3.1. General Procedures
Melting points were determined on a Koffler hot stage apparatus (Electrothermal 9100, Dubuque, IA, USA) and were uncorrected. Optical rotations were measured on a Jasco DIP 370 (Jasco Analytical Instruments, Easton, MD, USA) polarimeter in CHCl
3 at 20 °C. IR spectra were recorded on a Nicolet Nexus 470 FT-IR instrument (Thermo Electron Corporation, Whaltham, MA, USA). The NMR spectra were recorded on a Bruker Avance 400 (Bruker, Rheinstetten, Germany) spectrometer at 400 MHz for
1H and 100 MHz for
13C in CDCl
3. Chemical shifts are given in ppm with TMS as the internal standard. High-resolution mass spectra were measured on a VG Micromass ZAB-2F at 70 eV (Varian Inc., Palo Alto, CA, USA). Merck silica gel (0.063–0.2) was used for column chromatography, pre-coated Si gel plates (Merck, Kieselgel 60 F
254, 0.25 mm) were used for TLC analysis. TLC spots were visualized by spraying the chromatograms with
p-anisaldehyde-ethanol-acetic acid-H
2SO
4 (2:170:20:10 v/v) and heating at 110 °C for 3 min. Dicyclohexylcarbodiimide (DCC) and dimethylaminopyridine (DMAP) were from Merck (Germany). 4-Toluenesulfonyl chloride (TsCl) from Fluka (St. Louis, MO, USA), propargyl alcohol, 3-butyn-1-ol and 4-pentynoic acid were from Aldrich (Streinheim, Germany). 1,4-dibromobutane, 1,5-dibromopentane and 1,9-dibromononane were from Aldrich (St. Louis, MO, USA). Sodium azide (Sigma-Aldrich, St. Louis, MO, USA), copper (II) sulphatepentahydrate (Aldrich, St. Louis, MO, USA) and sodium ascorbate (Sigma, St. Louis, MO, USA). The diterpene15-hydroxy-labd-8(17)-en-19-oic acid (imbricatolic acid) was isolated from the resin of
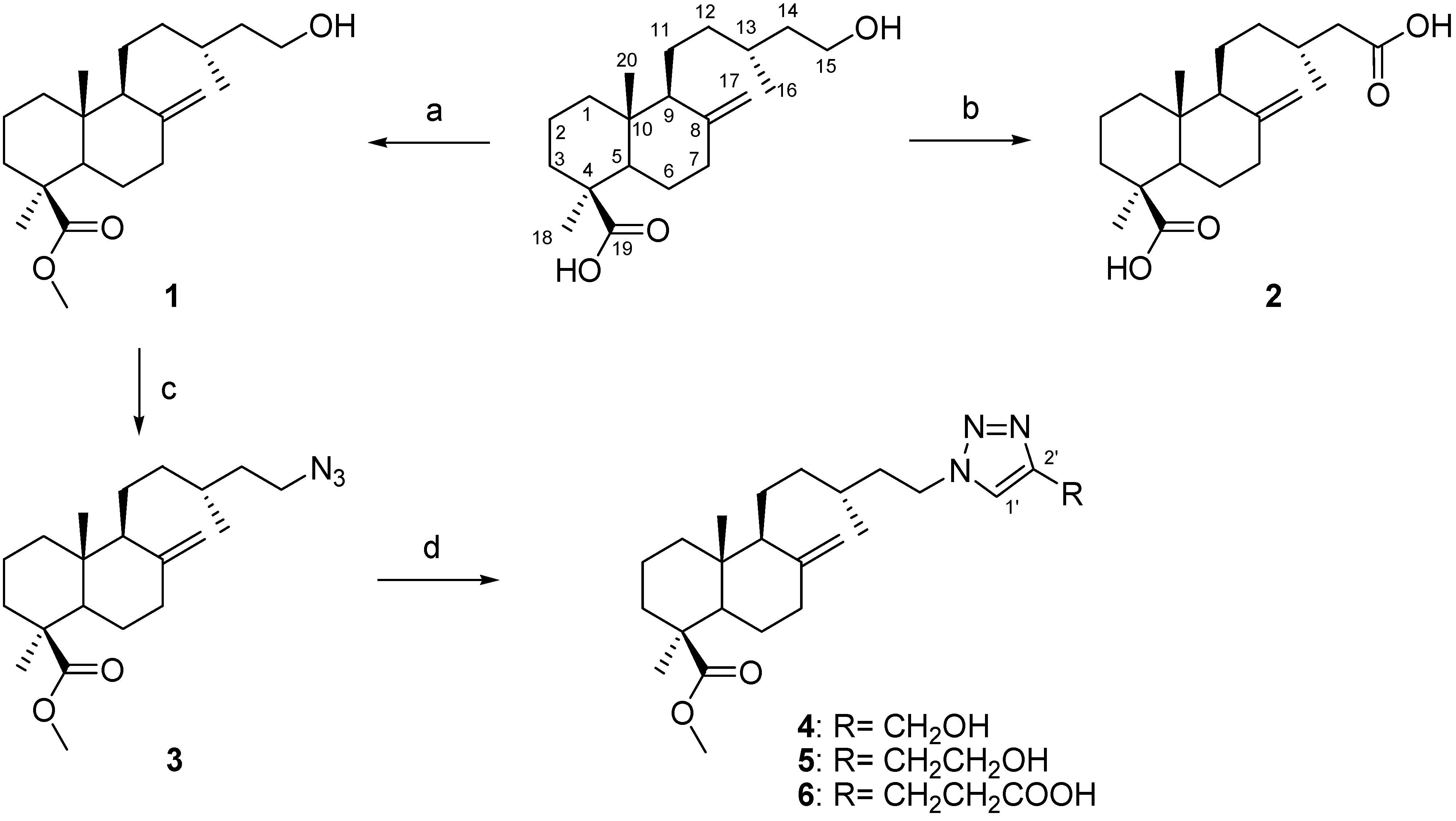
Araucaria araucana as previously described [
17,
22]. To obtain the starting compound for the synthesis, imbricatolic acid was methylated with diazomethane to afford compound
1. Compound
2 was obtained from imbricatolic acid by oxidation with Jones reagent (CrO
3/H
2SO
4/H
2O).
15-Hydroxyimbricatolic acid methyl ester (
1). Imbricatolic acid (3.00 g, 9.32 mmol) was dissolved in a solution of CH
2N
2/Et
2O. The mixture was stirred at room temperature for 3 h, taken to dryness under reduced pressure and purified by flash column chromatography (CC) on silica gel, eluting with hexane/EtOAc (9:1) to yield 1 (2.85 g, 91%): colorless resin; [α]20
D +50 (
c 0.016, CHCl
3); IR (film)
νmax 3440, 2937, 2873, 1722, 1426, 1147 cm
−1; The NMR data are in concordance with those informed previously [
22,
23]; HREIMS
m/z 337.2753 [M+H]
+ (calcd for C
21H
37O
3, 337.2742).
