Optimal Binary Solvent Extraction System for Phenolic Antioxidants from Mengkudu (Morinda citrifolia) Fruit
Abstract
:1. Introduction
2. Results and Discussion
2.1. Model Fitting
Y = β0 + ∑ βiXi + ∑ βiiXi2+ ∑ ∑βijXiXj
i = 1 i = 1
| Independent Variables | Regression coefficients | ||||
|---|---|---|---|---|---|
| TPC a | TFC b | ||||
| Full Quadratic Model | Reduced Quadratic Model | Full Quadratic Model | Reduced Quadratic Model | ||
| Intercept, X0 | 930.00 | 923.18 | 513.97 | 513.77 | |
| Linear | |||||
| X1, Ethanol concentration | −32.05 * | −32.05 * | 75.63 * | 75.63 * | |
| X2, Extraction time | −8.52 | - | −8.45 | −8.45 | |
| X3, Extraction temperature | 46.87* | 46.87* | 43.96 * | 43.96 * | |
| Quadratic | |||||
| X12 | −56.47 * | −55.64 * | −57.54 * | −57.51 * | |
| X22 | 0.79 | - | 19.75 * | 19.78 * | |
| X32 | −9.89 | - | −0.24 | - | |
| Interaction | |||||
| X12 | −11.71 | - | 4.08 | - | |
| X13 | −11.12 | - | −21.17 | −21.17 * | |
| X23 | 25.55 | - | −8.15 | - | |
| Model | |||||
| F value | 6.02 | 17.05 | 23.87 | 43.14 | |
| p value | 0.0067 | <0.0001 | <0.0001 | <0.0001 | |
| Lack of fit | |||||
| F value | 0.99 | 0.93 | 2.27 | 1.61 | |
| p value | 0.5177 | 0.5840 | 0.2239 | 0.3391 | |
| Mean | 879.62 | 879.62 | 480.49 | 480.49 | |
| Standard deviation | 42.79 | 41.82 | 27.89 | 25.35 | |
| R2 | 0.8576 | 0.7732 | 0.9598 | 0.9557 | |
| Adjusted R2 | 0.7151 | 0.7279 | 0.9196 | 0.9335 | |
| CV | 4.86 | 4.75 | 5.80 | 5.28 | |
| Independent Variables | Regression coefficients | ||||
|---|---|---|---|---|---|
| ABTS a | DPPH b | ||||
| Full Quadratic Model | Reduced Quadratic Model | Full Quadratic Model | Reduced Quadratic Model | ||
| Intercept, X0 | 746.24 | 746.24 | 2168.70 | 2168.70 | |
| Linear | |||||
| X1, Ethanol concentration | 24.45 * | 24.45 * | 198.52 * | 198.52 * | |
| X2, Extraction time | 1.33 | 1.33 | −5.04 | -5.04 | |
| X3, Extraction temperature | −3.17 | −3.17 | 61.83 * | 61.83 * | |
| Quadratic | |||||
| X12 | 14.89 * | 14.89 * | 47.96 * | 47.96 * | |
| X22 | 5.92 * | 5.92 * | −57.80 * | −57.80 * | |
| X32 | 14.62 * | 14.62 * | −55.31 * | −55.31 * | |
| Interaction | |||||
| X12 | 0.31 | - | −8.09 | - | |
| X13 | −0.10 | - | −55.68 * | −55.68 * | |
| X23 | 2.29 | - | −3.67 | - | |
| Model | |||||
| F value | 22.97 | 42.84 | 18.90 | 29.21 | |
| p value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| Lack of fit | |||||
