C-5 Hydroxyethyl and Hydroxypropyl Acyclonucleosides as Substrates for Thymidine Kinase of Herpes Simplex Virus Type 1 (HSV-1 TK): Syntheses and Biological Evaluation
Abstract
:1. Introduction
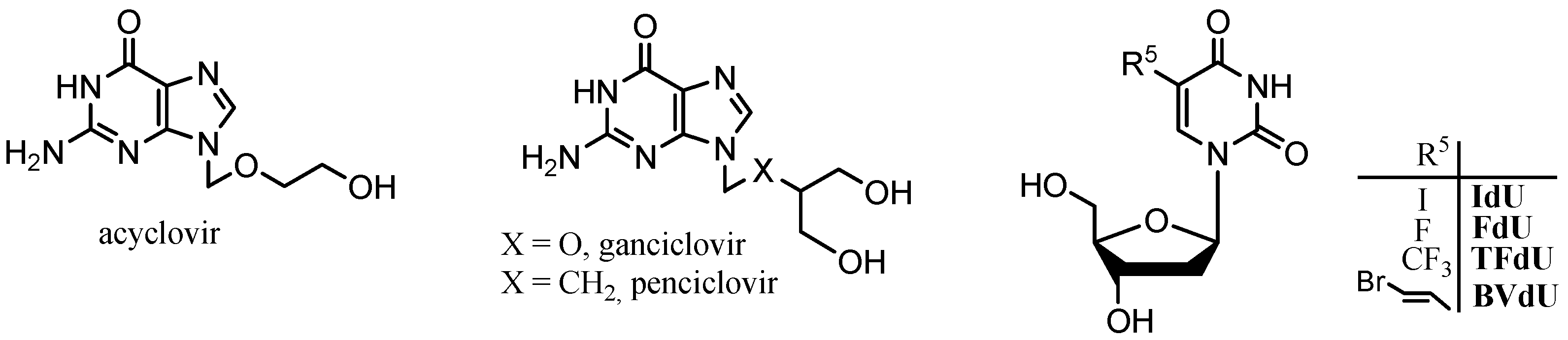
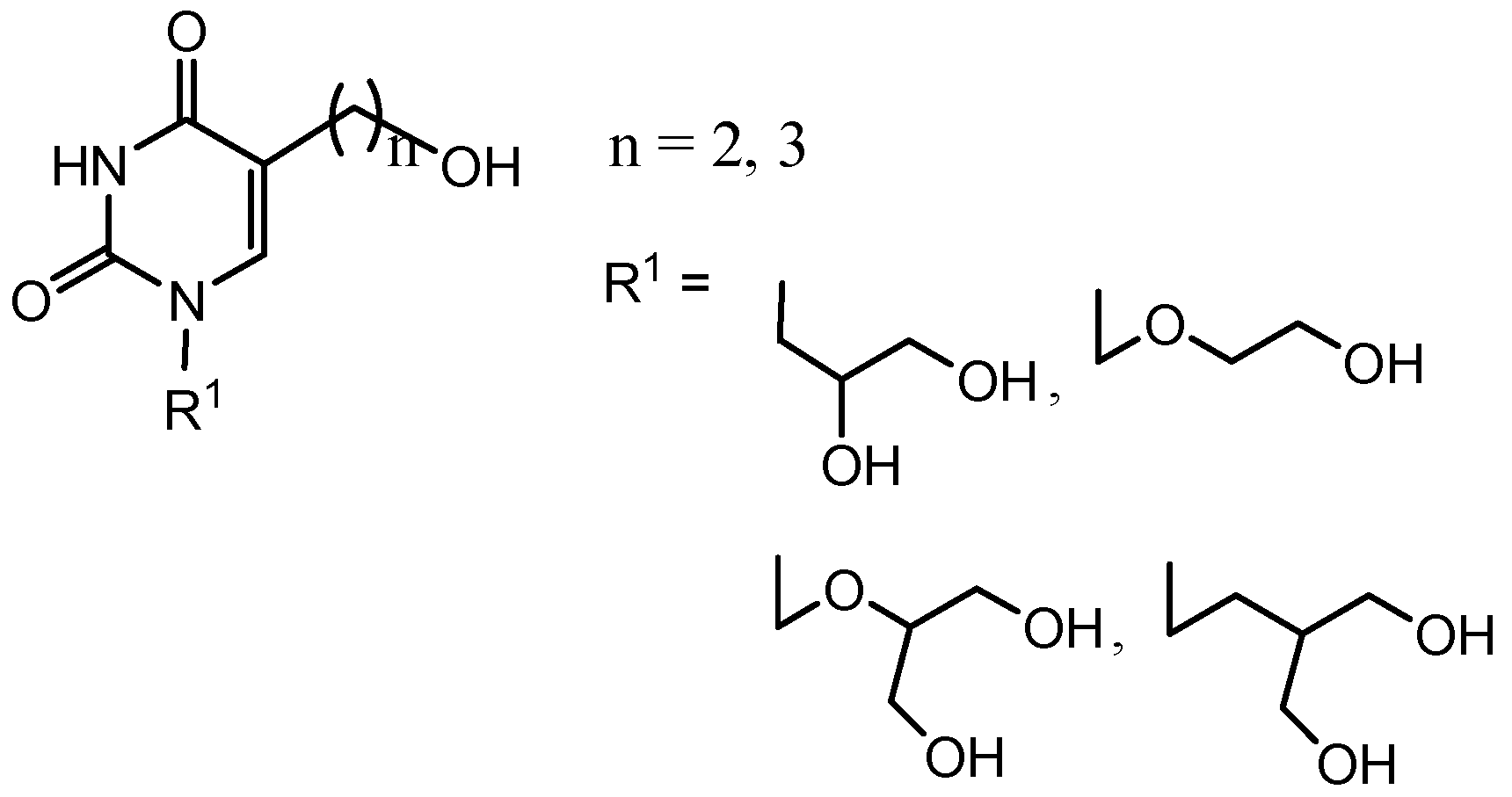
2. Results and Discussion
2.1. Chemistry
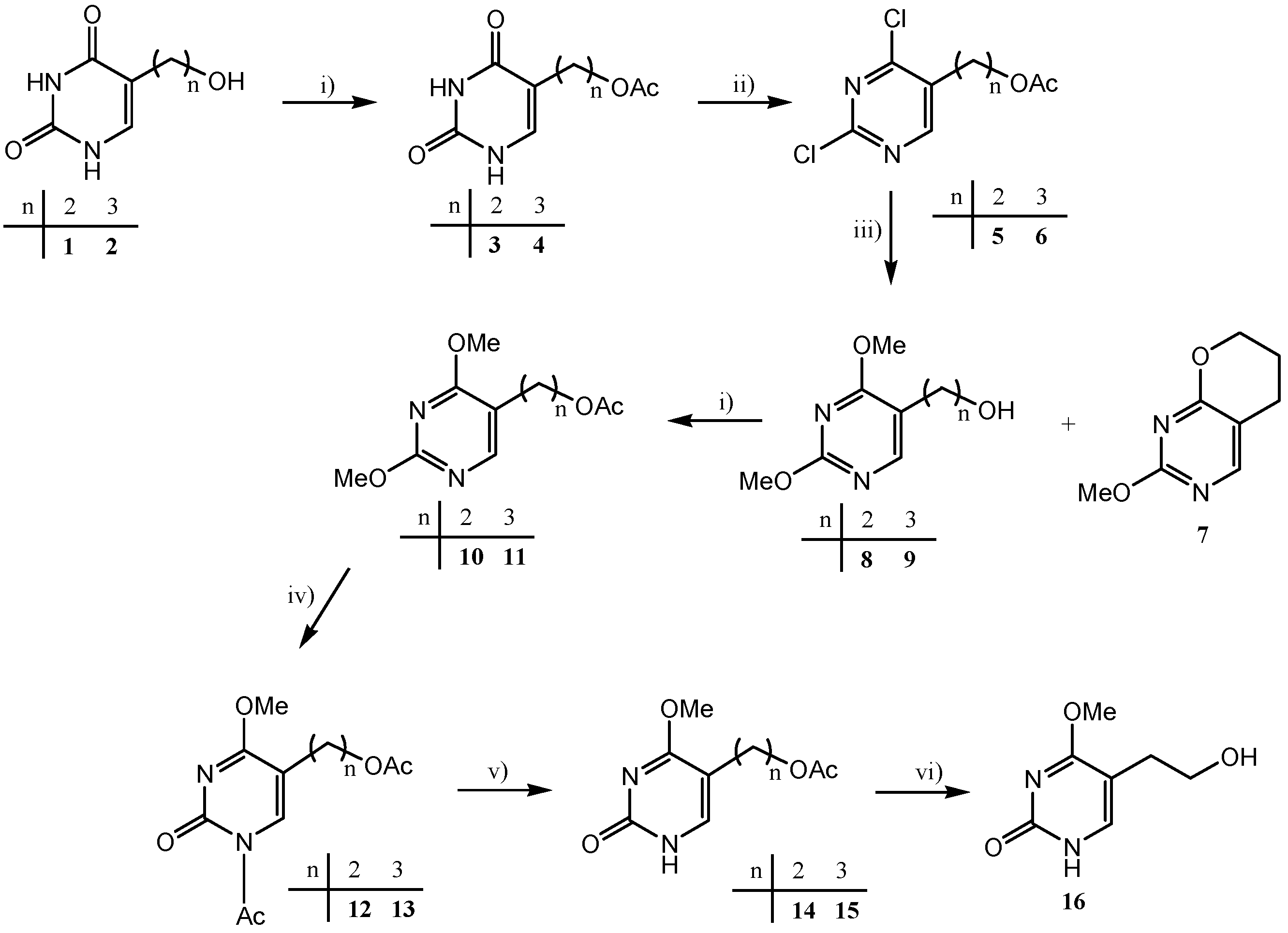

| Synthetic method A | 19 | 21 | 23 | |
| Yields | ||||
| 1. | Acetylation | 98% | 98% | 98% |
| 2. | Chlorination | 99% | 99% | 99% |
| 3. | Methoxylation | 95% | 95% | 95% |
| 4. | Acetylation | 90% | 90% | 90% |
| 5. | Alkylation/Hydroxylation | 73%/50% | 43% | 36% |
| 6. | Deprotection | 39% | 74% | 67% |
| Overall yield: | 12% | 26% | 20% | |
| Synthetic method B | 19 | 21 | 23 | |
| Yields | ||||
| 1. | Acetylation | 98% | 98% | - |
| 2. | Chlorination | 99% | 99% | - |
| 3. | Methoxylation | 95% | 95% | - |
| 4. | Acetylation | 90% | 90% | - |
| 5. | Demethoxylation | 80% | 80% | - |
| 6. | Deacetylation | - | 93% | - |
| 7. | Silylation/Akylation | 75% | 25% | 8% |
| 8. | Deprotection | 37% | 26% | 65% |
| Overall yield: | 18% | 4% | 5% | |
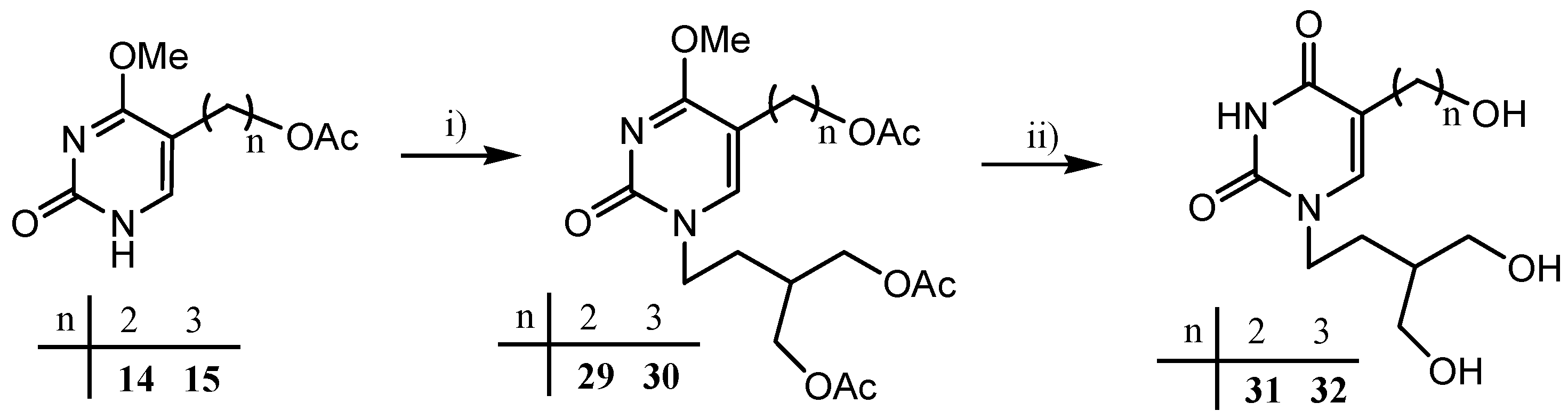
2.2. In Vitro Validation of Compounds 19, 21, 23, 31 and 32 as Substrates of HSV-1 TK and hTK



2.3. Cellular Activity Evaluation
3. Experimental
3.1. General
3.2. Procedures for the Preparation of Compounds
3.3. Phosphorylation Assay of 19, 21, 23, 31 and 32
3.3.1. Protein Expression
3.3.2. HPLC System and Conditions
3.3.3. The Phosphorylation Reaction
3.3.4. Cellular Activity Evaluations
4. Conclusions
Supplementary Materials
Acknowledgments
Conflicts of Interest
References
