Preparation and Characterization of Poly(ethyl hydrazide)-Grafted Oil Palm Empty Fruit Bunch Fibre for the Removal of Cu(II) Ions from an Aqueous Environment
Abstract
:1. Introduction
2. Results and Discussion
2.1. Graft Copolymerization of Methyl Acrylate onto Opefb
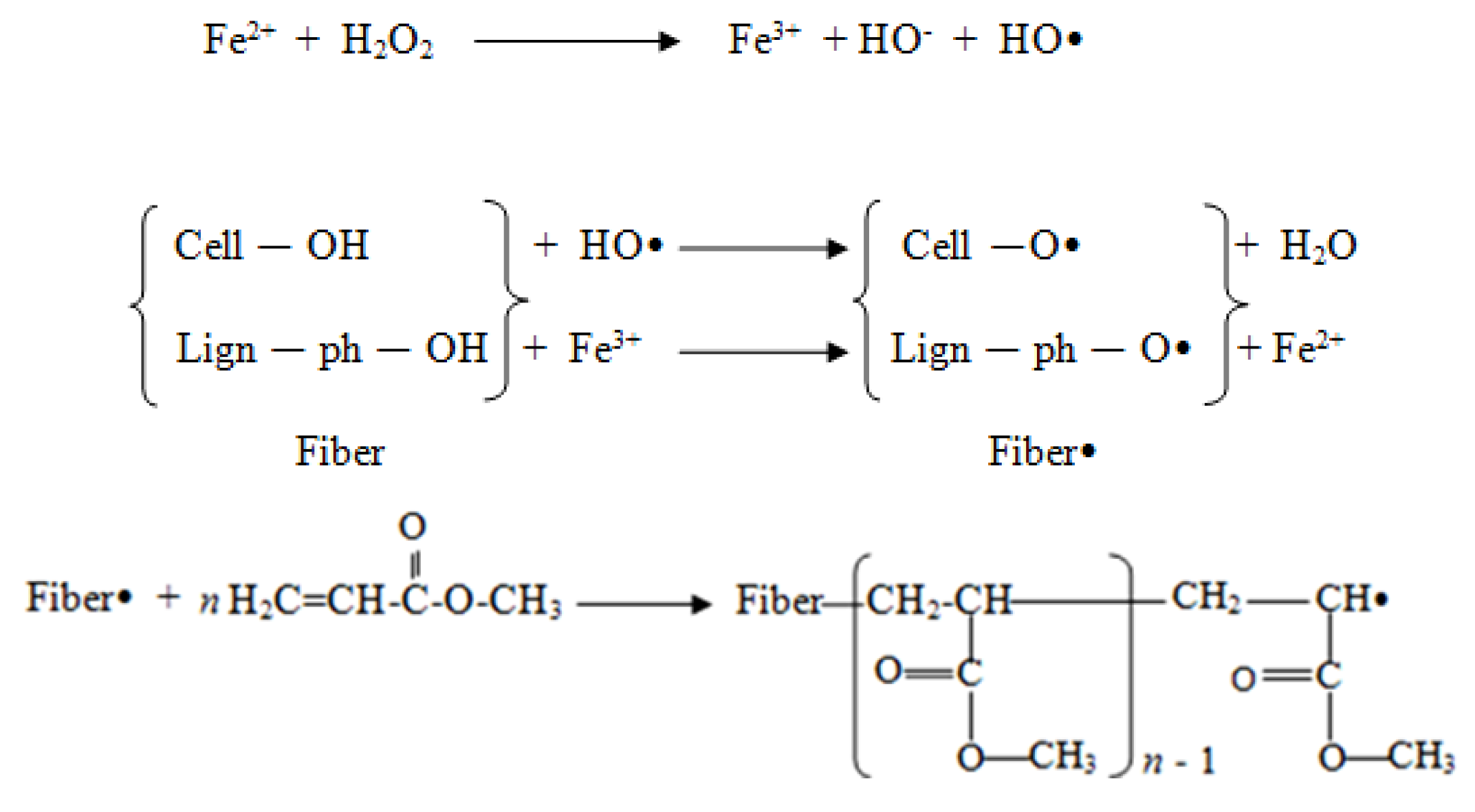
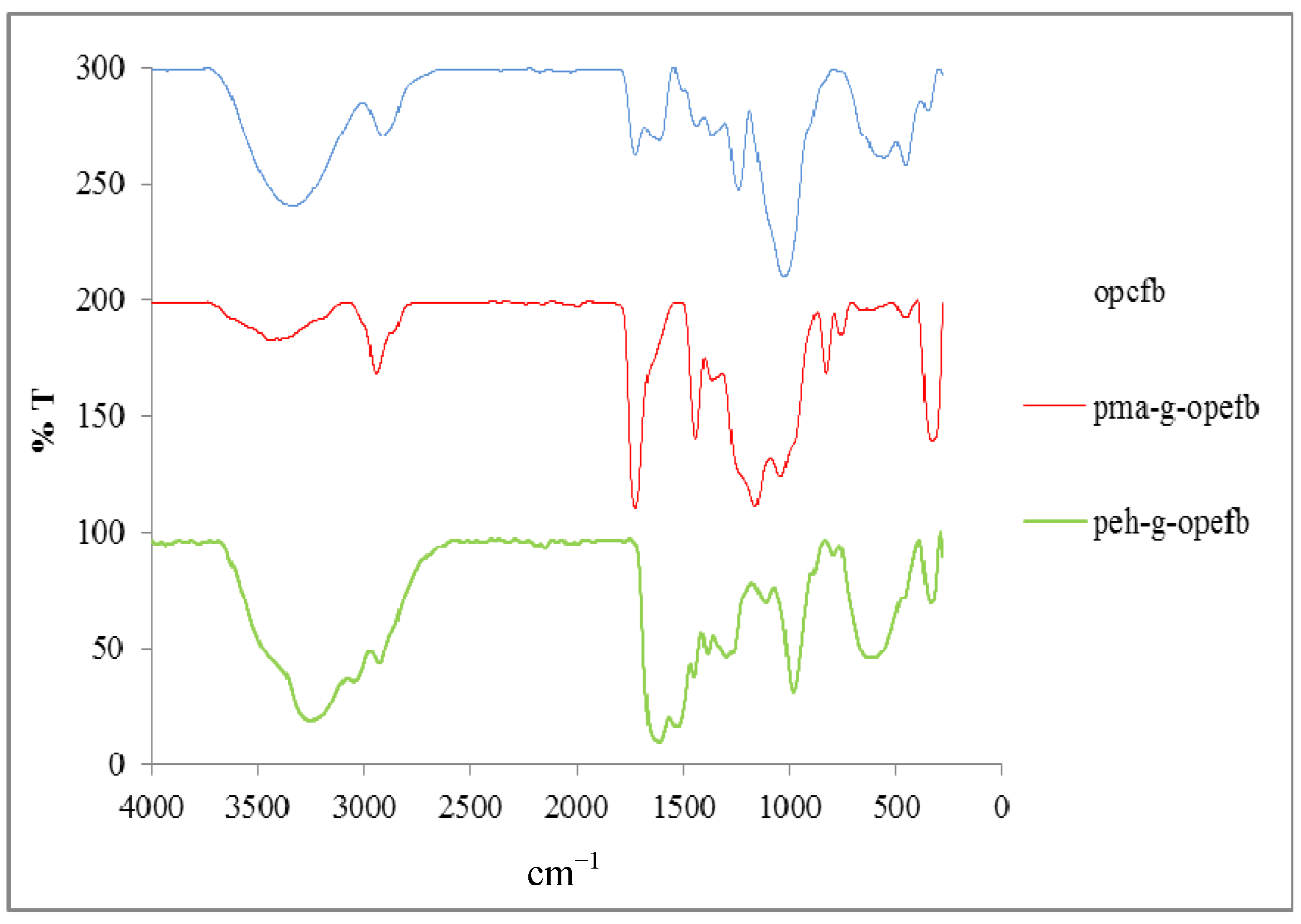
2.2. Characterization of Peh-g-opefb
Fourier Transform Infrared Analysis
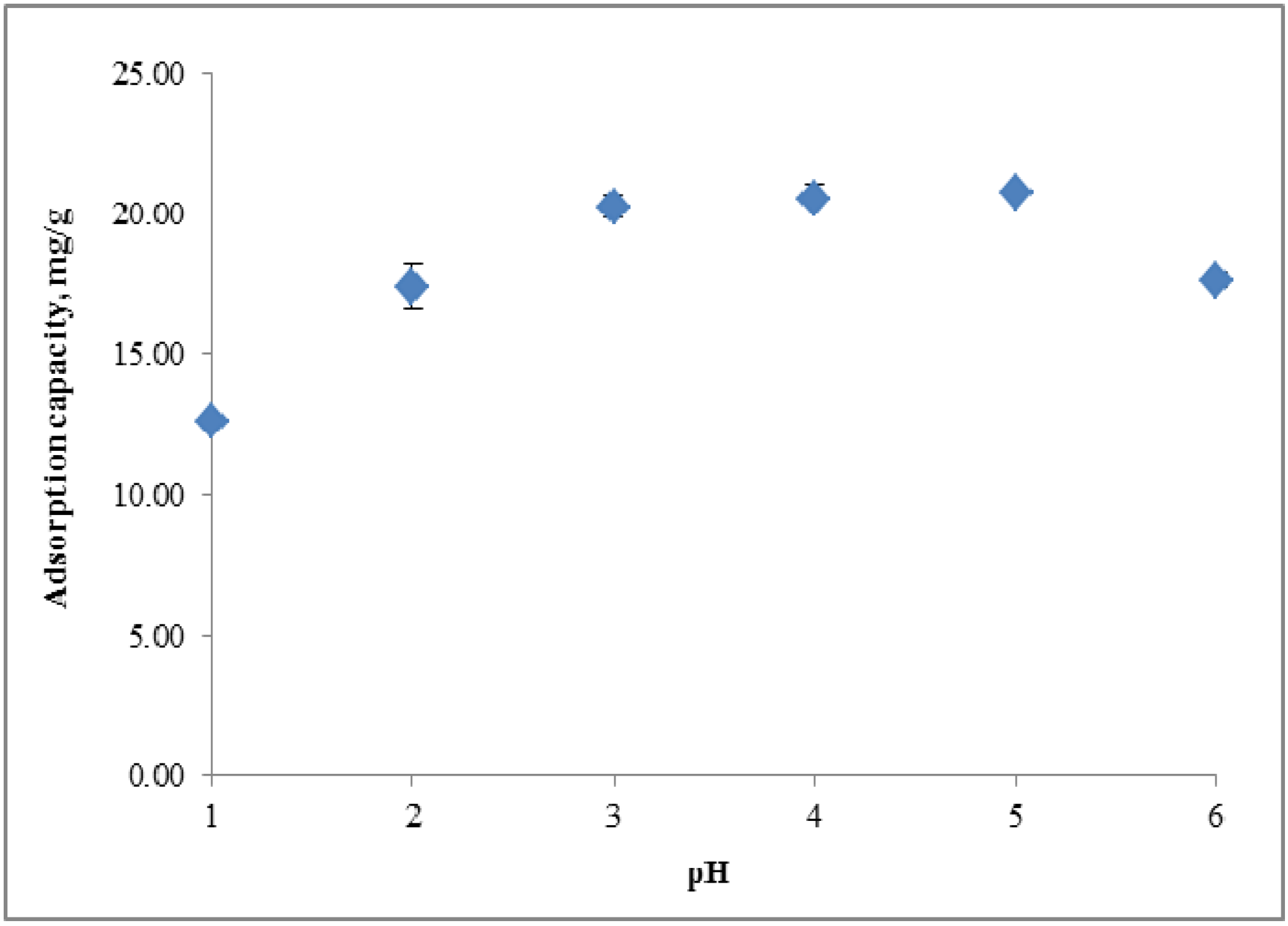
2.3. Copper (II) Ion Adsorption Study
2.3.1. Effect of pH
2.3.2. Effect of Initial Concentration
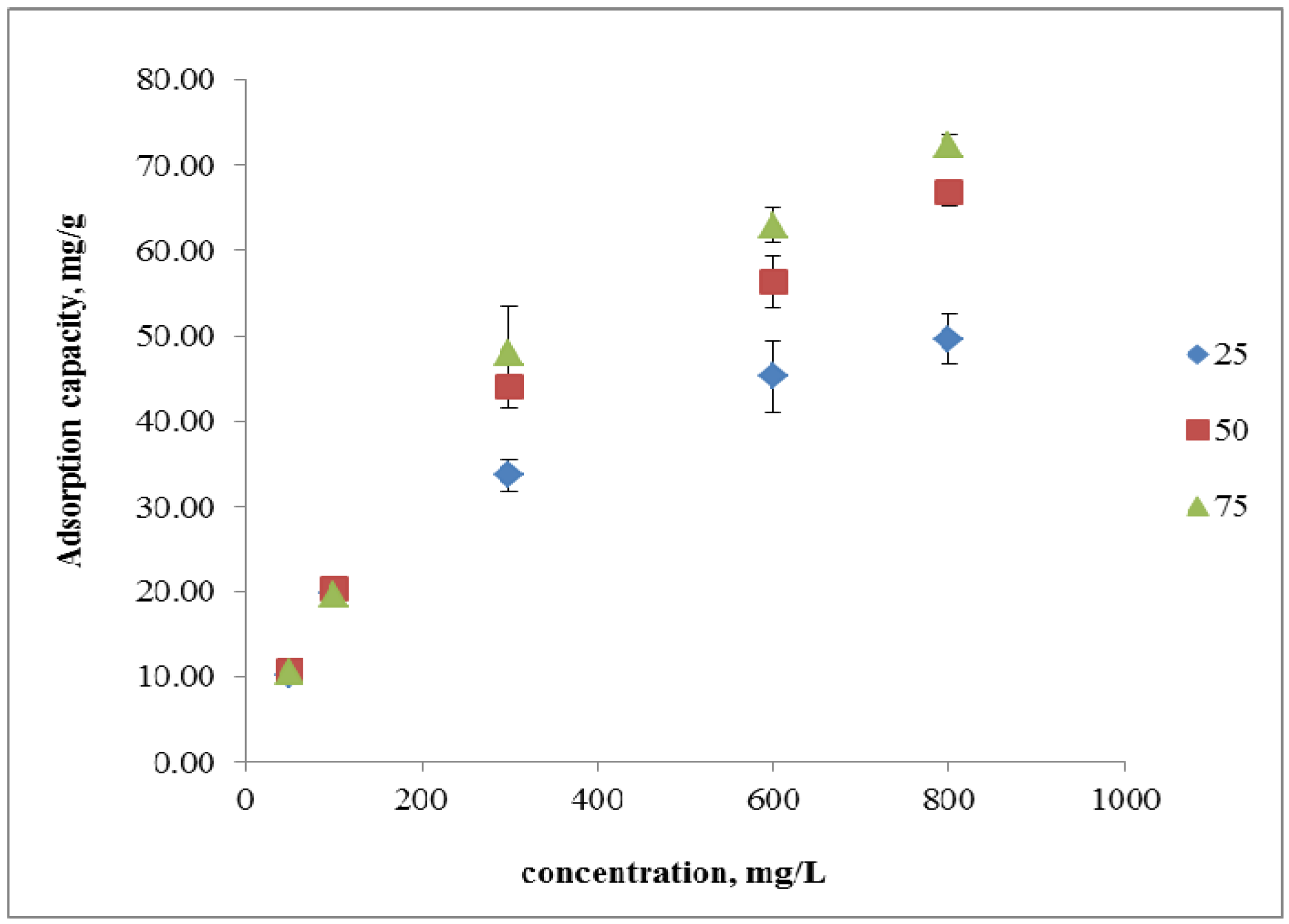
2.3.3. Adsorption Isotherms
| Langmuir isotherm | Freundlich isotherm | ||||||
|---|---|---|---|---|---|---|---|
| Temp. (°C) | Qmax (mg g−1) | b (L mg−1) | R2 | RL | KF (mg g−1) | n | R2 |
| 25 | 43.48 | 0.3382 | 0.9645 | 0.0161 | 10.87 | 4.4603 | 0.9350 |
| 50 | 59.17 | 0.0817 | 0.9863 | 0.0601 | 13.16 | 3.7078 | 0.9990 |
| 75 | 76.92 | 0.0289 | 0.9849 | 0.1460 | 14.02 | 4.3687 | 0.9648 |
| Adsorbent | qmax (mg g−1) | References |
|---|---|---|
| Cu(II) | ||
| Opefb (grafted with methyl acrylate and heated with hydrazine hydrate) | 43.48 | present study |
| Rape straw | 7.72 | [19] |
| Uncaria gambir (polymerized by formaldehyde) | 9.95 | [23] |
| Cashew nut shell | 20.00 | [17] |
| Opefb (grafted with methyl acrylate and reacted with hydroxylammonium chloride) | 74.10 | [9] |
| Rubber leaf powder | 14.97 | [12] |
| Lentil shell Wheat shell Rice shell | 9.59 17.42 2.95 | [16] |