| F value | 0.80 | 0.57 | 3.12 | 2.27 | |
| p value | 0.6031 | 0.7703 | 0.1466 | 0.2233 | |
| Mean | 771.94 | 771.94 | 2119.01 | 2119.01 | |
| Standard deviation | 8.29 | 7.43 | 66.39 | 60.53 | |
| R2 | 0.9583 | 0.9554 | 0.9497 | 0.9489 | |
| Adjusted R2 | 0.9166 | 0.9331 | 0.8995 | 0.9165 | |
| CV | 1.07 | 0.96 | 3.13 | 2.86 | |
| r | TPC | TFC | ABTS |
|---|---|---|---|
| TFC | 0.396 | ||
| ABTS | −0.637* | 0.147 | |
| DPPH | −0.228 | 0.522 * | 0.488 * |
2.2. Effects of Ethanol Concentration, Extraction time and Extraction Temperature
2.2.1. Phenolic Compounds (TPC and TFC)
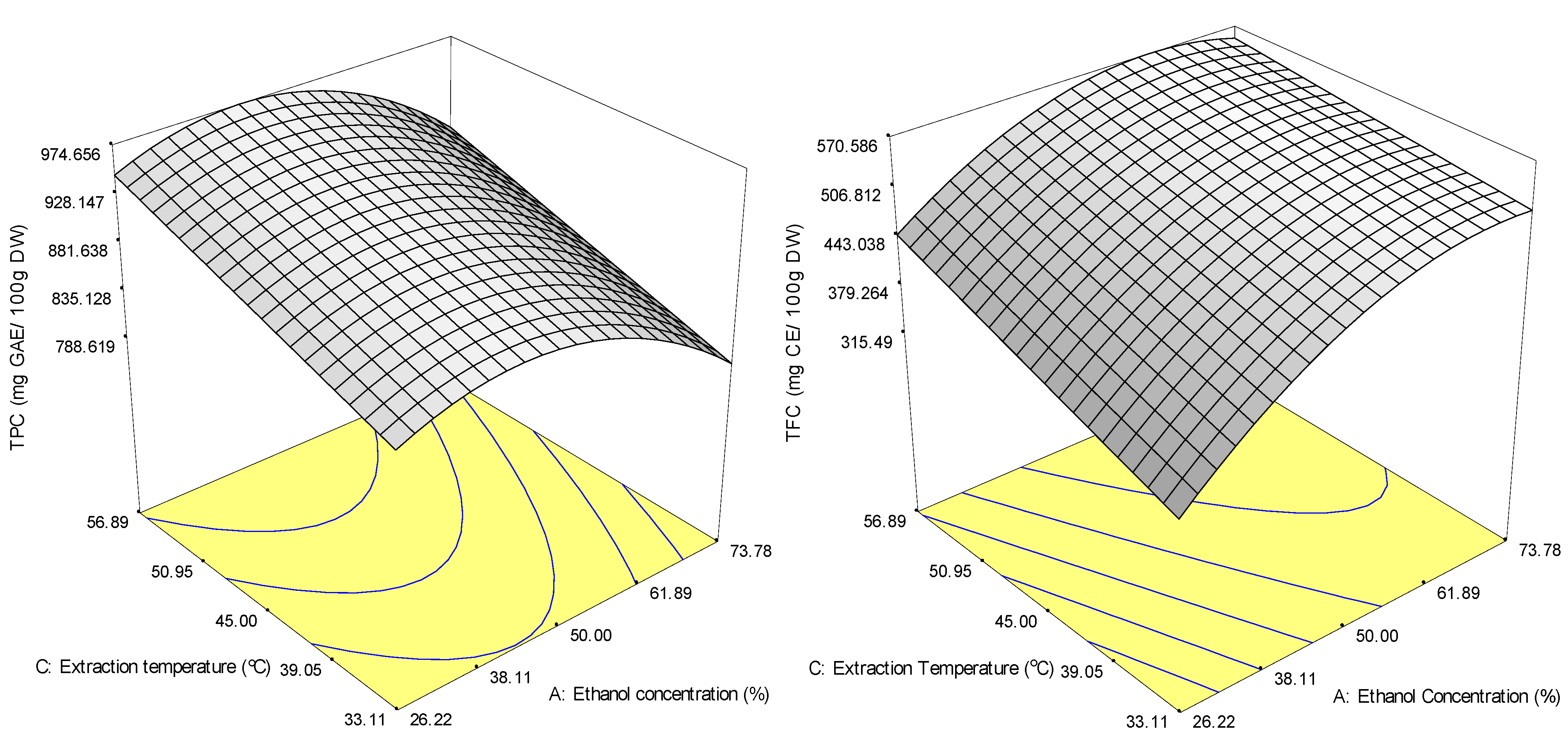
2.2.2. Antioxidant Capacity (ABTS and DPPH Radical-Scavenging Capacities)
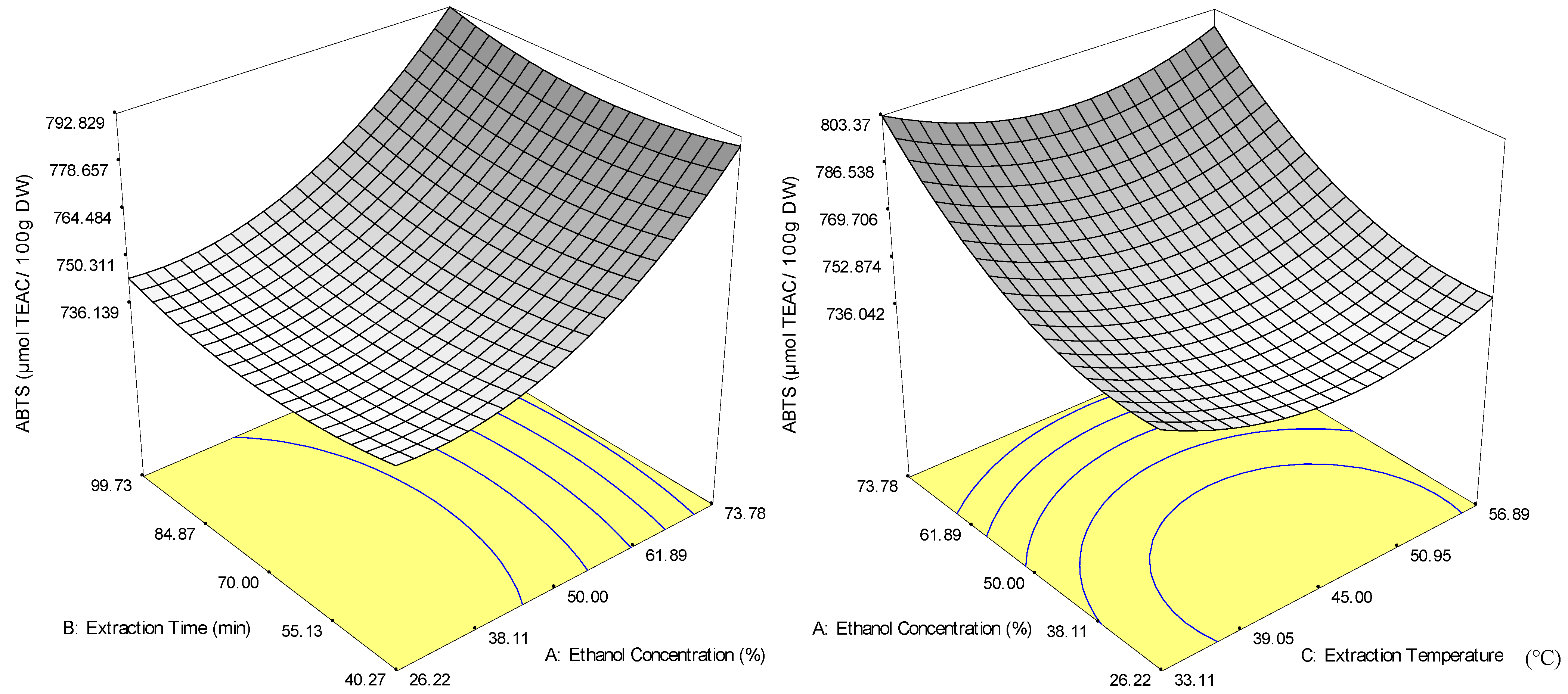
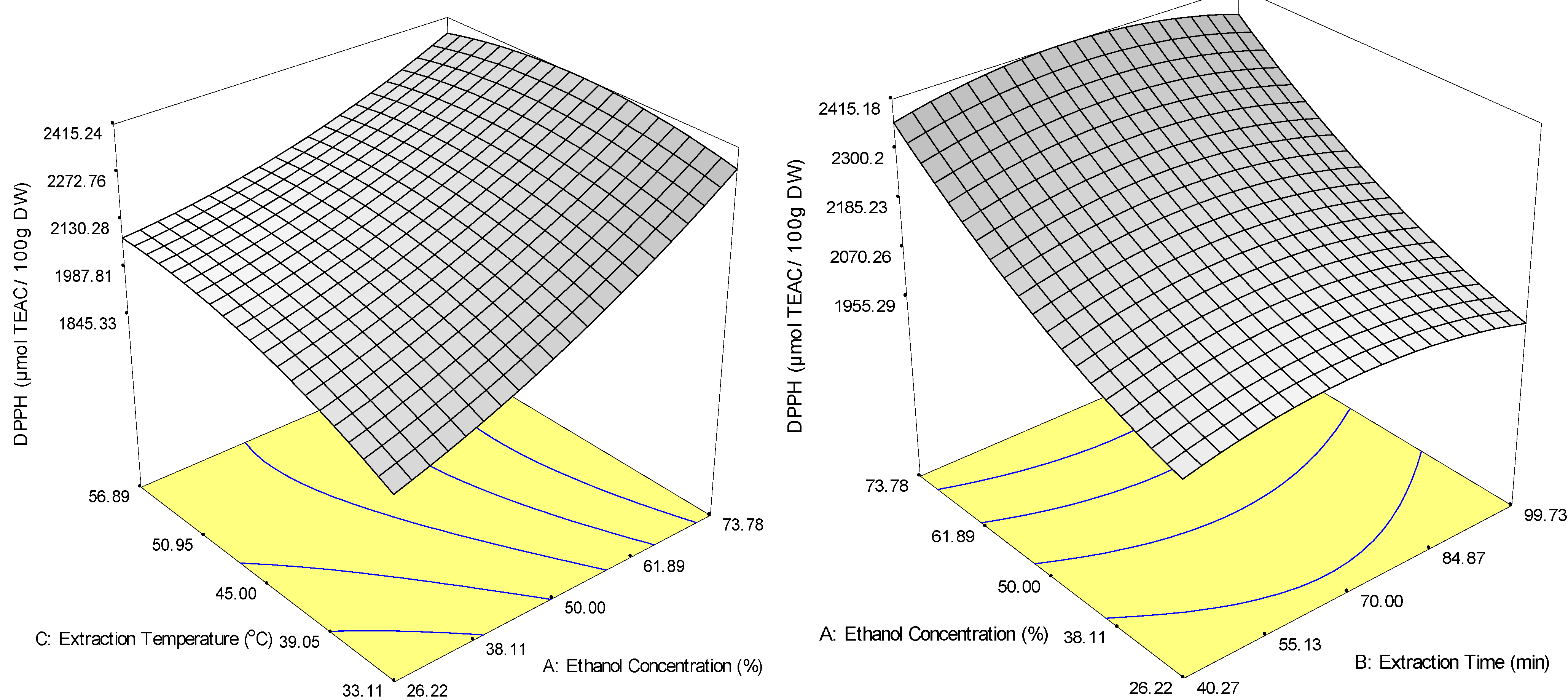
2.3. Optimisation and Model Verification
| Independent variables | Dependent variables (Responses) | Optimum value | |||
|---|---|---|---|---|---|
| X1 | X2 | X3 | Experimental b | Predicted | |
| 74.59 | 40.27 | 56.87 | Y1 | 881.57 ± 17.74 | 883.55 |
| Y2 | 552.53 ± 34.16 | 583.41 | |||
| Y3 | 799.20 ± 2.97 | 801.12 | |||
| Y4 | 2317.01 ± 18.13 | 2311.27 | |||
2.4. Quantitative Analysis of Quercetin and Rutin in Optimised extract.
3. Experimental
3.1. Chemicals
3.2. Plant Materials
3.3. Extraction of Plant Material
3.4. Experimental Design
3.4.1. Response Surface Procedures
| Independent variables | Units | Coded levels | ||||
|---|---|---|---|---|---|---|
| −α | −1 | 0 | +1 | +α | ||
| Actual levels | ||||||
| Ethanol concentration, X1 | % v/v | 10 | 26.22 | 50 | 73.78 | 90 |
| Extraction time, X2 | min | 20 | 40.27 | 70 | 99.73 | 120 |
| Extraction temperature, X3 | °C | 25 | 33.11 | 45 | 56.89 | 65 |
3.4.2. Determination of the Optimum Conditions and Validation of the Model

3.5. Analysis of the Response Variables
3.5.1. Total Phenolic Content (TPC)