- Christopherson, R.I.; Lyons, S.D.; Wilson, P.K. Inhibitors of de novo nucleotide biosynthesis as drugs. Acc. Chem. Res. 2002, 35, 961–971. [Google Scholar] [CrossRef]
- Blackburn, G.M. Nucleosides and Nucleotides. In Nucleic Acids in Chemistry and Biology, 3rd; Blackburn, G.M., Gait, M.J., Loakes, D., Williams, D.M., Eds.; Royal Society of Chemistry: Cambridge, UK, 2006; pp. 125–136. [Google Scholar]
- Schaeffer, J.H.; Beauchamp, L.; de Miranda, P.; Elion, G.; Bauer, D.J.; Collins, P. 9-(2-Hydroxyethoxymethyl)guanine activity against viruses of the herpes group. Nature 1978, 292, 583–585. [Google Scholar]
- Elion, G.B.; Furman, P.A.; Fyfe, J.A.; de Miranda, P.; Beauchamp, L.; Schaeffer, H.J. Selectivity of action of an antiherpetic agent, 9-(2-hydroxyethoxymethyl)guanine. Proc. Natl. Acad. Sci. USA 1977, 74, 5716–5720. [Google Scholar] [CrossRef]
- De Clerq, E.; Field, H.J. Antiviral prodrugs: The development of successful prodrug strategies for antiviral chemotheraphy. Br. J. Pharmacol. 2006, 147, 1–11. [Google Scholar] [CrossRef]
- Kim, H.S.; Barak, D.; Harden, K.T.; Boyer, J.L.; Jacobson, K.A. Acyclic and cyclopropyl analogues of adenosine bisphosphate antagonists of the P2Y1 receptor: Structure-activity relationships and receptor docking. J. Med. Chem. 2001, 44, 3092–3108. [Google Scholar] [CrossRef]
- Smee, D.F.; Boehme, R.; Chernow, M.; Binko, B.P.; Matthews, T.R. Intracellular metabolism and enzymatic phosphorylation of 9-(1,3-dihydroxy-2-propoxymethyl)guanine and acyclovir in herpes simplex virus-infected and uninfected cells. Biochem. Pharmacol. 1985, 34, 1049–1056. [Google Scholar] [CrossRef]