| opefb | 3.6 | [25] |
| Palm kernel fibre (treated with HCl) | 13.06 | [26] |
2.3.4. Adsorption Thermodynamics
| Temp, K | E nthalpy, ∆H⁰ (kJ mol−1) | Entropy, ∆S⁰ (J mol−1 K−1) | Free energy, −∆G⁰ (kJ mol−1) |
|---|---|---|---|
| 298 | 20.96 | 87.75 | 5.19 |
| 323 | 7.38 | ||
| 348 | 9.57 |
2.3.5. Adsorption Kinetics

| Kinetic models | qe exp (mg g−1) | Rate constant, k1 (min−1), k2 (g mg−1 min−1) (×10−3) | qe calc. (mg g−1) | Correlation coefficient, R2 |
|---|---|---|---|---|
| Pseudo-first order | 20.33 | 3.90 | 4.195 | 0.8952 |
| Pseudo-second order | 2.93 | 20.79 | 0.9999 |
3. Experimental
3.1. Instruments and Apparatus
3.2. Materials and Reagents
3.3. Graft Copolymerization of Pma-g-Opefb Chelating Fibres
3.4. Modification of the Pma-g-Opefb
3.5. Metal Ion Uptake Experiments Using a Batch Method
3.5.1. Effect of pH
3.5.2. Effect of Initial Concentration and Temperature
3.5.3. Kinetic Study
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Monier, M.; Nawar, N.; Abdel-latif, D.A. Preparation and characterization of chelating fibers based on natural wool for removal of Hg(II), Cu(II) and Co(II) metal ions from aqueous solutions. J. Hazard. Mater. 2010, 184, 118–125. [Google Scholar] [CrossRef]
- Argun, M.E.; Dursun, S.; Ozdemir, C.; Karatas, M. Heavy metal adsorption by modified oak sawdust: Thermodynamics and kinetics. J. Hazard. Mater. 2007, 141, 77–85. [Google Scholar] [CrossRef]
- Chang, S.H.; Teng, T.T.; Ismail, N. Extraction of Cu(II) from aqueous solutions by vegetable oil-based organic solvents. J. Hazard. Mater. 2010, 181, 868–872. [Google Scholar] [CrossRef]