3.5.2. Total Flavonoid Content (TFC)
3.5.3. ABTS Radical-Scavenging Capacity
3.5.4. DPPH Radical-Scavenging Capacity
3.5.5. Instrumentation
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Thitilertdecha, N.; Teerawutgulrag, A.; Rakariyatham, N. Antioxidant and antibacterial activities of Nephelium lappaceum L. extracts. LWT Food Sci. Technol. 2008, 41, 2029–2035. [Google Scholar] [CrossRef]
- Liu, X.; Jia, J.; Yang, L.; Yang, F.; Ge, H.; Zhao, C.; Zhang, L.; Zu, Y. Evaluation of Antioxidant Activities of Aqueous Extracts and Fractionation of Different Parts of Elsholtzia ciliata. Molecules 2012, 17, 5430–5441. [Google Scholar]
- Zin, Z. M.; Abdul-Hamid, A.; Osman, A. Antioxidative activity of extracts from Mengkudu (Morinda citrifolia L.) root, fruit and leaf. Food Chem. 2002, 78, 227–231. [Google Scholar] [CrossRef]
- Akihisa, T.; Matsumoto, K.; Tokuda, H.; Yasukawa, K.; Seino, K.-I.; Nakamoto, K.; Kuninaga, H.; Suzuki, T.; Kimura, Y. Anti-inflammatory and Potential Cancer Chemopreventive Constituents of the Fruits of Morinda citrifolia (Noni). J. Nat. Prod. 2007, 70, 754–757. [Google Scholar] [CrossRef]
- Kamiya, K.; Tanaka, Y.; Endang, H.; Umar, M.; Satake, T. Chemical Constituents of Morinda citrifolia Fruits Inhibit Copper-Induced Low-Density Lipoprotein Oxidation. J. Agric. Food Chem. 2004, 52, 5843–5848. [Google Scholar] [CrossRef]
- Serrano, J.; Goñi, I.; Saura-Calixto, F. Food antioxidant capacity determined by chemical methods may underestimate the physiological antioxidant capacity. Food Res. Int. 2007, 40, 15–21. [Google Scholar] [CrossRef]
- Sun-Waterhouse, D.; Wen, I.; Wibisono, R.; Melton, L.D.; Wadhwa, S. Evaluation of the extraction efficiency for polyphenol extracts from by-products of green kiwifruit juicing. Int. J. Food Sci. Technol. 2009, 44, 2644–2652. [Google Scholar] [CrossRef]