- Martin, J.C.; McGee, D.P.C.; Jeffrey, G.A.; Hobbs, D.W.; Smee, D.F.; Matthews, T.R.; Verheyden, J.P.H. Synthesis and anti-herpes virus activity of acyclic 2'-deoxyguanosine analogs related to 9-[(1,3-dihydroxy-2-propoxy)methyl]guanine. J. Med. Chem. 1986, 29, 1384–1389. [Google Scholar] [CrossRef]
- Vere Hodge, R.A.; Perkins, R.M. Mode of action of 9-(4-hydroxy-3-hydroxymethylbut-1-yl)guanine (BRL 39123) against herpes simplex virus in MRC-5 cells. Antimicrob. Agents Chemother. 1989, 33, 223–229. [Google Scholar] [CrossRef]
- Oldfield, E.H.; Ram, Z.; Culver, K.W.; Blaese, R.M.; de Vroom, H.L.; Anderson, W.F. Gene therapy for the treatment of brain tumors using intra-tumoral transduction with the thymidine kinase gene and intravenous ganciclovir. Hum. Gene Ther. 1993, 4, 39–69. [Google Scholar] [CrossRef]
- Verma, I.M.; Somia, N. Gene therapy - promises, problems and prospects. Nature 1997, 389, 239–242. [Google Scholar] [CrossRef]
- Freeman, S.M. Suicide gene therapy. Adv. Exp. Med. Biol. 2000, 465, 411–422. [Google Scholar] [CrossRef]
- Fillat, C.; Carrió, M.; Cascante, A.; Sangro, B. Suicide gene theraphy mediated by the herpes simplex virus thymidine kinase gene/ganciclovir system: Fifteen years of application. Curr. Gene Ther. 2003, 3, 13–26. [Google Scholar] [CrossRef]
- Shiue, G.G.; Shiue, C.-Y.; Lee, R.L.; MacDonald, D.; Hustinx, R.; Eck, S.L.; Alavi, A.A. A simplified one-pot synthesis of 9-[(3-[18F]Fluoro-1-hydroxy-2-propoxy)methyl]guanine([18F]FHPG) and 9-(4-[18F]Fluoro-3-hydroxymethylbutyl)guanine ([18F]FHBG) for gene therapy. Nucl. Med. Biol. 2001, 28, 875–883. [Google Scholar] [CrossRef]