- Liu, R.X.; Zhang, B.W.; Tang, H.X. Synthesis and characterization of poly(acrylaminophosphonic-carboxyl-hydrazide) chelating fibre. React. Funct. Polym. 1999, 39, 71–81. [Google Scholar] [CrossRef]
- Ahmad Zaini, M.A.; Amano, Y.; Machida, M. Adsorption of heavy metals onto activated carbons derived from polyacrylonitrile fiber. J. Hazard. Mater. 2010, 180, 552–560. [Google Scholar] [CrossRef]
- Raju, G.; Ratnam, C.T.; Ibrahim, N.A.; Ab Rahman, M.Z.; Wan Yunus, W.M.Z. Graft copolymerization of methyl acrylate onto oil palm empty fruit bunch (OPEFB) fiber. Polym. Plast. Technol. Eng. 2007, 46, 949–955. [Google Scholar] [CrossRef]
- David, L.; Rusu, M.; Cozar, O.; Rusu, D.; Todica, M.; Balan, C. Spectroscopic and magnetic investigations of some transition metal complexes with N-4-methoxyphenyl-N-4-chlorobenzoyl hydrazide as ligand. J. Mol. Struct. 1999, 482, 149–152. [Google Scholar]
- Bekheit, M.M.; Nawar, N.; Addison, A.W.; Abdel-Latif, D.A.; Monier, M. Preparation and characterization of chitosan-grafted-poly(2-amino-4,5-pentamethylene-thiophene-3-carboxylic acid N-acryloyl-hydrazide) chelating resin for removal of Cu(II), Co(II) and Ni(II) metal ions from aqueous solutions. Int. J. Biol. Macromol. 2011, 48, 558–565. [Google Scholar] [CrossRef]
- Haron, M.J.; Tiansin, M.; Ibrahim, N.A.; Kassim, A.; Wan Yunus, W.M.Z. Sorption of Cu(II) by polyhydroxamic acid chelating exchanger prepared from polymethyl acrylate grafted oil palm empty fruit bunch. Bioresources 2009, 4, 1305–1318. [Google Scholar]
