Antibacterial and Herbicidal Activity of Ring-Substituted 3-Hydroxynaphthalene-2-carboxanilides †
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
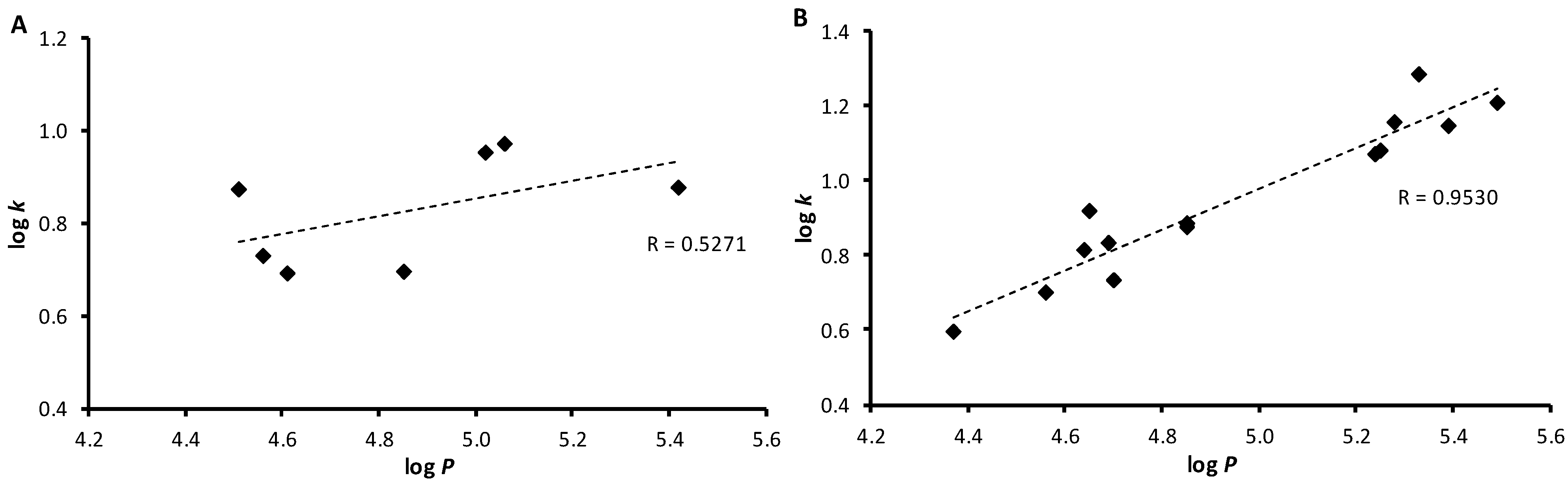
2.2. Lipophilicities
2.3. Inhibition of Photosynthetic Electron Transport (PET) in Spinach Chloroplasts
 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Comp. | R | log k | log P a | σ a | [μmol/L] | |||||||
| PET IC50 | MIC | LD50 | ||||||||||
| SA | MRSA 63718 | MRSA 630 | MRSA 3202 | MM | MK | |||||||
| 1 | H | 0.6310 | 4.52 | 0 | b | >972 | >972 | >972 | >972 | 122 | 972 | – |
| 2a | 2-OCH3 | 0.6916 | 4.61 | −0.28 | 59.5 | 55.0 | 55.0 | 55.0 | 55.0 | 873 | 218 | >30 |
| 2b | 3-OCH3 | 0.6971 | 4.56 | 0.12 | 53.4 | >873 | >873 | >873 | >873 | >873 | 54.6 | >30 |
| 2c | 4-OCH3 | 0.5951 | 4.37 | −0.27 | b | >873 | >873 | >873 | >873 | >873 | 873 | – |
| 3a | 2-CH3 | 0.6936 | 4.85 | −0.17 | b | >923 | >923 | >923 | 462 | 462 | 115 | – |
| 3b | 3-CH3 | 0.8831 | 4.85 | −0.07 | b | >923 | >923 | >923 | 462 | 923 | 57.7 | >30 |
| 3c | 4-CH3 | 0.8753 | 4.85 | −0.17 | b | >923 | >923 | 462 | 231 | >923 | 923 | – |
| 4a | 2-F | 0.7303 | 4.56 | 0.06 | b | >910 | >910 | 455 | 228 | 28.4 | 56.9 | >30 |
| 4b | 3-F | 0.8296 | 4.69 | 0.34 | b | >910 | >910 | 455 | 228 | 114 | 114 | – |
| 4c | 4-F | 0.7317 | 4.70 | 0.06 | b | >910 | >910 | 455 | 228 | 910 | 114 | – |
| 5a | 2-Cl | 0.9509 | 5.02 | 0.22 | b | >860 | >860 | >860 | 215 | 860 | 860 | – |
| 5b | 3-Cl | 1.0796 | 5.25 | 0.37 | b | >860 | >860 | 430 | 215 | >860 | 860 | – |
| 5c | 4-Cl | 1.0687 | 5.24 | 0.23 | b | >860 | >860 | >860 | >860 | >860 | 430 | – |
| 6a | 2-Br | 0.9715 | 5.06 | 0.22 | 43.2 | >748 | >748 | >748 | >748 | >748 | 748 | – |
| 6b | 3-Br | 1.1536 | 5.39 | 0.39 | b | >748 | >748 | >748 | 187 | >748 | 748 | – |
| 6c | 4-Br | 1.1459 | 5.28 | 0.23 | b | >748 | >748 | >748 | 187 | >748 | 374 | – |
| 7a | 2-CF3 | 0.8762 | 5.42 | 0.51 | 105.2 | >773 | >773 | >773 | 97 | 193 | 193 | 28.6 ± 0.5 |
| 7b | 3-CF3 | 1.2053 | 5.49 | 0.43 | b | >748 | >748 | 374 | 187 | >748 | 748 | – |
| 7c | 4-CF3 | 1.2835 | 5.33 | 0.51 | b | >748 | >748 | 374 | 187 | >748 | 187 | – |
| 8a | 2-NO2 | 0.8710 | 4.51 | 0.77 | 106.6 | >830 | >830 | 415 | 415 | 830 | 830 | – |
| 8b | 3-NO2 | 0.8143 | 4.64 | 0.71 | 16.9 | >830 | >830 | >830 | >830 | >830 | 415 | 2.5 ± 0.9 |