Labd-8(17)-en-15,19-dioic acid (junicedric acid) (
2). To a solution of imbricatolic acid (3.50 g, 10.54 mmol) in acetone (0.1 M), 3 eq of Jones reagent (1 eq CrO
3/35 eq H
2O/1 eq H
2SO
4 16 M) were added at 0 °C. After 10–20 min, the reaction was poured over saturated NaHCO
3 (50 mL) and extracted with ethyl ether (2 × 50 mL). The ethyl ether solution was washed with brine, dried over anhydrous Na
2SO
4, filtered and taken to dryness under reduced pressure. The residue was purified by flash CC on silica gel eluting with hexane/EtOAc (8:2) to yield
2 (2.97 g, 84%): colorless resin; [α]20
D +42 (
c 0.159, CHCl
3); IR (film)
νmax 3420, 2937, 2847, 1698, 1644, 1467, 1155 cm
−1; The NMR data are in concordance with those informed previously [
22,
23]; HREIMS
m/z 337.2342 [M+H]
+ (calcd for C
20H
33O
4, 337.2379).
15-Azidoimbricatolic acid methyl ester (3). (i) To a solution of compound 1 (2.00 g, 6.21 mmol) in pyridine (0.1 M), a solution of TsCl (1.30 g, 6.83 mmol) was added at 0 °C and the mixture was stirred at room temperature for 24 h. The reaction mixture was cooled in an ice bath, water was added, and the aqueous phase was extracted with EtOAc (3 × 20 mL), dried over anhydrous Na2SO4 and taken to dryness under reduced pressure. The residue was purified by silica gel CC eluting with hexane/EtOAc (8:2), yielding 2.37 g (78%) of the tosylated compound 1. (ii) The tosylated compound (2.30 g, 4.70 mmol) and NaN3 (0.61 g, 9.40 mmol) in DMF (0.1 M) were stirred at room temperature for 24 h. The reaction mixture was cooled in an ice-bath, water was added, and the aqueous phase was extracted with EtOAc (3 × 20 mL). The organic phase was dried over anhydrous Na2SO4, taken to dryness and the residue was purified by silica gel CC eluting with hexane/EtOAc (9:1), yielding 3 (1.42 g, 84%): yellow oil; [α]20 D +83 (c 0.068, CHCl3); IR (film) νmax 2931, 2843, 2094, 1725, 1644, 1464, 1153 cm−1; 1H-NMR (CDCl3): δ 0.46 (3H, s, H-20), 0.86 (3H, d, J = 6.5 Hz, H-16), 0.88 (1H, m, H-12), 1.02 (1H, m, H-3), 1.06 (1H, m, H-1), 1.14 s (3H, s, H-18), 1.21 (1H, m, H-11), 1.25 (1H, m, H-5), 1.34 (1H, m, H-14), 1.42–1.52 (5H, m, H-2, H-9, H-11, H-12 and H-13), 1.60 (1H, m, H-14), 1.72 (1H, m, H-6), 1.77–1.80 (1H, m, H-2), 1.80–1.84 (1H, m, H-1), 1.86–1.89 (1H, m, H-7), 1.90–1.95 (1H, m, H-6), 2.13 (1H, brd, J = 14.2 Hz, H-3), 2.36 (1H, dt, J = 12.1; 3.0 Hz, H-7), 3.23 (2H, m, H-15), 3.57 (3H, s, OMe), 4.44 (1H, s, H-17), 4.80 (1H, s, H-17); 13C-NMR (CDCl3): 39.17 (C-1), 19.97 (C-2), 38.26 (C-3), 44.27 (C-4), 56.35 (C-5), 26.25 (C-6), 38.78 (C-7), 148.24 (C-8), 56.54 (C-9), 40.34 (C-10), 21.05 (C-11), 35.93 (C-12), 31.02 (C-13), 35.44 (C-14), 49.51 (C-15), 19.54 (C-16), 106.27 (C-17), 28.79 (C-18), 177.64 (C-19), 12.54 (C-20), 51.05 (OMe); HREIMS m/z 362.2796 [M+H]+ (calcd for C21H36N3O2, 362.2807).
15-(4-(Hydroxymethyl)-1H-1,2,3-triazol-1-yl)-imbricatolic acid methyl ester (4). Compound 3 (100 mg, 0.28 mmol) and propargyl alcohol (24 μL, 0.42 mmol), were dissolved in t-BuOH/H2O (3 mL/3 mL) followed by the addition of CuSO4·5H2O (7 mg, 0.028 mmol, dissolved in 200 μL of water) and sodium ascorbate (11 mg, 0.056 mmol, dissolved in 200 μL of water). The solution was stirred at room temperature for 24 h. The reaction mixture was cooled in an ice-bath, water was added, and the aqueous phase was extracted with EtOAc (3 × 20 mL). The organic phase was dried over anhydrous Na2SO4, taken to dryness and the residue was purified by silica gel CC eluting with hexane/EtOAc (8:2) to yield 4 (85 mg, 73%): colorless oil; [α]20 D +55 (c 0.021, CHCl3); IR (film) νmax 3420, 2943, 2846, 1725, 1644, 1464, 1153 cm−1; 1H-NMR (CDCl3): δ 0.47 (3H, s, H-20), 0.94 (3H, d, J = 6.5 Hz, H-16), 0.95-1.01 (1H, m, H-12), 1.02 (1H, m, H-3), 1.02–1.07 (1H, m, H-1),1.16 s (3H, s, H-18), 1.23 (1H, m, H-11), 1.27 (1H, m, H-5), 1.40 (1H, m, H-14β), 1.48-1.54 (4H, m, H-2, H-9, H-12 and H-13), 1.68 (1H, m, H-14α), 1.73 (1H, m, H-6), 1.77–1.82 (1H, m, H-2), 1.79–1.84 (2H, m, H-1 and H-11), 1.87–1.90 (1H, m, H-7), 1.92–1.98 (1H, m, H-6), 2.15 (1H, brd, J = 12.9 Hz, H-3), 2.37 (1H, dt, J = 11.9; 3.0 Hz, H-7), 3.59 (3H, s, OMe), 4.32 (2H, m, H-15), 4.43 (1H, s, H-17), 4.76 (2H, s, H-3'), 4.82 (1H, s, H-17), 7.50 (1H, s, H-1'); 13C-NMR (CDCl3): 39.17 (C-1), 19.95 (C-2), 38.23 (C-3), 44.32 (C-4), 56.34 (C-5), 26.25 (C-6), 38.76 (C-7), 148.25 (C-8), 56.56 (C-9), 40.37 (C-10), 20.98 (C-11), 35.81 (C-12), 31.02 (C-13), 37.11 (C-14), 48.64 (C-15), 19.43 (C-16), 106.30 (C-17), 28.81 (C-18), 177.89 (C-19), 12.56 (C-20), 51.18 (OMe), 121.72 (C-1'), 147.66 (C-2'), 56.05 (C-3'); HREIMS m/z 418.3044 [M+H]+ (calcd for C24H40N3O3, 418.3069).
