Hydrophosphonylation of Nanoparticle Schiff Bases as a Mean for Preparation of Aminophosphonate-Functionalized Nanoparticles
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation of Modified Nanoparticles

2.2. Characterization of Nanoparticles
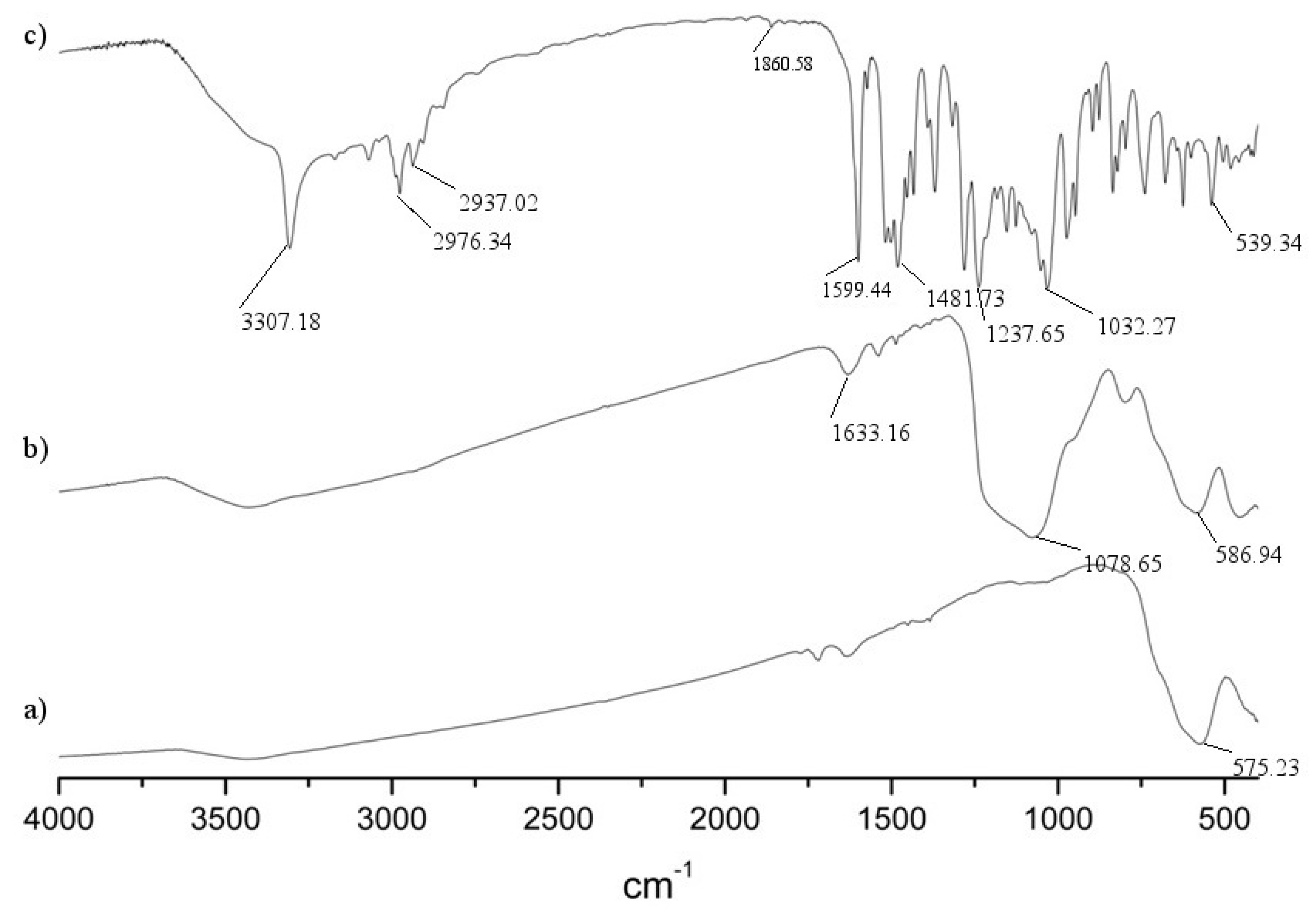
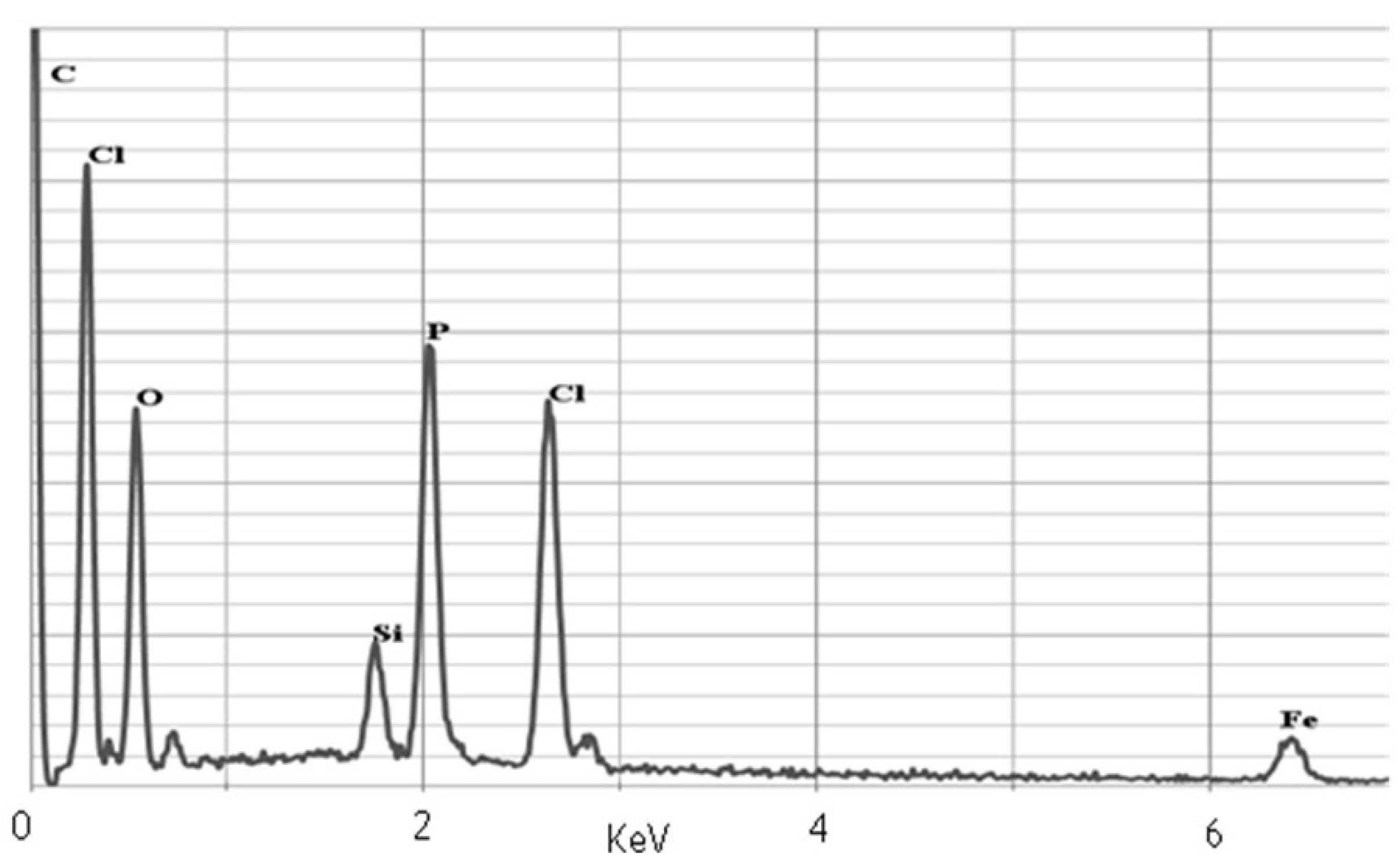

2.3. Nanoparticles Size and Dispersion
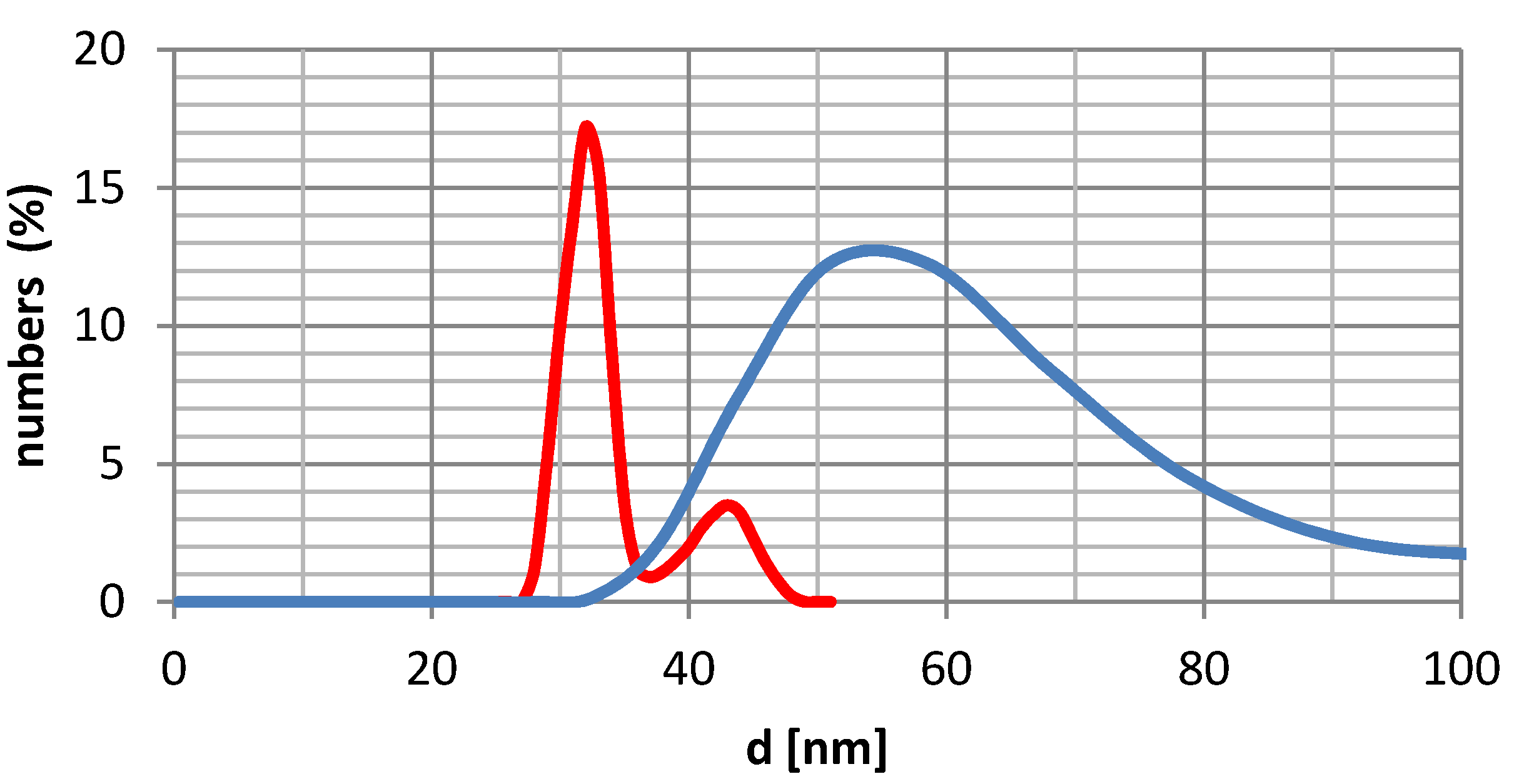
| No | Hydrodynamic size [nm] (fraction %) | |||
|---|---|---|---|---|
| Water | Methanol | Acetone | Toluene | |
| 1a | 168 (79%), 954 (21%) | 144 (100%) | 85 (100%) | Above 1 µm |
| 1b | 710 (100%) | 184 (100%) | 399 (100%) | 507 (10%) |
| 1c | 485 (100%) | 72 (100%) | 39 (49%), 81(51%) | 194 (36%), 808 (38%) |
| 1d | 74 (47%), 259 (53%) | 180 (100%) | 96 (100%) | 280 (23%), 870 (77%) |
| 1e | 180 (91%), 840 (8%) | 170 (100%) | 121 (100%) | 697 (100%) |
| 1f | 820 (100%) | 70 (40%), 337 (60%) | 73 (40%), 280 (60%) | 560 (40%) |
| 1g | 402 (25%), 930 (75%) | 159 (100%) | 294 (100%) | Above 1 µm |
| 1i | 296 (100%) | 65 (100%) | 114 (100%) | 495 (100%) |
| 1j | 519 (100%) | 139 (100%) | 294 (100%) | Above 1 µm |
| 2d | 177 (28%) | 202 (100%) | Powyżej 1 µm | Above 1 µm |
| 2g | 36 (15%), 116 (85%) | 178 (100%) | 95 (100%) | Above 1 µm |