- Alauddin, M.M.; Shahinian, A.; Gordon, E.M.; Bading, J.R.; Conti, P.S. Preclinical evaluation of the penciclovir analog 9-(4-[18F]fluoro-3-hydroxymethylbutyl)guanine for in vivo measurement of suicide gene expression with PET. J. Nucl. Med. 2001, 42, 1682–1690. [Google Scholar]
- Min, J.J.; Gambhir, S.S. Gene therapy progress and prospects: Noninvasive imaging of gene therapy in living subjects. Gene Ther. 2004, 11, 115–125. [Google Scholar]
- Degreve, B.; de Clercq, E.; Balzarini, J. Bystander effect of purine nucleoside analogues in HSV-1tk suicide gene therapy is superior to that of pyrimidine nucleoside analogues. Gene Ther. 1999, 6, 162–170. [Google Scholar] [CrossRef]
- Raić-Malić, S.; Johayem, A.; Ametamey, S.M.; Batinac, S.; de Clercq, E.; Folkers, G.; Scapozza, L. Synthesis, 18F-radiolabelling and biological evaluations of C-6 alkylated pyrimidine nucleoside analogues. Nucleosides Nucleotides Nucleic Acids 2004, 23, 1707–1721. [Google Scholar]
- Martić, M.; Pernot, L.; Westermaier, Y.; Perozzo, R.; Gazivoda Kraljević, T.; Krištafor, S.; Raić-Malić, S.; Scapozza, L.; Ametamey, S. Synthesis, crystal structure, and in vitro biological evaluation of C-6 pyrimidine derivatives: new lead structures for monitoring gene expression in vivo. Nucleosides Nucleotides Nucleic Acids 2011, 30, 293–315. [Google Scholar] [CrossRef]
- Krištafor, S.; Novaković, I.; Gazivoda Kraljević, T.; Kraljević Pavelić, S.; Lučin, P.; Westermaier, Y.; Scapozza, L.; Ametamey, S.M.; Raić-Malić, S. Synthetic approach to new N-methyl thymine derivative comprising dihydroxyisobutenyl unit as ligand for thymidine kinase of herpes simplex virus type 1 (HSV1-TK). Bioorg. Med. Chem. Lett. 2011, 21, 6161–6165. [Google Scholar] [CrossRef]