15-(4-(2-Hydroxyethyl)-1H-1,2,3-triazol-1-yl)-imbricatolic acid methyl ester (5). Compound 5 was synthesized as described for 4 starting from compound 3, using 3-butyn-1-ol instead of propargyl alcohol, to afford 82 mg (68%) of 5: colorless oil; [α]20 D +37 (c 0.034, CHCl3); IR (film) νmax 3422, 2946, 2846, 1723, 1644, 1464, 1153 cm−1; 1H-NMR (CDCl3): δ 0.46 (3H, s, H-20), 0.94 (3H, d, J = 6.5 Hz, H-16), 0.95–1.01 (1H, m, H-12), 1.02 (1H, m, H-3), 1.02–1.07 (1H, m, H-1), 1.16 (3H, s, H-18), 1.23 (1H, m, H-11), 1.27 (1H, m, H-5), 1.39 (1H, m, H-14β), 1.48–1.53 (4H, m, H-2, H-9, H-12 and H-13), 1.68 (1H, m, H-14α), 1.73 (1H, m, H-6), 1.77–1.82 (1H, m, H-2), 1.79-1.84 (2H, m, H-1 and H-11), 1.87–1.90 (1H, m, H-7), 1.93–1.99 (1H, m, H-6), 2.16 (1H, brd, J = 12.9 Hz, H-3), 2.38 (1H, dt, J = 11.9; 3.0 Hz, H-7), 2.92 (2H, t, J = 5.6 Hz, H-3'), 3.59 (3H, s, OMe), 3.93 (2H, brs, H-4'), 4.32 (2H, m, H-15), 4.42 (1H, s, H-17), 4.81 (1H, s, H-17), 7.37 (1H, s, H-1'); 13C-NMR (CDCl3): 39.21 (C-1), 19.90 (C-2), 38.27 (C-3), 44.33 (C-4), 56.37 (C-5), 26.27 (C-6), 38.79 (C-7), 148.31 (C-8), 56.57 (C-9), 40.39 (C-10), 21.03 (C-11), 35.83 (C-12), 31.08 (C-13), 37.16 (C-14), 48.50 (C-15), 19.51 (C-16), 106.28 (C-17), 28.85 (C-18), 177.79 (C-19), 12.59 (C-20), 51.17 (OMe), 121.44 (C-1'), 147.60 (C-2'), 28.70 (C-3'), 61.66 (C-4'); HREIMS m/z 432.3564 [M+H]+ (calcd for C25H42N3O3, 432.3226).
15-(4-(2-Carboxyethyl)-1H-1,2,3-triazol-1-yl)-imbricatolic acid methyl ester (6). 6 was synthesized as described for 4 from 3, using 4-pentynoic acid as the alkyne to afford 91 mg (71%) of 6: colorless oil; [α]20 D +56 (c 0.010, CHCl3); IR (film) νmax 3322, 2940, 2846, 1723, 1644, 1464, 1153 cm−1; 1H-NMR (CDCl3): δ 0.48 (3H, s, H-20), 0.94 (3H, d, J = 6.5 Hz, H-16), 0.95–1.01 (1H, m, H-12), 1.03 (1H, m, H-3), 1.02–1.07 (1H, m, H-1), 1.18 (3H, s, H-18), 1.23 (1H, m, H-11), 1.27 (1H, m, H-5), 1.38 (1H, m, H-14β), 1.47–1.55 (4H, m, H-2, H-9, H-12 and H-13), 1.68 (1H, m, H-14α ), 1.73 (1H, m, H-6), 1.77–1.82 (1H, m, H-2), 1.78–1.84 (2H, m, H-1 and H-11), 1.87–1.90 (1H, m, H-7), 1.93–1.99 (1H, m, H-6), 2.16 (1H, brd, J = 12.9 Hz, H-3), 2.37 (1H, dt, J = 11.9; 3.0 Hz, H-7), 2.79 (2H, t, J = 7.0 Hz, H-4'), 3.04 (2H, t, J = 7.0 Hz, H-3'), 3.61 (3H, s, OMe), 4.36 (2H, m, H-15), 4.44 (1H, s, H-17), 4.83 (1H, s, H-17), 7.34 (1H, s, H-1'); 13C-NMR (CDCl3): 39.18 (C-1), 19.96 (C-2), 38.24 (C-3), 44.29 (C-4), 56.32 (C-5), 26.25 (C-6), 38.76 (C-7), 148.30 (C-8), 56.54 (C-9), 40.36 (C-10), 21.01 (C-11), 35.79 (C-12), 31.07 (C-13), 37.13 (C-14), 48.57 (C-15), 19.47 (C-16), 106.26 (C-17), 28.82 (C-18), 177.79 (C-19), 12.56 (C-20), 51.16 (OMe), 121.11 (C-1'), 147.25 (C-2'), 20.65 (C-3'), 33.41 (C-4'), 176.23 (C-5'); HREIMS m/z 460.4086 [M+H]+ (calcd for C26H42N3O4, 460.4048).