- Liyana-Pathirana, C.; Shahidi, F. Optimization of extraction of phenolic compounds from wheat using response surface methodology. Food Chem. 2005, 93, 47–56. [Google Scholar] [CrossRef]
- Wang, J.; Sun, B.; Cao, Y.; Tian, Y.; Li, X. Optimisation of ultrasound-assisted extraction of phenolic compounds from wheat bran. Food Chem. 2008, 106, 804–810. [Google Scholar] [CrossRef]
- Karacabey, E.; Mazza, G. Optimization of Solid-Liquid Extraction of Resveratrol and Other Phenolic Compounds from Milled Grape Canes (Vitis vinifera). J. Agric. Food Chem. 2008, 56, 6318–6325. [Google Scholar] [CrossRef]
- Xie, J.-H.; Xie, M.-Y.; Shen, M.-Y.; Nie, S.-P.; Li, C.; Wang, Y.-X. Optimisation of microwave-assisted extraction of polysaccharides from Cyclocarya paliurus (Batal.) Iljinskaja using response surface methodology. J. Sci. Food Agric. 2010; 90, 1353–1360. [Google Scholar]
- Liu, T.; Ma, C.; Yang, L.; Wang, W.; Sui, X.; Zhao, C.; Zu, Y. Optimization of Shikonin Homogenate Extraction from Arnebia euchroma Using Response Surface Methodology. Molecules 2013, 18, 466–481. [Google Scholar]
- Ekren, O.; Ekren, B.Y.; Ozerdem, B. Break-even analysis and size optimization of a PV/wind hybrid energy conversion system with battery storage—A case study. Appl. Energy 2009, 86, 1043–1054. [Google Scholar] [CrossRef]
- Zhang, Q.-A.; Zhang, Z.-Q.; Yue, X.-F.; Fan, X.-H.; Li, T.; Chen, S.-F. Response surface optimization of ultrasound-assisted oil extraction from autoclaved almond powder. Food Chem. 2009, 116, 513–518. [Google Scholar] [CrossRef]
- Ji, Y.-B.; Dong, F.; Ma, D.-B.; Miao, J.; Jin, L.-N.; Liu, Z.-F.; Zhang, L.-W. Optimizing the Extraction of Anti-tumor Polysaccharides from the Fruit of Capparis spionosa L. by Response Surface Methodology. Molecules 2012, 17, 7323–7335. [Google Scholar]