| 8c | 4-NO2 | 0.9175 | 4.65 | 0.78 | 187.5 | >830 | >830 | >830 | >830 | >830 | 13.0 | <0.37 |
| DCMU | – | – | – | 1.9 | – | – | – | – | – | – | – | |
| APC | – | – | – | 5.7 | >45.8 | >45.8 | >45.8 | – | – | – | ||
| INH | – | – | – | – | 467 | 29.2 | – | |||||

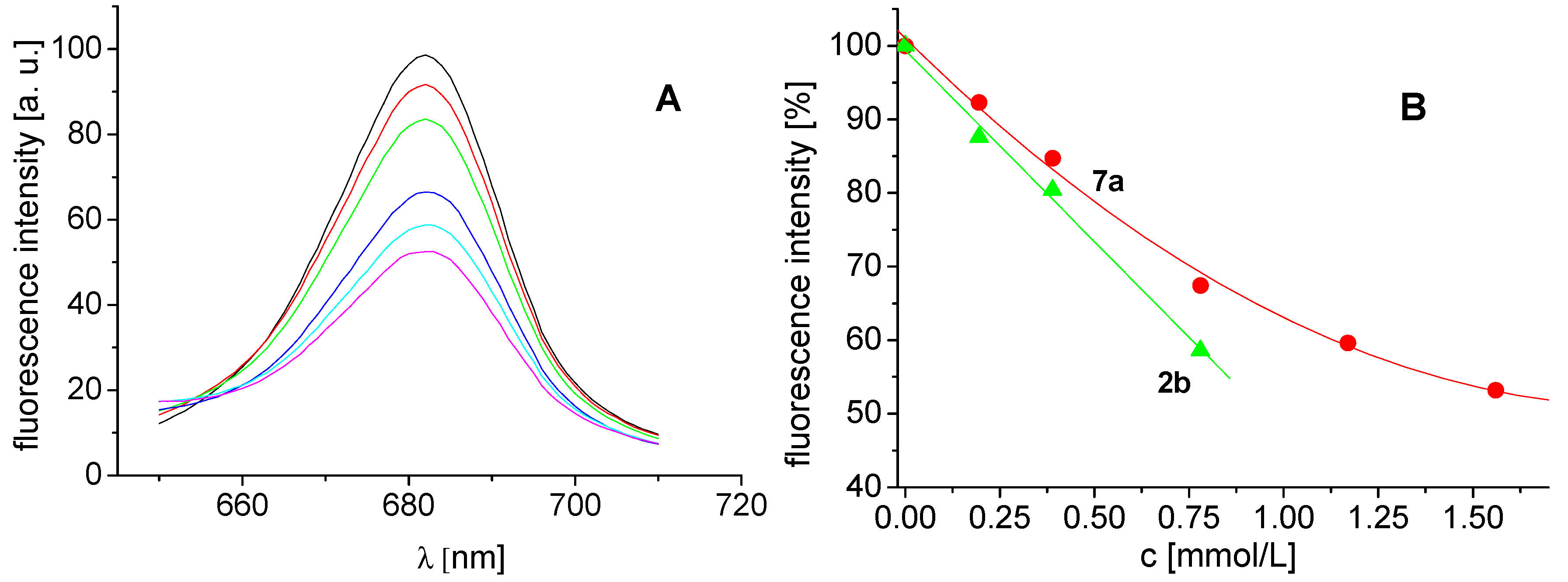
2.4. In Vitro Antibacterial Susceptibility Testing
2.5. In Vitro Antimycobacterial Evaluation
2.6. In Vitro Cytotoxicity Assay
3. Experimental
3.1. General
3.2. Synthesis
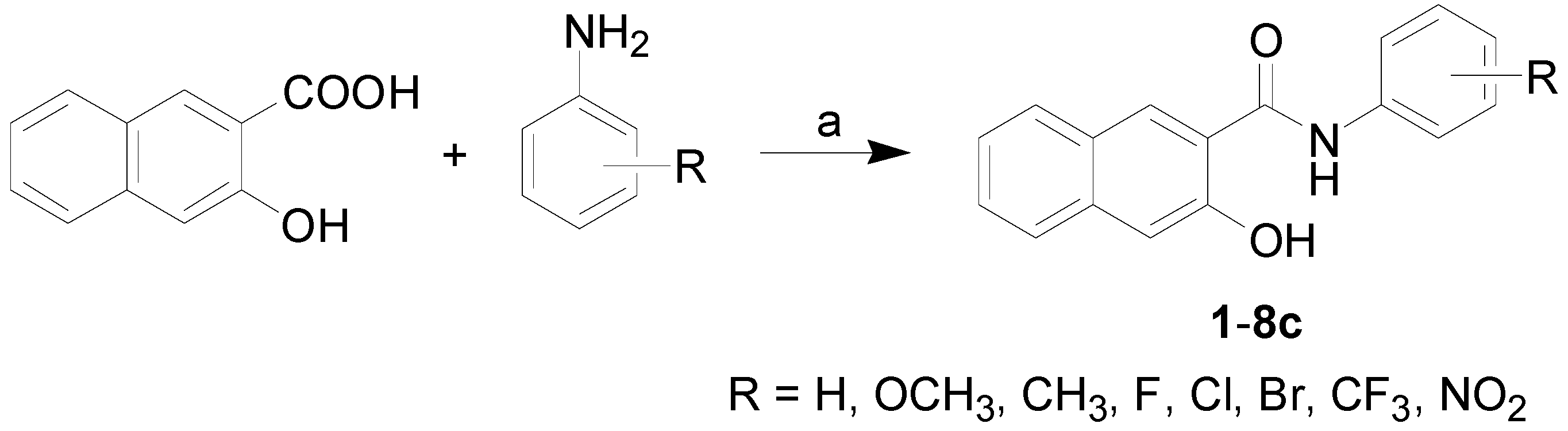
3.2.1. General Procedure for Synthesis of Carboxamide Derivatives 1–8c
3.3. Lipophilicity Determination by HPLC (Capacity Factor k/Calculated log k)
3.4. Study of Inhibition of Photosynthetic Electron Transport (PET) in Spinach Chloroplasts
3.5. Study of Chlorophyll a Fluorescence in Spinach Chloroplasts
3.6. In Vitro Antibacterial Susceptibility Testing
3.7. In Vitro Antimycobacterial Evaluation
3.8. In Vitro Cytotoxicity Assay
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Baquero, F.; Coque, T.M.; de la Cruz, F. Ecology and evolution as targets: the need for novel eco-evo drugs and strategies to fight antibiotic resistance. Antimicrob. Agents Chemother. 2011, 55, 3649–3660. [Google Scholar] [CrossRef] [Green Version]
- Acharya, N.; Varshney, U. Biochemical properties of single-stranded DNA-binding protein from Mycobacterium smegmatis, a fast-growing Mycobacterium and its physical and functional interaction with uracil DNA glycosylases. J. Mol. Biol. 2002, 318, 1251–1264. [Google Scholar] [CrossRef]
- Broussard, G.W.; Ennis, D.G. Mycobacterium marinum produces long-term chronic infections in medaka: A new animal model for studying human tuberculosis. Comp. Biochem. Phys. C 2007, 145, 45–54. [Google Scholar]
- Matveychuk, A.; Fuks, L.; Priess, R.; Hahim, I.; Shitrit, D. Clinical and radiological features of Mycobacterium kansasii and other NTM infections. Resp. Med. 2012, 106, 1472–1477. [Google Scholar] [CrossRef]
- Laughon, E.L. New tuberculosis drugs in development. Curr. Top. Med. Chem. 2007, 7, 463–473. [Google Scholar] [CrossRef]
- Levy, S.B. Antibiotic resistance-the problem intensifies. Adv. Drug. Delivery Rev. 2005, 57, 1446–1450. [Google Scholar] [CrossRef]
- Kallen, A.J.; Mu, Y.; Bulens, S.; Reingold, A.; Petit, S.; Gershman, K.; Ray, S.M.; Harrison, L.H.; Lynfield, R.; Dumyati, G.; et al. Health care-associated invasive MRSA infections, 2005–2008. JAMA 2010, 304, 641–647. [Google Scholar] [CrossRef]