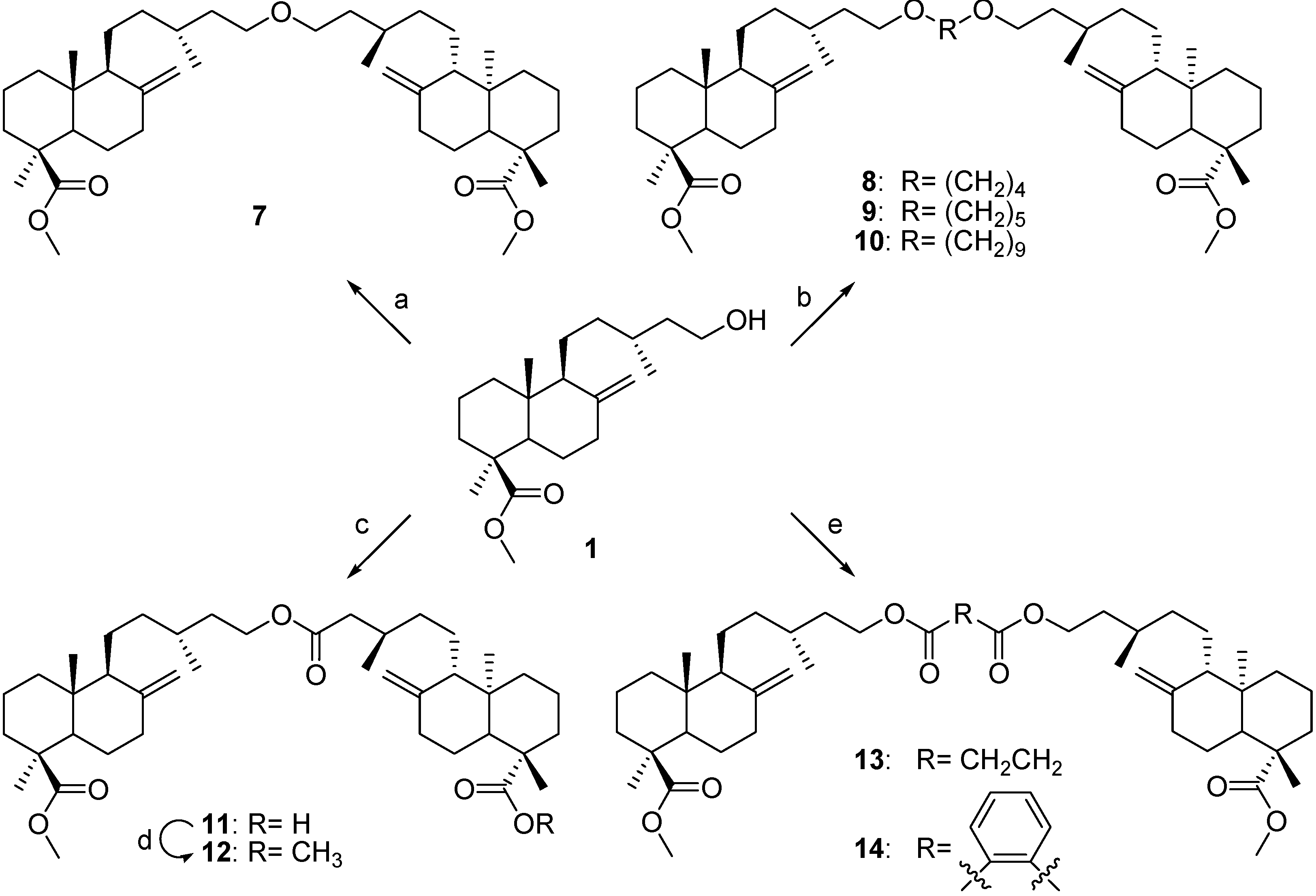
Dimer A (
7). To a solution of
1 (150 mg, 0.45 mmol) in DMF (10 mL) was added NaH (16 mg, 0.68 mmol) and tosylated compound
1 (220 mg, 0.45 mmol, for preparation see compound
3). The mixture was stirred at room temperature for 4 h, cooled in an ice-bath and after addition of water, the product was extracted with EtOAc (3 × 10 mL), washed with brine, and dried over anhydrous Na
2SO
4. The residue was purified by flash CC on silica gel eluting with hexane/EtOAc (8:2) to yield
7 (153 mg, 51%): pale yellow oil; [α]20
D +41 (
c 0.009, CHCl
3); IR (film)
νmax 2949, 2867, 1723, 1465, 1151 cm
−1;
1H-NMR (CDCl
3): δ 0.49 (6H, s, 2xH-20), 0.87 (6H, d,
J = 6.5 Hz, 2 × H-16), 0.90 (2H, m, 2 × H-12), 1.00–1.04 (2H, m, 2 × H-3), 1.03-1.08 (2H, m, 2 × H-1), 1.17 (6H, s, 2 × H-18), 1.23–1.28 (2H, m, 2 × H-11), 1.26–1.33 (2H, m, 2 × H-5), 1.37 (2H, m, 2 × H-14β), 1.46–1.54 (10H, m, 2 × H-2, 2 × H-9, 2 × H-11, 2 × xH-12 and 2 × H-13), 1.63 (2H, m, 2 × H-14α), 1.75–1.80 (4H, m, 2 × H-2 and 2 × H-6), 1.80–1.85 (2H, m, 2 × H-1), 1.84–1.90 (2H, m, 2 × H-7), 1.91–1.97 (2H, m, 2 × H-6), 2.13 (2H, brd,
J = 14.2 Hz, 2 × H-3), 2.38 (2H, dt,
J = 12.1; 3.0 Hz, 2 × H-7), 3.40 (4H, t,
J = 7.0 Hz, 2 × H-15), 3.61 (6H, s, 2 × OMe), 4.48 (2H, s, 2 × H-17), 4.82 (2H, s, 2 × H-17);
13C-NMR (CDCl
3): see
Table 1; HREIMS
m/z 655.5412 [M+H]
+ (calcd for C
42H
71O
5, 655.5301).
Dimer B (
8). To a solution of
1 (150 mg, 0.45 mmol) in DMF (10 mL) was added NaH (16 mg, 0.68 mmol) and 1,4-dibromobutane (54
μL, 0.45 mmol). The mixture was stirred at room temperature for 4 h, cooled in an ice-bath and after addition of water, the product was extracted with EtOAc (3 × 10 mL), washed with brine, and dried over anhydrous Na
2SO
4. The residue was purified by flash CC on silica gel eluting with hexane/EtOAc (9:1) to yield
7 (78 mg, 48%): pale yellow oil; [α]20
D +32 (
c 0.037; CHCl
3); IR (film)
νmax 2942, 2864, 1723, 1449, 1151 cm
−1;
1H-NMR (CDCl
3): δ 0.49 (6H, s, 2 × H-20), 0.87 (6H, d,
J = 6.5 Hz, 2 × H-16), 0.88 (2H, m, 2 × H-12), 1.00-1.04 (2H, m, 2 × H-3), 1.03–1.08 (2H, m, 2 × H-1), 1.17 (6H, s, 2 × H-18), 1.24–1.30 (2H, m, 2 × H-11), 1.27–1.34 (2H, m, 2 × H-5),1.34 (2H, m, 2 × H-14β), 1.45–1.53 (10H, m, 2 × H-2, 2 × H-9, 2 × H-11, 2 × H-12 and 2 × H-13), 1.63 (2H, m, 2 × H-14α), 1.75–1.80 (4H, m, 2 × H-2 and 2 × H-6), 1.80–1.85 (2H, m, 2 × H-1), 1.84–1.89 (2H, m, 2 × H-7), 1.88–1.95 (2H, m, 2 × H-6 and 4H, m, 2 × H-2''), 2.15 (2H, brd,
J = 14.2 Hz, 2 × H-3), 2.38 (2H, dt,
J = 12.1; 3.0 Hz, 2 × H-7), 3.40 (4H, m, 2 × H-1''), 3.43 (4H, t,
J = 7.0 Hz, 2 × H-15), 3.60 (6H, s, 2 × OMe), 4.48 (2H, s, 2 × H-17), 4.82 (2H, s, 2 × H-17);
13C-NMR (CDCl
3): see
Table 1; HREIMS
m/z 727.5793 [M+H]
+ (calcd for C
46H
79O
6, 727.5876).