- Müller, U.; Martić, M.; Gazivoda-Kraljević, T.; Krištafor, S.; Ranadheera, C.; Müller, A.; Born, M.; Krämer, S.D.; Raić-Malić, S.; Ametamey, M.S. Synthesis and evaluation of a C-6 alkylated pyrimidine derivative for in vivo imaging of HSV1-TK gene expression. Nucl. Med. Biol. 2012, 39, 235–246. [Google Scholar] [CrossRef]
- Fissekis, J.D.; Myles, A.; Brown, G.B. Synthesis of 5-hydroxyalkylpyrimidines from lactones. J. Org. Chem. 1964, 29, 2670–2673. [Google Scholar] [CrossRef]
- Fissekis, J.D.; Sweet, F. Chemistry of some-5-(2-hydroxyalkyl)uracil derivatives and a synthesis of 5-vinyluracil. J. Org. Chem. 1973, 38, 264–269. [Google Scholar] [CrossRef]
- Denny, G.H.; Ryder, M.A. α-(Ureidomethylene)lactones and derived 5-(hyroxyalkyl)uracils. J. Med. Chem. 1974, 17, 1230–1231. [Google Scholar] [CrossRef]
- Griengl, H.; Wanek, E.; Schwarz, W.; Strecher, W.; Rosenwirth, B.; de Clercq, E. 2'-Fluorinated arabinonucleosides of 5-(2-haloalkyl)uracil: Synthesis and antiviral activity. J. Med. Chem. 1987, 30, 1199–1204. [Google Scholar] [CrossRef]
- Yu, C.-S.; Oberdorfer, F. Synthesis of 4-O-methyl-protected 5-(2-hydroxyethyl)-2′-deoxyuridine derivatives and their nucleophilic fluorination to 5-(2-fluoroethyl)-2′-deoxyuridine. Synthesis 1999, 12, 2057–2064. [Google Scholar]
- Hilbert, G.E.; Johnson, T.B. Researches on pyrimidines. CXVII. A method for the synthesis of nucleosides. J. Am. Chem. Soc. 1930, 52, 4489–4494. [Google Scholar] [CrossRef]
- Ogilvie, K.K.; Hamilton, R.G.; Gillen, M.F.; Radatus, B.K.; Smith, K.O.; Galloway, K.S. Uracil analogues of the acyclonucleoside 9-[[2-hydroxy-1-(hydroxymethyl)ethoxy]methyl]guanine (BIOLF-62). Can. J. Chem. 1984, 62, 16–21. [Google Scholar] [CrossRef]