- Liu, C.; Bayer, A.; Cosgrove, S.E.; Daum, R.S.; Fridkin, S.K.; Gorwitz, R.J.; Kaplan, S.L.; Karchmer, A.W.; Levine, D.P.; Murray, B.E.; et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin. Infect. Dis. 2011, 52, 285–292. [Google Scholar] [CrossRef]
- Shaner, D.L. Herbicide safety relative to common targets in plants and mammals. Pest. Manag. Sci. 2004, 60, 17–24. [Google Scholar] [CrossRef]
- Delaney, J.; Clarke, E.; Hughes, D.; Rice, M. Modern agrochemical research: A missed opportunity for drug discovery? Drug Discov. Today 2006, 11, 839–845. [Google Scholar] [CrossRef]
- Duke, S.O. Herbicide and pharmaceutical relationships. Weed Sci. 2010, 58, 334–339. [Google Scholar] [CrossRef]
- Kralova, K.; Sersen, F.; Cizmarik, J. Inhibitory effect of piperidinoethylesters of alkoxyphenylcarbamic acids on photosynthesis. Gen. Physiol. Biophys. 1992, 11, 261–267. [Google Scholar]
- Kralova, K.; Bujdakova, H.; Kuchta, T.; Loos, D. Correlation between biological activity and the structure of 6-amino-2-R-thiobenzothiazoles. Anti-yeast activity and inhibition of photochemical activity of chloroplasts. Pharmazie 1994, 49, 460–461. [Google Scholar]
- Kralova, K.; Kallova, J.; Loos, D.; Devinsky, F. Correlation between biological activity and the structure of N,N'-bis(alkyldimethyl)-1,6-hexanediammonium dibromides. Antibacterial activity and inhibition of photochemical activity of chloroplasts. Pharmazie 1994, 49, 857–858. [Google Scholar]
- Kralova, K.; Bujdakova, H.; Cizmarik, J. Antifungal and antialgal activity of piperidinopropyl esters of alkoxy substituted phenylcarbamic acids. Pharmazie 1995, 50, 440–441. [Google Scholar]
- Musiol, R.; Tabak, D.; Niedbala, H.; Podeszwa, B.; Jampilek, J.; Kralova, K.; Dohnal, J.; Finster, J.; Mencel, A.; Polanski, J. Investigating biological activity spectrum for novel quinoline analogues 2: Hydroxyquinolinecarboxamides with photosynthesis inhibiting activity. Bioorg. Med. Chem. 2008, 16, 4490–4499. [Google Scholar] [CrossRef]
- Imramovsky, A.; Vinsova, J.; Monreal-Ferriz, J.; Buchta, V.; Jampilek, J. Salicylanilide esters of N-protected amino acids as novel antimicrobial agents. Bioorg. Med. Chem. Lett. 2009, 19, 348–351. [Google Scholar] [CrossRef]