Dimer C (
9).
9 was synthesized as described for
8 from
1 using 1,5-dibromopentane to afford 93 mg (56%) of
9: colorless oil; [α]20
D +35 (
c 0.030, CHCl
3); IR (film)
νmax 2942, 2864, 1723, 1468, 1148 cm
−1;
1H-NMR (CDCl
3): δ 0.49 (6H, s, 2 × H-20), 0.87 (6H, d,
J = 6.5 Hz, 2 × H-16), 0.90 (2H, m, 2 × H-12), 1.00–1.04 (2H, m, 2 × H-3), 1.02–1.06 (2H, m, 2 × H-1), 1.17 (6H, s, 2 × H-18), 1.24–1.31 (2H, m, 2 × H-11), 1.27–1.33 (2H, m, 2 × H-5), 1.34 (2H, m, 2 × H-14β), 1.45–1.55 (10H, m, 2 × H-2, 2 × H-9, 2 × H-11, 2 × H-12, 2 × H-13 and 2H, m, H-3''), 1.62 (2H, m, 2 × H-14α), 1.75-1.80 (4H, m, 2 × H-2 and 2 × H-6), 1.79–1.84 (2H, m, 2 × H-1), 1.84–1.89 (2H, m, 2 × H-7 and 4H, m, 2 × H-2''), 1.91–1.96 (2H, m, 2 × H-6), 2.16 (2H, brd,
J = 14.2 Hz, 2 × H-3), 2.39 (2H, dt,
J = 12.1; 3.0 Hz, 2 × H-7), 3.39 (4H, m, 2 × H-1''), 3.40 (4H, t,
J = 7.0 Hz, 2 × H-15), 3.60 (6H, s, 2xOMe), 4.48 (2H, s, 2 × H-17), 4.82 (2H, s, 2 × H-17);
13C-NMR (CDCl
3): see
Table 1; HREIMS
m/z 741.6012 [M+H]
+ (calcd for C
47H
81O
6, 741.6033).
Dimer D (
10).
10 was synthesized as described for
8 from
1 using 1,9-dibromononane to afford 73 mg (41%) of
10: yellow oil; [α]20
D +20 (
c 0.098, CHCl
3); IR (film)
νmax 2923, 2849, 1723, 1462, 1152 cm
−1;
1H-NMR (CDCl
3): δ 0.47 (6H, s, 2 × H-20), 0.86 (6H, d,
J = 6.5 Hz, 2 × H-16), 0.89 (2H, m, 2 × H-12), 1.00–1.04 (2H, m, 2 × H-3), 1.01–1.05 (2H, m, 2 × H-1), 1.16 (6H, s, 2 × H-18), 1.24–1.30 (2H, m, 2 × H-11 and 6H, m, 2 × H-4'' and H-5''), 1.27–1.34 (2H, m, 2 × H-5), 1.34 (2H, m, 2 × H-14β), 1.47–1.55 (10H, m, 2 × H-2, 2 × H-9, 2 × H-11, 2 × H-12, 2 × H-13 and 4H, m, 2 × H-3''), 1.62 (2H, m, 2 × H-14α), 1.75–1.80 (4H, m, 2 × H-2 and 2 × H-6), 1.81–1.85 (2H, m, 2 × H-1 and 4H, m, 2 × H-2''), 1.86–1.90 (2H, m, 2 × H-7), 1.92–1.97 (2H, m, 2 × H-6), 2.14 (2H, brd,
J = 14.2 Hz, 2 × H-3), 2.38 (2H, dt,
J = 12.1; 3.0 Hz, 2 × H-7), 3.36 (4H, m, 2 × H-1''), 3.38 (4H, t,
J = 7.0 Hz, 2 × H-15), 3.60 (6H, s, 2xOMe), 4.47 (2H, s, 2 × H-17), 4.80 (2H, s, 2 × H-17);
13C-NMR (CDCl
3): see
Table 1; HREIMS
m/z 797.6721 [M+H]
+ (calcd for C
51H
89O
6, 797.6659).
Dimer E (
11).
2 (120 mg, 0.36 mmol), DCC (111 mg, 0.54 mmol), catalytic amount of DMAP and
1 (120 mg, 0.36 mmol) in dry CH
2Cl
2 (10 mL) were stirred at room temperature for 2 h. The reaction mixture was cooled in an ice-bath, water was added, and the aqueous phase was extracted with EtOAc (3 × 20 mL), and dried over anhydrous Na
2SO
4, taken to dryness and the residue was purified by silica gel CC eluting with hexane/EtOAc (8:2), yielding
11 (153 mg, 65%): colorless oil; [α]20
D +34 (
c 0.148, CHCl
3); IR (film)
νmax 3322, 2930, 2847, 1724, 1644, 1465, 1152 cm
−1;
1H-NMR (CDCl
3): δ 0.46 (3H, s, H-20), 0.55 (3H, s, H-20'), 0.86 (3H, d,
J = 6.5 Hz, H-16), 0.89 (3H, d,
J = 6.5 Hz, H-16'), 0.86-0.91 (2H, m, H-12 and H-12'), 0.98–1.08 (4H, m, H-1, H-1', H-3 and H-3'), 1.14 (3H, s, H-18), 1.19 (3H, s, H-18'), 1.20–1.25 (4H, m, H-5, H-5', H-11 and H-11'), 1.34 (1H, m, H-14β), 1.45–1.50 (10H, m, H-2, H-2', H-9, H-9', H-11, H-11', H-12, H-12', H-13 and H-13'), 1.64 (1H, m, H-14α), 1.70–1.75 (2H, m, H-6 and H-6'), 1.76-1.84 (4H, m, H-1, H-1', H-2 and H-2'), 1.86–1.93 (4H, m, H-6, H-6', H-7 and H-7'), 2.04 (1H, dd,
J = 14.5; 8.2 Hz, H-14'β), 2.13 (2H, m, H-3 and H-3'), 2.27 (1H, dd,
J = 14.5; 5.9 Hz, H-14'α), 2.36 (2H, m, H-7 and H-7'), 3.57 (3H, s, OMe), 4.04 (4H, m, H-15 and H-15'), 4.44 (2H, s, H-17 and H-17'), 4.79 (2H, s, H-17 and H-17');
13C-NMR (CDCl
3): see
Table 2; HREIMS
m/z 655.4924 [M+H]
+ (calcd for C
41H
67O
6, 655.4937).