- Tabart, J.; Kevers, C.; Pincemail, J.; Defraigne, J.-O.; Dommes, J. Comparative antioxidant capacities of phenolic compounds measured by various tests. Food Chem. 2009, 113, 1226–1233. [Google Scholar] [CrossRef]
- Sin, H.N.; Yusof, S.; Sheikh Abdul Hamid, N.; Rahman, R.A. Optimization of enzymatic clarification of sapodilla juice using response surface methodology. J. Food Eng. 2006, 73, 313–319. [Google Scholar] [CrossRef]
- Juntachote, T.; Berghofer, E.; Bauer, F.; Siebenhandl, S. The application of response surface methodology to the production of phenolic extracts of lemon grass, galangal, holy basil and rosemary. Int. J. Food Sci. Technol. 2006, 41, 121–133. [Google Scholar] [CrossRef]
- Thoo, Y.Y.; Ho, S. K.; Liang, J.Y.; Ho, C.W.; Tan, C.P. Effects of binary solvent extraction system, extraction time and extraction temperature on phenolic antioxidants and antioxidant capacity from mengkudu (Morinda citrifolia). Food Chem. 2010, 120, 290–295. [Google Scholar] [CrossRef]
- Pazos, M.; Andersen, M.L.; Medina, I.; Skibsted, L.H. Efficiency of Natural Phenolic Compounds Regenerating α-Tocopherol from α-Tocopheroxyl Radical. J. Agric. Food Chem. 2007, 55, 3661–3666. [Google Scholar] [CrossRef]
- Yusri, N.; Chan, K.; Iqbal, S.; Ismail, M. Phenolic Content and Antioxidant Activity of Hibiscus cannabinus L. Seed Extracts after Sequential Solvent Extraction. Molecules 2012, 17, 12612–12621. [Google Scholar] [CrossRef]
- Durling, N.E.; Catchpole, O.J.; Grey, J.B.; Webby, R.F.; Mitchell, K.A.; Foo, L.Y.; Perry, N.B. Extraction of phenolics and essential oil from dried sage (Salvia officinalis) using ethanol–water mixtures. Food Chem. 2007, 101, 1417–1424. [Google Scholar] [CrossRef]
- Lim, Y.Y.; Murtijaya, J. Antioxidant properties of Phyllanthus amarus extracts as affected by different drying methods. LWT Food Sci. Technol. 2007, 40, 1664–1669. [Google Scholar] [CrossRef]