| 3a | 105 (100%) | 120 (78%) | 86 (100%) | Above 1 µm |
| 3c | 100 (39%), 319 (61%) | 130 (100%) | 82 (100%) | 349 (100%) |
| 3d | 93 (38%), 277 (62%) | 109 (100%) | 77 (100%) | Above 1 µm |
| 3e | 158 (15%), 406 (85%) | 82 (22%), 125 (78%) | 96 (100%) | 377 (58%), 840 (42%) |
| 3j | 630 (100%) | 209 (100%) | 86 (100%) | 635 (100%) |
| 4d | 602 (100%) | 196 (100%) | 80 (100%) | 249 (46%), 748(54%) |
| 4g | 128 (52%), 380 (48%) | 150 (100%) | 198 (100%) | 175 (100%) |
| 5b | 490 (30%), 770 (70%) | 71 (100%) | 86 (100%) | 215 (25%), 743 (75%) |
| 5g | 123 (100%) | 130 (18%), 346 (82%) | 93 (100%) | Above 1 µm |
| 5h | 105 (100%) | 118 (100%) | 160 (100%) | 222 (100%) |


3. Experimental
3.1. General
3.2. The Synthesis of Iron Oxide Nanoparticles Coated with Silica
3.3. The Functionalization of Silica Coated Nanoparticles with (3-aminopropyl)trimethoxysilane
3.4. The General Procedure Followed for the Synthesis of Aminophosphonate-Functionalized Magnetic Nanoparticles
3.5. The General Synthesis of Monoesters
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Allen, T.M.; Cullis, P.R. Drug delivery systems: Entering the mainstream. Science 2004, 303, 1818–1822. [Google Scholar] [CrossRef]