Dimer F (
12). Compound
11 (100 mg, 0.15 mmol), was methylated with a solution of CH
2N
2 in ethyl ether, yielding 92 mg (92%) of
12: colorless oil; [α]20
D +45 (
c 0.029, CHCl
3); IR (film)
νmax 2949, 2842, 1723, 1644, 1465, 1152 cm
−1;
1H-NMR (CDCl
3): δ 0.48 (6H, s, H-20 and H-20'), 0.89 (3H, d,
J = 6.5 Hz, H-16), 0.92 (3H, d,
J = 6.5 Hz, H-16'), 0.88–0.93 (2H, m, H-12 and H-12'), 1.00–1.08 (4H, m, H-1, H-1', H-3 and H-3'), 1.17 (6H, s, H-18 and H-18'), 1.22–1.30 (4H, m, H-5, H-5', H-11 and H-11'), 1.36 (1H, m, H-14β), 1.46–1.52 (10H, m, H-2, H-2', H-9, H-9', H-11, H-11', H-12, H-12', H-13 and H-13'), 1.65 (1H, m, H-14α), 1.72–1.78 (2H, m, H-6 and H-6'), 1.80–1.87 (4H, m, H-1, H-1', H-2 and H-2'), 1.88–1.95 (4H, m, H-6, H-6', H-7 and H-7'), 2.06 (1H, dd,
J = 14.5; 8.3 Hz, H-14'β), 2.14 (2H, m, H-3 and H-3'), 2.29 (1H, dd,
J = 14.5; 5.9 Hz, H-14'α), 2.38 (2H, m, H-7 and H-7'), 3.60 (6H, s, OMe and OMe'), 4.06 (4H, m, H-15 and H-15'), 4.46 (2H, s, H-17 and H-17'), 4.82 (2H, s, H-17 and H-17');
13C-NMR (CDCl
3): see
Table 2; HREIMS
m/z 669.5131 [M+H]
+ (calcd for C
42H
69O
6, 669.5094).
Dimer G (
13).
13 was synthesized as described for
11 from
1 using succinic acid to afford 76 mg (45%) of
13: pale yellow oil; [α]20
D +34 (
c 0.042, CHCl
3); IR (film)
νmax 3320, 2949, 2841, 1721, 1645, 1465, 1155 cm
−1;
1H-NMR (CDCl
3): δ 0.49 (6H, s, 2 × H-20), 0.89 (6H, d,
J = 6.5 Hz, 2 × H-16), 0.87–0.93 (2H, m, 2x H-12), 0.99–1.07 (4H, m, 2 × H-1 and 2 × H-3), 1.18 (6H, s, 2 × H-18), 1.23–1.29 (4H, m, 2 × H-5 and 2 × H-11), 1.30 (2H, m, 2 × H-14β), 1.46–1.52 (10H, m, 2 × H-2, 2 × H-9, 2 × H-11, 2 × H-12 and 2 × H-13), 1.66 (2H, m, 2 × H-14α), 1.70–1.76 (2H, m, 2 × H-6), 1.77–1.84 (4H, m, 2 × H-1 and 2 × H-2), 1.86–1.94 (4H, m, 2 × H-6 and 2 × H-7), 2.16 (2H, m, 2 × H-3), 2.38 (2H, m, 2 × H-7), 2.64 (4H, brd,
J = 6.1 Hz, 2 × H-2''), 3.61 (6H, s, 2 × OMe), 4.11 (4H, m, 2 × H-15), 4.47 (2H, s, 2 × H-17), 4.82 (2H, s, 2 × H-17);
13C-NMR (CDCl
3): see
Table 1; HREIMS
m/z 755.5546 [M+H]
+ (calcd for C
46H
75O
8, 755.5462).
Dimer H (
14).
14 was synthesized as described for
11 from
1 using phthalic acid to afford 95 mg (53%) of 14: yellow oil; [α]20
D +33 (
c 0.053, CHCl
3); IR (film)
νmax 2949, 2842, 1723, 1644, 1465, 1148 cm
−1;
1H-NMR (CDCl
3): δ 0.42 (6H, s, 2 × H-20), 0.88 (6H, d,
J = 6.5 Hz, 2 × H-16), 0.88–0.95 (2H, m, 2 × H-12), 0.97–1.06 (4H, m, 2 × H-1 and 2 × H-3), 1.11 (6H, s, 2 × H-18), 1.20–1.25 (4H, m, 2 × H-5 and 2 × H-11), 1.30 (2H, m, 2 × H-14β), 1.40–1.48 (10H, m, 2 × H-2, 2 × H-9, 2 × H-11, 2 × H-12 and 2 × H-13), 1.68 (2H, m, 2 × H-14α), 1.70–1.76 (2H, m, 2 × H-6), 1.76–1.82 (4H, m, 2 × H-1 and 2 × H-2), 1.84–1.92 (4H, m, 2 × H-6 and 2 × H-7), 2.08 (2H, m, 2 × H-3), 2.33 (2H, m, 2 × H-7), 3.55 (6H, s, 2 × OMe), 4.28 (4H, m, 2 × H-15), 4.42 (2H, s, 2 × H-17), 4.76 (2H, s, 2 × H-17), 7.58 (2H, m, 2 × H-4''), 7.76 (2H, m, 2 × H-3'');
13C-NMR (CDCl
3): see
Table 1; HREIMS
m/z 803.5437 [M+H]
+ (calcd for C
50H
74O
8, 803.5462).