- Silva, E.M.; Rogez, H.; Larondelle, Y. Optimization of extraction of phenolics from Inga edulis leaves using response surface methodology. Sep. Purif. Technol. 2007, 55, 381–387. [Google Scholar] [CrossRef]
- Mohamed, H.; Ons, M.; Yosra, E.-T.; Rayda, S.; Neji, G.; Moncef, N. Chemical composition and antioxidant and radical-scavenging activities of Periploca laevigata root bark extracts. J. Sci. Food Agric. 2009, 89, 897–905. [Google Scholar] [CrossRef]
- Musa, K.; Abdullah, A.; Jusoh, K.; Subramaniam, V. Antioxidant Activity of Pink-Flesh Guava (Psidium guajava L.): Effect of Extraction Techniques and Solvents. Food Anal. Methods 2011, 4, 100–107. [Google Scholar]
- Singh, A.; Sabally, K.; Kubow, S.; Donnelly, D.J.; Gariepy, Y.; Orsat, V.; Raghavan, G.S.V. Microwave-Assisted Extraction of Phenolic Antioxidants from Potato Peels. Molecules 2011, 16, 2218–2232. [Google Scholar] [CrossRef]
- Ho, C.H.L.; Cacace, J.E.; Mazza, G. Extraction of lignans, proteins and carbohydrates from flaxseed meal with pressurized low polarity water. LWT Food Sci. Technol. 2007, 40, 1637–1647. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Zhang, Z.-S.; Li, D.; Wang, L.-J.; Ozkan, N.; Chen, X.D.; Mao, Z.-H.; Yang, H.-Z. Optimization of ethanol-water extraction of lignans from flaxseed. Sep. Purif. Technol. 2007, 57, 17–24. [Google Scholar] [CrossRef]
- Al-Farsi, M.A.; Lee, C.Y. Optimization of phenolics and dietary fibre extraction from date seeds. Food Chem. 2008, 108, 977–985. [Google Scholar] [CrossRef]
- Falleh, H.; Ksouri, R.; Chaieb, K.; Karray-Bouraoui, N.; Trabelsi, N.; Boulaaba, M.; Abdelly, C. Phenolic composition of Cynara cardunculus L. organs, and their biological activities. Comptes. Rendus Biol. 2008, 331, 372–379. [Google Scholar] [CrossRef]
- Myers, R.H.; Montgomery, D.C. Response Surface Methodology: Process and Product in Optimization Using Designed Experiments; Wiley: New York, NY, USA, 1995; pp. 273–286. [Google Scholar]