- Hasar, H. Adsorption of nickel (II) from aqueous solution onto activated carbon prepared from almond husk. J. Hazard. Mater. 2003, B97, 49–57. [Google Scholar] [CrossRef]
- Kobya, M.; Demirbas, E.; Senturk, E.; Ince, M. Adsorption of heavy metal ions from aqueous solutions by activated carbon prepared from apricot stone. Bioresour. Technol. 2005, 96, 1518–1521. [Google Scholar] [CrossRef]
- Wan Ngah, W.S.; Hanafiah, M.A.K.M. Biosorption of copper ions from dilute aqueous solutions on base treated rubber (Hevea brasiliensis) leaves powder: Kinetics, isotherm, and biosorption mechanisms. J. Environ. Sci. 2008, 20, 1168–1176. [Google Scholar] [CrossRef]
- Dai, J.; Yan, H.; Yang, H.; Cheng, R. Simple method for preparation of chitosan/poly(acrylic acid) blending hydrogel beads and adsorption of copper (II) from aqueous solutions. Chem. Eng. J. 2010, 165, 240–249. [Google Scholar] [CrossRef]
- Ozcan, A.; Ozcan, A.S.; Tunali, S.; Akar, T.; Kiran, I. Determination of the equilibrium, kinetic and thermodynamic parameters of adsorption of copper (II) ions onto seeds of Capsicum annuum. J. Hazard. Mater. B 2005, 124, 200–208. [Google Scholar] [CrossRef]
- Gok, O.; Ozcan, A.; Erdem, B.; Ozcan, A.S. Prediction of the kinetics, equilibrium and thermodynamic parameters of adsorption of copper (II) ions onto 8-hydroxy quinolone immobilized bentonite. Colloids Surf. A Physicochem. Eng. Aspects 2008, 317, 174–185. [Google Scholar]