- Boyer, C.; Whittaker, M.R.; Bulmus, V.; Liu, J.; Davis, T.P. The design and utility of polymer-stabilized iron-oxide nanoparticles for nanomedicine applications. NPG Asia Mater. 2010, 2, 23–30. [Google Scholar] [CrossRef]
- Morteza, M.; Sant, S.; Wang, B.; Laurent, S.; Sen, T. Superparamagnetic iron oxide nanoparticles (SPIONs): Development, Surface modification and applications in chemotherapy. Adv. Drug Delivery Rev. 2011, 63, 24–46. [Google Scholar] [CrossRef]
- Coti, K.K.; Belowich, M.E.; Liong, M.; Ambrogio, M.W.; Lau, Y.A.; Khatib, H.A.; Zink, J.I.; Khashab, N.M.; Stoddart, J.F. Mechanised nanoparticles for drug delivery. Nanoscale 2009, 1, 16–39. [Google Scholar] [CrossRef]
- Mout, R.; Moyano, D.R.; Rana, S.; Rotello, V.M. Surface functionalization of nanoparticles for nanomedicine. Chem. Rev. 2012, 41, 2539–2544. [Google Scholar] [CrossRef]
- Cho, K.; Wang, X.; Nie, S.; Chen, Z.; Shin, D.M. Therapeutic nanoparticles for drug delivery in cancer. Clin. Cancer Res. 2008, 14, 1310–1316. [Google Scholar] [CrossRef]
- Davis, M.E.; Chen, Z.G.; Shin, D.M. Nanoparticle therapeutics: An emerging treatment modality for cancer. Nature Rev. Drug Discov. 2012, 7, 771–782. [Google Scholar]
- Wang, A.Z.; Langer, L.; Farokhzad, O.C. Nanoparticle delivery of cancer drugs. Ann. Rev. Med. 2012, 63, 185–198. [Google Scholar] [CrossRef]