- Otevrel, J.; Mandelova, Z.; Pesko, M.; Guo, J.; Kralova, K.; Sersen, F.; Vejsova, M.; Kalinowski, D.; Kovacevic, Z.; Coffey, A.; et al. Investigating the spectrum of biological activity of ring-substituted salicylanilides and carbamoylphenylcarbamates. Molecules 2010, 15, 8122–8142. [Google Scholar] [CrossRef]
- Imramovsky, A.; Pesko, M.; Kralova, K.; Vejsova, M.; Stolarikova, J.; Vinsova, J.; Jampilek, J. Investigating spectrum of biological activity of 4- and 5-chloro-2-hydroxy-N-[2-(arylamino)-1-alkyl-2-oxoethyl]benzamides. Molecules 2011, 16, 2414–2430. [Google Scholar] [CrossRef]
- Gonec, T.; Bobal, P.; Sujan, J.; Pesko, M.; Guo, J.; Kralova, K.; Pavlacka, L.; Vesely, L.; Kreckova, E.; Kos, J.; et al. Investigating the spectrum of biological activity of substituted quinoline-2-carboxamides and their isosteres. Molecules 2012, 17, 613–644. [Google Scholar] [CrossRef]
- Draber, W.; Tietjen, K.; Kluth, J.F.; Trebst, A. Herbicides in photosynthesis research. Angew. Chem. 1991, 3, 1621–1633. [Google Scholar]
- Tischer, W.; Strotmann, H. Relationship between inhibitor binding by chloroplasts and inhibition of photosynthetic electron-transport. Biochim. Biophys. Acta 1977, 460, 113–125. [Google Scholar] [CrossRef]
- Trebst, A.; Draber, W. Structure activity correlations of recent herbicides in photosynthetic reactions. In Advances in Pesticide Science; Greissbuehler, H., Ed.; Pergamon Press: Oxford, UK, 1979; pp. 223–234. [Google Scholar]
- Bowyer, J.R.; Camilleri, P.; Vermaas, W.F.J. Photosystem II and its interaction with herbicides. In Herbicides, Topics in Photosynthesis; Baker, N.R., Percival, M.P., Eds.; Elsevier: Amsterdam, The Netherlands, 1991; Volume 10, pp. 27–85. [Google Scholar]
- Koreyoshi, S.; Masato, M.; Tetsuji, I. (Yoshitomi Pharmaceutical Industries) Hydroxynaphthoic acid anilides, microbicides and slimicides. Japan. Kokai JP 52 076 430, 27 June 1977. [Google Scholar]
- Macielag, M.J.; Demers, J.P.; Fraga-Spano, S.A.; Hlasta, J.D.; Johnson, G.S.; Kanojia, M.R.; Russell, K.R.; Sui, Z.; Weidner-Wells, A.M.; Werblood, H.; et al. Substituted salicylanilides as inhibitors of two-component regulatory systems in bacteria. J. Med. Chem. 1998, 41, 2939–2945. [Google Scholar] [CrossRef]
- Kauppi, A.M.; Nordfelth, R.; Uvell, H.; Wolf-Watz, H.; Elofsson, M. Targeting bacterial virulence: Inhibitors of type III secretion in Yersinia. Chem. Biol. 2003, 10, 241–249. [Google Scholar] [CrossRef]