- Aydin, H.; Bulut, Y.; Yerliyaka, C. Removal of copper (II) from aqueous solution by adsorption onto low-cost adsorbents. J. Environ. Manage. 2008, 87, 37–45. [Google Scholar]
- Senthilkumar, P.; Ramalingam, S.; Sathyaselvabala, V.; Dinesh Kirupa, S.; Sivanesan, S. Removal of copper (II) ions from aqueous solution by adsorption using cashew nut shell. Desalination 2011, 266, 63–71. [Google Scholar] [CrossRef]
- Aloma, I.; Martin-Lara, M.A.; Rodriguez, I.L.; Balzquez, G.; Calero, M. Removal of nickel (II) ions from aqueous solutions by biosorption on sugarcane bagasse. J. Taiwan Inst. Chem. Eng. 2012, 43, 275–281. [Google Scholar] [CrossRef]
- Wang, J.; Chen, T.; Li, S.; Yue, Z.; Jin, J.; He, G.; Zhang, H. Biosorption of copper (II) from aqueous solutions with rape straw. Geomicrobiol. J. 2012, 29, 250–254. [Google Scholar] [CrossRef]
- Kamari, A; Wan Ngah, W.S. Isotherm, kinetic and thermodynamic studies of lead and copper uptake by H2SO4 modified chitosan. Colloids Surf. B Biointerfaces 2009, 73, 257–266. [Google Scholar] [CrossRef]
- Fil, B.A.; Boncukcuoglu, R.; Yilmaz, A.E.; Bayar, S. Adsorption of Ni(II) on ion exchange resin: Kinetics, equilibrium and thermodynamic studies. Korean J. Chem. Eng. 2012, 29, 1232–1238. [Google Scholar]
- Bhatnagar, A.; Minocha, A.K. Biosorption optimization of nickel removal from water using Punica granatum peel waste. Colloids Surf. B Biointerfaces 2010, 76, 544–548. [Google Scholar] [CrossRef]
