahedron Asymmetry 1994, 5, 1321–1326. [Google Scholar] [CrossRef]
- Muzart, J.; Hénin, F.; Aboulhoda, S.J. Asymmetric protonation of enolic species: Dramatic increase in the selectivity with temperature and unexpected eyring diagram. Tetrahedron Asymmetry 1997, 8, 381–389. [Google Scholar] [CrossRef]
- Detalle, J.F.; Riahi, A.; Steinmetz, V.; Hénin, F.; Muzart, J. Mechanistic insights into the palladium-induced domino reaction leading to ketones from benzyl β-ketoesters: First characterization of the enol as an intermediate. J. Org. Chem. 2004, 69, 6528–6532. [Google Scholar] [CrossRef]
- Roy, O.; Diekmann, M.; Riahi, A.; Hénin, F.; Muzart, J. Access to optically active linear ketones by one-pot catalytic deprotection, decarboxylation, asymmetric tautomerization from racemic benzyl β-ketoesters. Chem. Commun. 2001, 2001, 533–534. [Google Scholar]
- Roy, O.; Riahi, A.; Hénin, F.; Muzart, J. Catalysed asymmetric protonation of simple linear keto-enolic species—A route to chiral α-arylpropionic acids. Eur. J. Org. Chem. 2002, 2002, 3986–3994. [Google Scholar] [CrossRef]
- Kukula, P.; Matoušek, V.; Mallat, T.; Baiker, A. Enantioselective decarboxylation of β-keto esters with Pd/amino alcohol systems: Successive metal catalysis and organocatalysis. Chem. Eur. J. 2008, 14, 2699–2708. [Google Scholar] [CrossRef]
- Mohr, J.T.; Nishimata, T.; Behenna, D.C.; Stoltz, B.M. Catalytic enantioselective decarboxylative protonation. J. Am. Chem. Soc. 2006, 128, 11348–11349. [Google Scholar] [CrossRef]
- Marinescu, S.C.; Nishimata, T.; Mohr, J.T.; Stoltz, B.M. Homogeneous Pd-catalyzed enantioselective decarboxylative protonation. Org. Lett. 2008, 8, 1039–1042. [Google Scholar]
- Pellissier, H. Stereocontrolled domino reactions. Chem. Rev. 2013, 113, 442–524. [Google Scholar] [CrossRef]
- Patil, N.T.; Shinde, V.S.; Gajula, B. A one-pot catalysis: The strategic classification with some recent examples. Org. Biomol. Chem. 2012, 10, 211–224. [Google Scholar] [CrossRef]
- Lu, L.Q.; Chen, J.R.; Xiao, W.J. Development of cascade reactions for the concise construction of diverse heterocyclic architectures. Acc. Chem. Res. 2012, 45, 1278–1293. [Google Scholar] [CrossRef]
- Yang, T.; Ferrali, A.; Campbell, L.; Dixon, D.J. Combination iminium, enamine and copper(I) cascade catalysis: A carboannulation for the synthesis of cyclopentenes. Chem. Commun. 2008, 2008, 2923–2925. [Google Scholar]
- Zhao, G.L.; Ullah, F.; Deiana, L.; Lin, S.; Zhang, Q.; Sun, J.; Ibrahem, I.; Dziedzic, P.; Córdova, A. Dynamic kinetoc asymmetric transformation (DYKAT) by combined amine- and transition-metal-catalyzed enantioselective cycloisomerization. Chem. Eur. J. 2010, 16, 1585–1591. [Google Scholar] [CrossRef]
- Lin, S.; Zhao, G.L.; Deiana, L.; Sun, J.; Zhang, Q.; Leijonmarck, H.; Córdova, A. Dynamic kinetic asymmetric domino oxa-michael/carbocyclization by combination of transition-metal and amine catalysis: Catalytic enantioselective synthesis of dihydrofurans. Chem. Eur. J. 2010, 16, 13930–13934. [Google Scholar]
- Sun, W.; Zhu, G.; Hong, L.; Wang, R. The marriage of organocatalysis with metal catalysis: access to multisubstituted chiral 2,5-dihydropyrroles by cascade iminium/enamine-metal cooperative catalysis. Chem. Eur. J. 2011, 17, 13958–13962. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Wang, Y.; Hu, X.Q.; Xu, P.F. Merging organocatalysis with transition metal catalysis and using O2 as the oxidant for enantioselective C-H functionalization of aldehydes. Chem. Commun. 2013, 49, 7555–7557. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Fernández-Ibañez, M.Á.; Maciá, B.; Alonso, D.A.; Pastor, I.M. Palladium and Organocatalysis: An Excellent Recipe for Asymmetric Synthesis. Molecules 2013, 18, 10108-10121. https://doi.org/10.3390/molecules180910108
Fernández-Ibañez MÁ, Maciá B, Alonso DA, Pastor IM. Palladium and Organocatalysis: An Excellent Recipe for Asymmetric Synthesis. Molecules. 2013; 18(9):10108-10121. https://doi.org/10.3390/molecules180910108
Chicago/Turabian StyleFernández-Ibañez, M. Ángeles, Beatriz Maciá, Diego A. Alonso, and Isidro M. Pastor. 2013. "Palladium and Organocatalysis: An Excellent Recipe for Asymmetric Synthesis" Molecules 18, no. 9: 10108-10121. https://doi.org/10.3390/molecules180910108
APA StyleFernández-Ibañez, M. Á., Maciá, B., Alonso, D. A., & Pastor, I. M. (2013). Palladium and Organocatalysis: An Excellent Recipe for Asymmetric Synthesis. Molecules, 18(9), 10108-10121. https://doi.org/10.3390/molecules180910108