- Robins, M.J.; Hatfield, P.W. Nucleic acid related compounds. 37. Convenient and high-yield syntheses of N-[(2-hydroxyethoxy)methyl] heterocycles as “acylic nucleoside” analogues. Can. J. Chem. 1982, 60, 547–553. [Google Scholar] [CrossRef]
- Brand, B.; Reese, C.B.; Song, Q.; Visintin, C. Convenient syntheses of 9-[4-hydroxy-3-(hydroxymethyl)butyl]guanine (penciclovir) and 9-[4-acetoxy-3-(acetoxymethyl)butyl]2-amino-9H-purine (famciclovir). Tetrahedron 1999, 55, 5239–5252. [Google Scholar]
- Geen, G.R.; Grinter, T.J.; Kincey, P.M.; Jarvest, R.L. The effect of the C-6 substituent on the regioselectivity of N-alkylation of 2-aminopurines. Tetrahedron 1990, 46, 6903–6914. [Google Scholar] [CrossRef]
- Schelling, P.; Claus, M.T.; Johner, R.; Marquez, V.E.; Schulz, G.E.; Scapozza, L. Biochemical and structural characterization of (south)-methanocarbathymidine that specifically inhibits growth of herpes simplex virus type 1 thymidine kinase-transduced osteosarcoma cells. J. Biol. Chem. 2004, 279, 32832–32838. [Google Scholar]
- Vogt, J.; Perozzo, R.; Pautsch, A.; Prota, A.; Schelling, P.; Pilger, B.; Folkers, G.; Scapozza, L.; Schulz, G.E. Nucleoside binding site of herpes simplex type 1 thymidine kinase analyzed by X-ray crystallography. Proteins 2000, 41, 545–553. [Google Scholar] [CrossRef]
- Birringer, M.S.; Perozzo, R.; Kut, E.; Stillhart, C.; Surber, W.; Scapozza, L.; Folkers, G. High-level expression and purification of human thymidine kinase 1: Quaternary structure, stability, and kinetics. Protein Expr. Purif. 2006, 47, 506–515. [Google Scholar] [CrossRef]