- Wang, D.; Tejerina, B.; Lagzi, I.; Kowalczyk, B.; Grzybowski, B. Bridging interactions and selective nanoparticle aggregation mediated by monovalent cations. ACS Nano 2011, 5, 530–536. [Google Scholar] [CrossRef]
- Kafarski, P.; Lejczak, B. Aminophosphonic acids of potential medical importance. Curr. Med. Chem.–Anti-Cancer Agents 2001, 1, 301–312. [Google Scholar] [CrossRef]
- Mucha, A.; Kafarski, P.; Berlicki, Ł. Remarkable potential of the -aminophosphonate/phosphinate structural motif in medicinal chemistry. J. Med. Chem. 2011, 54, 5955–5980. [Google Scholar] [CrossRef]
- Kiss, T.; Lazar, I.; Kafarski, P. Chelating tendencies of bioactive aminophosphonates. Metal-Based Drugs 1994, 1, 247–264. [Google Scholar] [CrossRef]
- Massart, R. Preparation of aqueous magnetic liquids in alkaline and acidic media. IEEE Trans. Magn. 1981, MAG-17, 1247–1248. [Google Scholar] [CrossRef]
- Liu, X.; Ma, Z.; Xing, J.; Liu, H. Preparation and characterization of amino–silane modified superparamagnetic silica nanospheres. J. Magn. Mag. Mat. 2004, 270, 1–6. [Google Scholar] [CrossRef]
- Tyka, R. Novel Synthesis of α-Aminophosphonic Acids. Tetrahedron Lett. 1970, 677–680. [Google Scholar] [CrossRef]