Dimer I (
15). (i)
2 (120 mg, 0.36 mmol), DCC (111 mg, 0.54 mmol), catalytic amount of DMAP and propargyl alcohol (31 μL, 0.54 mmol) in dry CH
2Cl
2 (10 mL) were stirred at room temperature for 2 h. Yielding the propargyl ester of
2 (92 mg, 66%) after the extraction and purification. (ii)
3 (76 mg, 0.21 mmol) and propargyl ester of
2 (80 mg, 0. 21 mmol), were dissolved in
t-BuOH/H
2O (3 mL/3 mL) followed by the addition of 5 mg CuSO
4.5H
2O (0.021 mmol, dissolved in 200 μL of water) and 8 mg of sodium ascorbate (0.042 mmol, dissolved in 200 μL of water). The solution was stirred at room temperature for 24 h. The reaction mixture was cooled in an ice-bath, water was added, and the aqueous phase was extracted with EtOAc (3 × 20 mL), and dried over anhydrous Na
2SO
4, taken to dryness and the residue was purified by silica gel CC eluting with hexane/EtOAc (8:2), yielding
15 (120 mg, 78%): yellow oil; [α]20
D +37 (
c 0.075, CHCl
3); IR (film)
νmax 3320, 2936, 2842, 1718, 1641, 1466, 1152 cm
−1;
1H-NMR (CDCl
3): δ 0.44 (3H, s, H-20), 0.54 (3H, s, H-20'), 0.85 (3H, d,
J = 6.5 Hz, H-16), 0.90 (3H, d,
J = 6.5 Hz, H-16'), 0.94–0.99 (2H, m, H-12 and H-12'), 0.98–1.07 (4H, m, H-1, H-1', H-3 and H-3'), 1.13 (3H, s, H-18), 1.18 (3H, s, H-18'), 1.20–1.28 (4H, m, H-5, H-5', H-11 and H-11'), 1.34 (1H, m, H-14β), 1.45–1.51 (10H, m, H-2, H-2', H-9, H-9', H-11, H-11', H-12, H-12', H-13 and H-13'), 1.65 (1H, m, H-14α), 1.72–1.78 (2H, m, H-6 and H-6'), 1.79–1.85 (4H, m, H-1, H-1', H-2 and H-2'), 1.86–1.96 (4H, m, H-6, H-6', H-7 and H-7'), 2.05 (1H, dd,
J = 14.8; 8.5 Hz, H-14'β), 2.11 (2H, m, H-3 and H-3'), 2.29 (1H, dd,
J = 14.8; 5.6 Hz, H-14'α), 2.33 (2H, m, H-7 and H-7'), 3.56 (3H, s, OMe), 4.30 (2H, m, H-15), 4.39 (2H, s, H-17 and H-17'), 4.75 (1H, s, H-17'), 4.78 (1H, s, H-17), 5.16 (2H, s, H-3''), 7.55 (1H, s, H-1'');
13C-NMR (CDCl
3): see
Table 2; HREIMS
m/z 736.5359 [M+H]
+ (calcd for C
44H
70N
3O
6, 736.5265).
Dimer J (
16). Compound
15 (60 mg, 0.08 mmol), was methylated with a solution of CH
2N
2 in ethyl ether, yielding 57 mg (94%) of
16: colorless oil; [α]20
D +40 (
c 0.008, CHCl
3); IR (film)
νmax 2929, 2835, 1720, 1643, 1454, 1153 cm
−1;
1H-NMR (CDCl
3): δ 0.46 (6H, s, H-20 and H-20'), 0.84 (3H, d,
J = 6.5 Hz, H-16), 0.88 (3H, d,
J = 6.5 Hz, H-16'), 0.93–0.98 (2H, m, H-12 and H-12'), 0.99–1.06 (4H, m, H-1, H-1', H-3 and H-3'), 1.12 (3H, s, H-18), 1.19 (3H, s, H-18'), 1.20–1.28 (4H, m, H-5, H-5', H-11 and H-11'), 1.33 (1H, m, H-14β), 1.49–1.53 (10H, m, H-2, H-2', H-9, H-9', H-11, H-11', H-12, H-12', H-13 and H-13'), 1.63 (1H, m, H-14α), 1.70–1.77 (2H, m, H-6 and H-6'), 1.79–1.86 (4H, m, H-1, H-1', H-2 and H-2'), 1.88–1.98 (4H, m, H-6, H-6', H-7 and H-7'), 2.02 (1H, dd,
J = 14.8; 8.5 Hz, H-14'β), 2.12 (2H, m, H-3 and H-3'), 2.28 (1H, dd,
J = 14.8; 5.6 Hz, H-14'α), 2.34 (2H, m, H-7 and H-7'), 3.59 (6H, s, OMe and OMe'), 4.33 (2H, m, H-15), 4.42 (2H, s, H-17 and H-17'), 4.79 (1H, s, H-17'), 4.81 (1H, s, H-17), 5.18 (2H, s, H-3''), 7.55 (1H, s, H-1'');
13C-NMR (CDCl
3): see
Table 2; HREIMS
m/z 750.5205 [M+H]
+ (calcd for C
45H
72N
3O
6, 750.5421).
Dimer K (
17).
17 was synthesized as described for
15 (i) from
2 using 3-butyn-1-ol to afford 84 mg (58%) of the butyn ester of
2. (ii) from
3 using butyn ester of
2 to afford 115 mg (74%) of
17: colorless oil; [α]20
D +27 (
c 0.122, CHCl
3); IR (film)
νmax 3315, 2933, 2864, 1723, 1638, 1462, 1152 cm
−1;
1H-NMR (CDCl
3): δ 0.44 (3H, s, H-20), 0.55 (3H, s, H-20'), 0.85 (3H, d,
J = 6.5 Hz, H-16), 0.90 (3H, d,
J = 6.5 Hz, H-16'), 0.92–0.98 (2H, m, H-12 and H-12'), 0.99–1.07 (4H, m, H-1, H-1', H-3 and H-3'), 1.14 (3H, s, H-18), 1.19 (3H, s, H-18'), 1.20–1.28 (4H, m, H-5, H-5', H-11 and H-11'), 1.34 (1H, m, H-14β), 1.45–1.51 (10H, m, H-2, H-2', H-9, H-9', H-11, H-11', H-12, H-12', H-13 and H-13'), 1.64 (1H, m, H-14α), 1.71–1.77 (2H, m, H-6 and H-6'), 1.78–1.84 (4H, m, H-1, H-1', H-2 and H-2'), 1.86–1.95 (4H, m, H-6, H-6', H-7 and H-7'), 2.03 (1H, dd,
J = 14.8; 8.5 Hz, H-14'β), 2.11 (2H, m, H-3 and H-3'), 2.28 (1H, dd,
J = 14.8; 5.6 Hz, H-14'α), 2.33 (2H, m, H-7 and H-7'), 3.02 (2H, t,
J = 6.5 Hz, H-3''), 3.57 (3H, s, OMe), 4.29 (2H, t,
J = 6.5 Hz, H-4''), 4.31 (2H, m, H-15), 4.40 (1H, s, H-17'), 4.42 (1H, s, H-17), 4.79 (2H, s, H-17 and H-17'), 7.32 (1H, s, H-1'');
13C-NMR (CDCl
3): see
Table 2; HREIMS
m/z 750.5205 [M+H]
+ (calcd for C
45H
72N
3O
6, 750.5421).