- Vinsova, J.; Imramovsky, A.; Buchta, V.; Ceckova, M.; Dolezal, M.; Staud, F.; Jampilek, J.; Kaustova, J. Salicylanilide acetates: Synthesis and antibacterial evaluation. Molecules 2007, 12, 1–12. [Google Scholar] [CrossRef]
- Imramovsky, A.; Vinsova, J.; Monreal-Ferriz, J.; Dolezal, R.; Jampilek, J.; Kaustova, J.; Kunc, F. New antituberculotics originated from salicylanilides with promising in vitro activity against atypical mycobacterial strains. Bioorg. Med. Chem. 2009, 17, 3572–3579. [Google Scholar] [CrossRef]
- Imramovsky, A.; Pesko, M.; Monreal-Ferriz, J.; Kralova, K.; Vinsova, J.; Jampilek, J. Photosynthesis-Inhibiting efficiency of 4-chloro-2-(chlorophenylcarbamoyl)phenyl alkylcarbamates. Bioorg. Med. Chem. Lett. 2011, 21, 4564–4567. [Google Scholar] [CrossRef]
- Kratky, M.; Vinsova, J. Salicylanilide ester prodrugs as potential antimicrobial agents – a review. Curr. Pharm. Des. 2011, 17, 3494–3505. [Google Scholar] [CrossRef]
- Kratky, M.; Vinsova, J.; Buchta, V. In vitro antibacterial and antifungal activity of salicylanilide benzoates. Sci. World J. 2012, 2012, 290628. [Google Scholar]
- Kratky, M.; Vinsova, J.; Novotna, E.; Mandikova, J.; Wsol, V.; Trejtnar, F.; Ulmann, V.; Stolarikova, J.; Fernandes, S.; Bhat, S.; et al. Salicylanilide derivatives block Mycobacterium tuberculosis through inhibition of isocitrate lyase and methionine aminopeptidase. Tuberculosis 2012, 92, 434–439. [Google Scholar] [CrossRef]
- Hartrodt, V.W.; Woohsmann, H. Ein Beitrag zur Darstellun von Alkalisalzen saurer Schwefelsäureester verschiedener Naphthol AS-Verbindungen. J. Prakt. Chem. 1965, 27, 99–107. [Google Scholar] [CrossRef]
- Tripathy, H.; Pradhan, D.G.; Dash, B.C.; Mahapatra, G.N. Synthesis of some new halogenated N-thiazolyl substituted hydroxy acid amides and their use as possible fungicides. Agric. Biol. Chem. 1973, 6, 1375–1383. [Google Scholar]
- Hitch, F.E.; Dahlen, A.M.; Friedrich, M.E. (E.I. du Pont de Nemours & Company). Fluorinated arylamides. US 1982661, 4 December 1934. [Google Scholar]
- Liechty, C.H.; Sequin, U.; Bold, G.; Furet, P.; Meyer, T.; Traxler, P. Salicylanilides as inhibitors of the protein tyrosine kinase epidermal growth factor receptor. Eur. J. Med. Chem. 2004, 39, 11–26. [Google Scholar] [CrossRef]