- Netto, C.G.C.M.; Andrade, L.H.; Toma, E.H. Enantioselective transesterification catalysis by Candida antarctica lipase immobilized on superparamagnetic nanoparticles. Tetrahedron: Asymmetry 2009, 20, 2299–2304. [Google Scholar] [CrossRef]
- Benyettou, F.; Lalatonne, Y.; Chebbi, I.; Di Benedetto, M.; Serfay, J.-M.; Lecouvey, M.; Motte, L. A multimodal magnetic resonance imaging nanoplatform for cancer theranostics. PhysChemChemPhys 2011, 13, 10020–10027. [Google Scholar]
- Sample Availability: Samples of the nanoparticles are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Siemieniec, J.; Kafarski, P.; Plucinski, P. Hydrophosphonylation of Nanoparticle Schiff Bases as a Mean for Preparation of Aminophosphonate-Functionalized Nanoparticles. Molecules 2013, 18, 8473-8484. https://doi.org/10.3390/molecules18078473
Siemieniec J, Kafarski P, Plucinski P. Hydrophosphonylation of Nanoparticle Schiff Bases as a Mean for Preparation of Aminophosphonate-Functionalized Nanoparticles. Molecules. 2013; 18(7):8473-8484. https://doi.org/10.3390/molecules18078473
Chicago/Turabian StyleSiemieniec, Justyna, Pawel Kafarski, and Pawel Plucinski. 2013. "Hydrophosphonylation of Nanoparticle Schiff Bases as a Mean for Preparation of Aminophosphonate-Functionalized Nanoparticles" Molecules 18, no. 7: 8473-8484. https://doi.org/10.3390/molecules18078473