- Li, H.-B.; Wong, C.-C.; Cheng, K.-W.; Chen, F. Antioxidant properties in vitro and total phenolic contents in methanol extracts from medicinal plants. LWT Food Sci. Technol. 2008, 41, 385–390. [Google Scholar] [CrossRef]
- Yoo, K.M.; Lee, C.H.; Lee, H.; Moon, B.; Lee, C.Y. Relative antioxidant and cytoprotective activities of common herbs. Food Chem. 2008, 106, 929–936. [Google Scholar] [CrossRef]
- Liu, M.; Li, X.Q.; Weber, C.; Lee, C.Y.; Brown, J.; Liu, R.H. Antioxidant and Antiproliferative Activities of Raspberries. J. Agric. Food Chem. 2002, 50, 2926–2930. [Google Scholar] [CrossRef]
- Arancibia-Avila, P.; Toledo, F.; Park, Y.-S.; Jung, S.-T.; Kang, S.-G.; Heo, B.G.; Lee, S.-H.; Sajewicz, M.; Kowalska, T.; Gorinstein, S. Antioxidant properties of durian fruit as influenced by ripening. LWT Food Sc. Technol. 2008, 41, 2118–2125. [Google Scholar] [CrossRef]
- Cai; Sun, M.; Xing, J.; Corke, H. Antioxidant Phenolic Constituents in Roots of Rheum officinale and Rubia cordifolia: Structure-Radical Scavenging Activity Relationships. J. Agric. Food Chem. 2004, 52, 7884–7890. [Google Scholar] [CrossRef]
- Surveswaran, S.; Cai, Y.-Z.; Corke, H.; Sun, M. Systematic evaluation of natural phenolic antioxidants from 133 Indian medicinal plants. Food Chem. 2007, 102, 938–953. [Google Scholar] [CrossRef]
- Saafi, E.B.; Arem, A.E.; Issaoui, M.; Hammami, M.; Achour, L. Phenolic content and antioxidant activity of four date palm (Phoenix dactylifera L.) fruit varieties grown in Tunisia. Int. J. Food Sci. Technol. 2009, 44, 2314–2319. [Google Scholar]