- Izawa, S. Acceptors and donors for chloroplast electron transpor. In Methods in Enzymology; Colowick, P., Kaplan, N.O., Eds.; Academic Press: London, UK, 1980; Volume 69, Part C; pp. 413–434. [Google Scholar]
- Kralova, K.; Sersen, F.; Pesko, M.; Klimesova, V.; Waisser, K. Photosynthesis-inhibiting effects of 2-benzylsulphanylbenzimidazoles in spinach chloroplasts. Chem. Pap. 2012, 66, 795–799. [Google Scholar] [CrossRef]
- Kralova, K.; Sersen, F.; Sidoova, E. Effect of 2-alkylthio-6-aminobenzothiazoles and their 6-N-substituted derivatives on photosynthesis inhibition in spinach chloroplasts. Gen. Phys. Biophys. 1993, 12, 421–427. [Google Scholar]
- Kralova, K.; Sersen, F.; Miletin, M.; Hartl, J. Inhibition of photosynthetic electron transport by some anilides of 2-alkylpyridine-4-carboxylic acids in spinach chloroplasts. Chem. Pap. 1998, 52, 52–55. [Google Scholar]
- Kralova, K.; Sersen, F.; Pesko, M.; Waisser, K.; Kubicova, L. 5-Bromo- and 3,5-dibromo-2-hydroxy-N-phenylbenzamides—Inhibitors of photosynthesis. Chem. Pap. 2013, 67. in press. [Google Scholar]
- Kralova, K.; Sersen, F.; Klimesova, V.; Waisser, K. 2-Alkylsulphanyl-4-pyridine-carbothioamides—Inhibitors of oxygen evolution in freshwater alga Chlorella vulgaris. Chem. Pap. 2011, 65, 909–912. [Google Scholar] [CrossRef]
- Atal, N.; Saradhi, P.P.; Mohanty, P. Inhibition of the chloroplast photochemical reactions by treatment of wheat seedlings with low concentrations of cadmium: Analysis of electron transport activities and changes in fluorescence yields. Plant Cell Physiol. 1995, 32, 943–951. [Google Scholar]
- Sersen, F.; Kralova, K.; Bumbalova, A. Action of mercury on the photosynthetic apparatus of spinach chloroplasts. Photosynthetica 1998, 35, 551–559. [Google Scholar] [CrossRef]
- Apostolova, E.L.; Ivanov, A.G. Influence of Triton X-100on the structure and functions of pea thylakoid membranes. J. Plant Physiol. 1995, 145, 239–244. [Google Scholar] [CrossRef]
- Kralova, K.; Sersen, F.; Kubicova, L.; Waisser, K. Inhibitory effects of substituted benzanilides on photosynthetic electron transport in spinach chloroplasts. Chem. Pap. 1999, 53, 328–331. [Google Scholar]
- Kubicova, L.; Kralova, K.; Sersen, F.; Gregor, J.; Waisser, K. Effects of substituted salicylanilides on the photosynthetic apparatus of spinach chloroplasts. Folia Pharm. Univ. Carol. 2000, 25, 89–96. [Google Scholar]