- Gazivoda, T.; Raić-Malić, S.; Krištafor, V.; Makuc, D.; Plavec, J.; Bratulić, S.; Kraljević-Pavelić, S.; Pavelić, K.; Naesens, L.; Andrei, G.; et al. Synthesis, cytostatic and anti-HIV evaluations of the new unsaturated acyclic C-5 pyrimidine nucleoside analogues. Bioorg. Med. Chem. 2008, 16, 5624–5634. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 1−32 are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Meščić, A.; Krištafor, S.; Novaković, I.; Osmanović, A.; Müller, U.; Završnik, D.; Ametamey, S.M.; Scapozza, L.; Raić-Malić, S. C-5 Hydroxyethyl and Hydroxypropyl Acyclonucleosides as Substrates for Thymidine Kinase of Herpes Simplex Virus Type 1 (HSV-1 TK): Syntheses and Biological Evaluation. Molecules 2013, 18, 5104-5124. https://doi.org/10.3390/molecules18055104
Meščić A, Krištafor S, Novaković I, Osmanović A, Müller U, Završnik D, Ametamey SM, Scapozza L, Raić-Malić S. C-5 Hydroxyethyl and Hydroxypropyl Acyclonucleosides as Substrates for Thymidine Kinase of Herpes Simplex Virus Type 1 (HSV-1 TK): Syntheses and Biological Evaluation. Molecules. 2013; 18(5):5104-5124. https://doi.org/10.3390/molecules18055104
Chicago/Turabian StyleMeščić, Andrijana, Svjetlana Krištafor, Ivana Novaković, Amar Osmanović, Ursina Müller, Davorka Završnik, Simon M. Ametamey, Leonardo Scapozza, and Silvana Raić-Malić. 2013. "C-5 Hydroxyethyl and Hydroxypropyl Acyclonucleosides as Substrates for Thymidine Kinase of Herpes Simplex Virus Type 1 (HSV-1 TK): Syntheses and Biological Evaluation" Molecules 18, no. 5: 5104-5124. https://doi.org/10.3390/molecules18055104