Dimer L (
18). Compound
17 (60 mg, 0.08 mmol), was methylated with a solution of CH
2N
2 in ethyl ether, yielding 54 mg (88%) of
18: colorless oil; [α]20
D +41 (
c 0.024, CHCl
3); IR (film)
νmax 2947, 2839, 1720, 1643, 1463, 1149 cm
−1;
1H-NMR (CDCl
3): δ 0.47 (6H, s, H-20 and H-20'), 0.89 (3H, d,
J = 6.5 Hz, H-16), 0.92 (3H, d,
J = 6.5 Hz, H-16'), 0.94–1.00 (2H, m, H-12 and H-12'), 1.01–1.09 (4H, m, H-1, H-1', H-3 and H-3'), 1.17 (6H, s, H-18 and H-18'), 1.22–1.30 (4H, m, H-5, H-5', H-11 and H-11'), 1.35 (1H, m, H-14β), 1.46–1.53 (10H, m, H-2, H-2', H-9, H-9', H-11, H-11', H-12, H-12', H-13 and H-13'), 1.65 (1H, m, H-14α), 1.71–1.77 (2H, m, H-6 and H-6'), 1.79–1.86 (4H, m, H-1, H-1', H-2 and H-2'), 1.87–1.93 (4H, m, H-6, H-6', H-7 and H-7'), 2.06 (1H, dd,
J = 14.8; 8.5 Hz, H-14'β), 2.12 (2H, m, H-3 and H-3'), 2.30 (1H, dd,
J = 14.8; 5.6 Hz, H-14'α), 2.35 (2H, m, H-7 and H-7'), 3.05 (2H, t,
J = 6.5 Hz, H-3''), 3.60 (6H, s, OMe and OMe'), 4.32 (2H, t,
J = 6.5 Hz, H-4''), 4.33 (2H, m, H-15), 4.43 (1H, s, H-17'), 4.44 (1H, s, H-17), 4.82 (2H, s, H-17 and H-17'), 7.32 (1H, s, H-1'');
13C-NMR (CDCl
3): see
Table 2; HREIMS
m/z 764.5391 [M+H]
+ (calcd for C
46H
74N
3O
6, 764.5577).
Dimer M (
19).
19 was synthesized as described for 15 (i) from imbricatolic acid using 5-pentynoic acidto afford 97 mg (62%) of the pentynoic ester of imbricatolic acid. (ii) from
3 using pentynoic ester of imbricatolic acid to afford 107 mg (57%) of
19: yellow oil; [α]20
D +28 (
c 0.046, CHCl
3); IR (film)
νmax 3318, 2947, 2840, 1720, 1639, 1463, 1149 cm
−1;
1H-NMR (CDCl
3): δ 0.48 (3H, s, H-20), 0.59 (3H, s, H-20'), 0.88 (3H, d,
J = 6.5 Hz, H-16), 0.93 (3H, d,
J = 6.5 Hz, H-16'), 0.95–0.99 (2H, m, H-12 and H-12'), 1.00–1.07 (4H, m, H-1, H-1', H-3 and H-3'), 1.17 (3H, s, H-18), 1.23 (3H, s, H-18'), 1.21–1.29 (4H, m, H-5, H-5', H-11 and H-11'), 1.32–1.36 (2H, m, H-14β and H-14'β), 1.47–1.52 (10H, m, H-2, H-2', H-9, H-9', H-11, H-11', H-12, H-12', H-13 and H-13'), 1.65 (2H, m, H-14α and H-14'α), 1.74–1.79 (2H, m, H-6 and H-6'), 1.80–1.86 (4H, m, H-1, H-1', H-2 and H-2'), 1.87–1.95 (4H, m, H-6, H-6', H-7 and H-7'), 2.15 (2H, m, H-3 and H-3'), 2.39 (2H, m, H-7 and H-7'), 2.70 (2H, t,
J = 7.3 Hz, H-4''), 3.02 (2H, t,
J = 7.3 Hz, H-3''), 3.60 (3H, s, OMe), 4.08 (2H, m, H-15'), 4.30 (2H, m, H-15),4.43 (1H, s, H-17'), 4.46 (1H, s, H-17), 4.81 (1H, s, H-17'), 4.82 (1H, s, H-17), 7.31 (1H, s, H-1'');
13C-NMR (CDCl
3): see
Table 2; HREIMS
m/z 764.5516 [M+H]
+ (calcd for C
46H
74N
3O
6, 764.5577).
Dimer N (
20). Compound
19 (50 mg, 0.065 mmol), was methylated with a solution of CH
2N
2 in ethyl ether, yielding 43 mg (86%) of
20: pale yellow oil; [α]20
D +30 (
c 0.047, CHCl
3); IR (film)
νmax 2947, 2860, 1724, 1639, 1463, 1149 cm
−1;
1H-NMR (CDCl
3): δ 0.43 (3H, s, H-20), 0.44 (3H, s, H-20'), 0.84 (3H, d,
J = 6.5 Hz, H-16), 0.89 (3H, d,
J = 6.5 Hz, H-16'), 0.92–0.97 (2H, m, H-12 and H-12'), 0.98–1.05 (4H, m, H-1, H-1', H-3 and H-3'), 1.13 (6H, s, H-18 and H-18'), 1.20–1.27 (4H, m, H-5, H-5', H-11 and H-11'), 1.30–1.35 (2H, m, H-14β and H-14'β), 1.45–1.51 (10H, m, H-2, H-2', H-9, H-9', H-11, H-11', H-12, H-12', H-13 and H-13'), 1.66 (2H, m, H-14α and H-14'α), 1.73–1.78 (2H, m, H-6 and H-6'), 1.79–1.84 (4H, m, H-1, H-1', H-2 and H-2'), 1.86–1.93 (4H, m, H-6, H-6', H-7 and H-7'), 2.11 (2H, m, H-3 and H-3'), 2.34 (2H, m, H-7 and H-7'), 2.66 (2H, t,
J = 7.3 Hz, H-4''), 2.97 (2H, t,
J = 7.3 Hz, H-3''), 3.56 (6H, s, OMe and OMe'), 4.04 (2H, m, H-15'), 4.27 (2H, m, H-15), 4.39 (1H, s, H-17'), 4.41 (1H, s, H-17), 4.78 (2H, s, H-17 and H-17'), 7.30 (1H, s, H-1'');
13C-NMR (CDCl
3): see
Table 2; HREIMS
m/z 778.5566 [M+H]
+ (calcd for C
46H
73N
3O
6, 778.5734).