- Kollar, P.; Zavalova, V.; Barta, T.; Smejkal, K.; Hampl, A. Geranylated flavanone tomentodiplacone B inhibits proliferation of human monocytic leukaemia (THP-1) cells. Br. J. Pharmacol. 2011, 162, 1534–1541. [Google Scholar] [CrossRef]
- Fajkusova, D.; Pesko, M.; Keltosova, S.; Guo, J.; Oktabec, Z.; Vejsova, M.; Kollar, P.; Coffey, A.; Csollei, J.; Kralova, K.; Jampilek, J. Anti-infective and herbicidal activity of N-substituted 2-aminobenzothiazoles. Bioorg. Med. Chem. 2012, 20, 7059–7068. [Google Scholar] [CrossRef]
- Masarovicova, E.; Kralova, K. Approaches to Measuring Plant Photosynthesis Activity. In Handbook of Photosynthesis, 2nd ed.; Pessarakli, M., Ed.; Taylor & Francis Group: Boca Raton, FL, USA, 2005; pp. 617–656. [Google Scholar]
- Kralova, K.; Sersen, F.; Sidoova, E. Photosynthesis inhibition produced by 2-alkylthio-6-R-benzothiazoles. Chem. Pap. 1992, 46, 348–350. [Google Scholar]
- National Committee for Clinical Laboratory Standards, Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, 5th Edition ed; Approved Standard M7-A5; NCCLS: Wayne, PA, USA, 2000.
- National Committee for Clinical Laboratory Standards, Performance Standards for Antimicrobial Susceptibility Testing; 12th Informational Supplement M100-S12; NCCLS: Wayne, PA, USA, 2002.
- Schwalbe, R.; Steele-Moore, L.; Goodwin, A.C. Antimicrobial Susceptibility Testing Protocols. CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kos, J.; Zadrazilova, I.; Pesko, M.; Keltosova, S.; Tengler, J.; Gonec, T.; Bobal, P.; Kauerova, T.; Oravec, M.; Kollar, P.; et al. Antibacterial and Herbicidal Activity of Ring-Substituted 3-Hydroxynaphthalene-2-carboxanilides. Molecules 2013, 18, 7977-7997. https://doi.org/10.3390/molecules18077977
Kos J, Zadrazilova I, Pesko M, Keltosova S, Tengler J, Gonec T, Bobal P, Kauerova T, Oravec M, Kollar P, et al. Antibacterial and Herbicidal Activity of Ring-Substituted 3-Hydroxynaphthalene-2-carboxanilides. Molecules. 2013; 18(7):7977-7997. https://doi.org/10.3390/molecules18077977
Chicago/Turabian StyleKos, Jiri, Iveta Zadrazilova, Matus Pesko, Stanislava Keltosova, Jan Tengler, Tomas Gonec, Pavel Bobal, Tereza Kauerova, Michal Oravec, Peter Kollar, and et al. 2013. "Antibacterial and Herbicidal Activity of Ring-Substituted 3-Hydroxynaphthalene-2-carboxanilides" Molecules 18, no. 7: 7977-7997. https://doi.org/10.3390/molecules18077977