- Tong, K.S.; Jain Kassim, M.; Azraa, A. Adsorption of copper ion from its aqueous solution by a novel biosorbent Uncaria gambir: Equilibrium, kinetics, and thermodynamic studies. Chem. Eng. J. 2011, 170, 145–153. [Google Scholar]
- Ozer, A.; Gurbuz, G.; Calimli, A.; Korbahti, B.K. Investigation of nickel (II) biosorption on Enteromorpha prolifera: Optimization using response surface analysis. J. Hazard. Mater. 2008, 152, 778–788. [Google Scholar]
- Salamatinia, B.; Kamaruddin, A.H.; Abdullah, A.Z. Removal of Zn and Cu from wastewater by sorption on oil palm tree-derived biomasses. J. Appl. Sci. 2007, 7, 2020–2027. [Google Scholar] [CrossRef]
- Ho, Y.S.; Ofomaja, A.E. Kinetic studies of copper ion adsorption on palm kernel fibre. J. Hazard. Mater. 2006, B137, 1796–1802. [Google Scholar]
- Reddy, D.H.K.; Seshaiah, K.; Reddy, A.V.R.; Lee, S.M. Optimization of Cd(II), Cu(II) and Ni(II) biosorption by chemically modified Moringa oleifera leaves powder. Carbohydr. Polym. 2012, 88, 1077–1086. [Google Scholar]
- Reddy, D.H.K.; Ramana, D.K.V.; Seshaiah, K.; Reddy, A.V.R. Biosorption of Ni(II) from aqueous phase by Moringa oleifera bark, a low cost biosorbent. Desalination 2011, 268, 150–157. [Google Scholar]
- Pimentel, P.M.; Melo, M.A.F.; Melo, D.M.A.; Assuncao, A.L.C.; Henrique, D.M.; Silva, C.N., Jr.; Gonzalez, G. Kinetics and thermodynamics of Cu(II) adsorption on oil shale wastes. Fuel Process. Technol. 2008, 89, 62–67. [Google Scholar] [CrossRef]