- Cai, Y.-Z.; Mei, S.; Jie, X.; Luo, Q.; Corke, H. Structure-radical scavenging activity relationships of phenolic compounds from traditional Chinese medicinal plants. Life Sci. 2006, 78, 2872–2888. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Saura-Calixto, F. Effect of solvent and certain food constituents on different antioxidant capacity assays. Food Res. Int. 2006, 39, 791–800. [Google Scholar] [CrossRef]
- Faller, A.L.K.; Fialho, E. The antioxidant capacity and polyphenol content of organic and conventional retail vegetables after domestic cooking. Food Res. Int. 2009, 42, 210–215. [Google Scholar] [CrossRef]
- Noor Hashim, N.H.; Abas, F.; Shaari, K.; Lajis, N.H. LC–DAD–ESIMS/MS characterization of antioxidant and anticholinesterase constituents present in the active fraction from Persicaria hydropiper. LWT Food Sc. Technol. 2012, 46, 468–476. [Google Scholar] [CrossRef]
- Abas, F.; Shaari, K.; Israf, D.A.; Syafri, S.; Zainal, Z.; Lajis, N. H. LC–DAD–ESI-MS analysis of nitric oxide inhibitory fractions of tenggek burung (Melicope ptelefolia Champ. ex Benth.). J. Food Compos. Anal. 2010, 23, 107–112. [Google Scholar]
- Sample Availability: Powdered dried fruits o f M. citrifolia is available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Thoo, Y.Y.; Ho, S.K.; Abas, F.; Lai, O.M.; Ho, C.W.; Tan, C.P. Optimal Binary Solvent Extraction System for Phenolic Antioxidants from Mengkudu (Morinda citrifolia) Fruit. Molecules 2013, 18, 7004-7022. https://doi.org/10.3390/molecules18067004
Thoo YY, Ho SK, Abas F, Lai OM, Ho CW, Tan CP. Optimal Binary Solvent Extraction System for Phenolic Antioxidants from Mengkudu (Morinda citrifolia) Fruit. Molecules. 2013; 18(6):7004-7022. https://doi.org/10.3390/molecules18067004
Chicago/Turabian StyleThoo, Yin Yin, Swee Kheng Ho, Faridah Abas, Oi Ming Lai, Chun Wai Ho, and Chin Ping Tan. 2013. "Optimal Binary Solvent Extraction System for Phenolic Antioxidants from Mengkudu (Morinda citrifolia) Fruit" Molecules 18, no. 6: 7004-7022. https://doi.org/10.3390/molecules18067004