- Padmavathy, V. Biosorption of nickel (II) ions by baker’s yeast: Kinetic, thermodynamic and desorption studies. Bioresour. Technol. 2008, 99, 3100–3109. [Google Scholar] [CrossRef]
- Aksu, Z.; Isoglu, I.A. Removal of copper (II) ions from aqueous solution by biosorption onto agricultural waste sugar beet pulp. Process Biochem. 2005, 40, 3031–3044. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. The kinetics of sorption of divalent metal ions onto sphagnum moss peat. Water Res. 1999, 34, 735–742. [Google Scholar]
- Sample Availability: A sample of the compound is available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Johari, I.S.; Yusof, N.A.; Haron, M.J.; Nor, S.M.M. Preparation and Characterization of Poly(ethyl hydrazide)-Grafted Oil Palm Empty Fruit Bunch Fibre for the Removal of Cu(II) Ions from an Aqueous Environment. Molecules 2013, 18, 8461-8472. https://doi.org/10.3390/molecules18078461
Johari IS, Yusof NA, Haron MJ, Nor SMM. Preparation and Characterization of Poly(ethyl hydrazide)-Grafted Oil Palm Empty Fruit Bunch Fibre for the Removal of Cu(II) Ions from an Aqueous Environment. Molecules. 2013; 18(7):8461-8472. https://doi.org/10.3390/molecules18078461
Chicago/Turabian StyleJohari, Ili Syazana, Nor Azah Yusof, Md Jelas Haron, and Siti Mariam Mohd Nor. 2013. "Preparation and Characterization of Poly(ethyl hydrazide)-Grafted Oil Palm Empty Fruit Bunch Fibre for the Removal of Cu(II) Ions from an Aqueous Environment" Molecules 18, no. 7: 8461-8472. https://doi.org/10.3390/molecules18078461




