Microwave-Assisted Synthesis of New Selenazole Derivatives with Antiproliferative Activity
Abstract
:1. Introduction
2. Results and Discussion
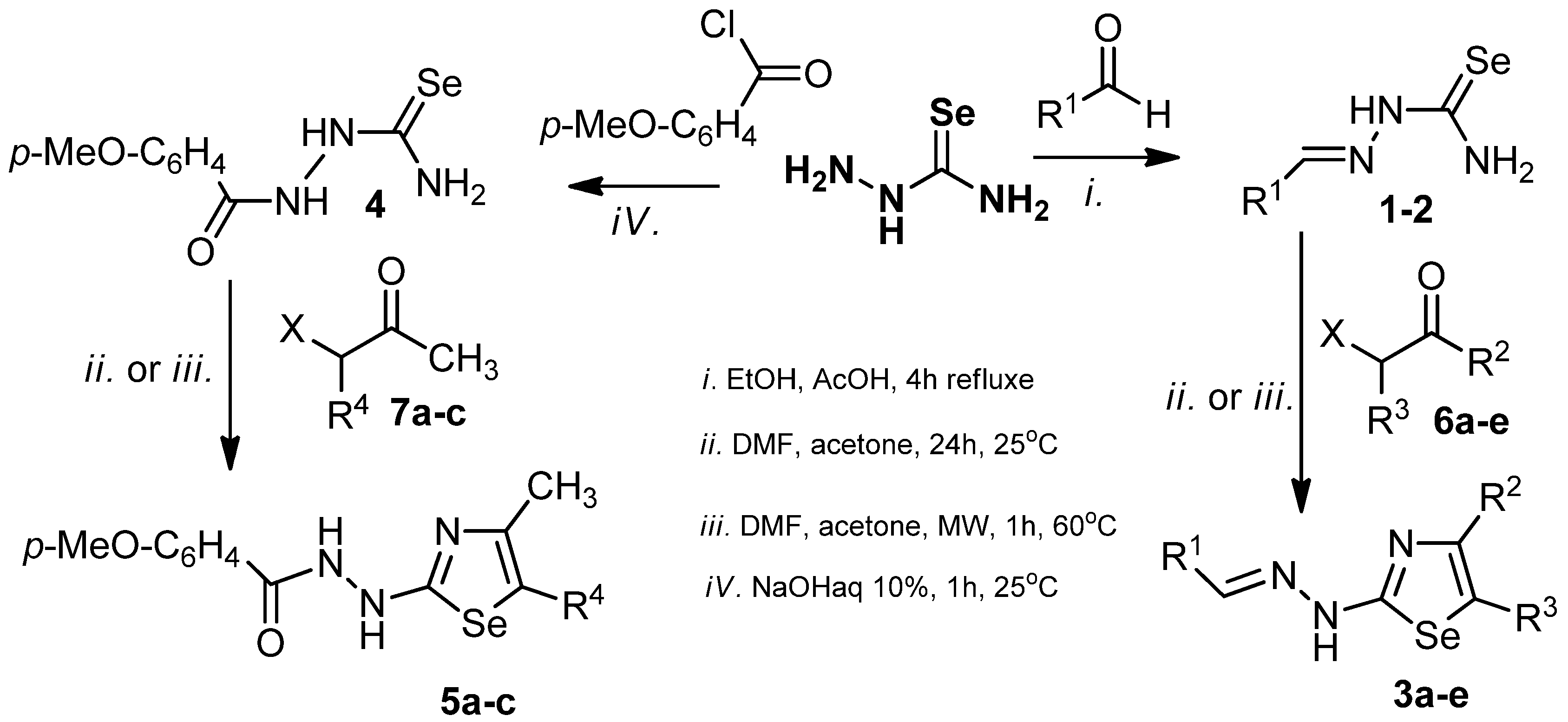
| Comp. | R1 | R2 | R3 | X | Comp. | R1 | R4 | X |
|---|---|---|---|---|---|---|---|---|
| 3a | p-MeO-C6H4 | COOEt | H | 1 | p-Cl-C6H4 | - | - | |
| 3b | p-MeO-C6H4 | Me | COMe | 2 | p-MeO-C6H4 | - | - | |
| 3c | p-MeO-C6H4 | CH2Cl | H | 5a | - | COOEt | - | |
| 3d | p-Cl-C6H4 | Me | COMe | 5b | - | COMe | - | |
| 3e | p-Cl-C6H4 | COOEt | H | 5c | - | H | - | |
| 6a | - | COOEt | H | Br | 7a | - | COOEt | Br |
| 6b | - | Me | COMe | Cl | 7b | - | COMe | Cl |
| 6c | - | CH2Cl | H | Cl | 7c | - | H | Cl |
| 6d | - | Me | COMe | Cl | ||||
| 6e | - | COOEt | H | Br |
| Compounds | 3a | 3b | 3c | 3d | 3e | 5a | 5b | 5c |
|---|---|---|---|---|---|---|---|---|
| Yield (%) MW a | 93 | 92 | 95 | 94 | 91 | 94 | 93 | 87 |
| Yield (%) b | 52 | 51 | 56 | 56 | 60 | 57 | 63 | 67 |
| Compounds | Cell lines and IC50 values (µM) | ||||
|---|---|---|---|---|---|
| CCRF-CEM | HL60 | MDA-MB231 | HCT116 | U87MG | |
| 3a | 6.36 ± 0.66 | 48.44 ± 11.14 | >113.31 | >113.31 | >113.31 |
| 3b | 8.87 ± 2.52 | 14.42 ± 234.31 | 72.60 ± 47.56 | 53.37 ± 8.67 | 66.53 ± 6.36 |
| 3c | 5.11 ± 0.30 | 27.67 ± 8.45 | 85.24 ± 6.00 | 35.96 ± 4.17 | 65.41 ± 0.47 |
| 3d | 9.97 ± 1.58 | 17.24 ± 1.66 | 42.68 ± 1.18 | 35.13 ± 3.77 | 30.32 ± 1.08 |
| 3e | 8.40 ± 2.15 | 12.86 ± 1.99 | 65.72 ± 0.37 | 46.14 ± 0.97 | 59.12 ± 5.97 |
| 4 | 6.88 ± 1.53 | 10.62 ± 0.88 | 21.98 ± 0.63 | 23.51 ± 0.86 | 27.56 ± 10.02 |
| 5a | 8.33 ± 2.03 | 29.88 ± 0.17 | 61.19 ± 4.86 | 24.99 ± 2.58 | 29.80 ± 1.68 |
| 5b | 6.43 ± 0.96 | 13.23 ± 0.12 | 16.90 ± 4.55 | 22.25 ± 1.66 | 20.95 ± 1.62 |
| 5c | 5.67 ± 3.87 | 11.94 ± 0.72 | 29.19 ± 1.92 | 34.66 ± 3.21 | 25.22 ± 7.23 |
| Doxorubicin | 0.20 ± 0.06 | 0.73 ± 0.20 | 1.10 ± 0.28 | 1.41 ± 0.29 | 1.06 ± 0.15 |
3. Experimental
3.1. General
3.2. General Procedure for the Preparation of Arylhydrazinoselenazoles 3a–e
3.3. General Procedure for the Preparation of Aroylhydrazinoselenazoles 5a–c
3.4. Cytotoxicity Assay
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Muller, D.; Desel, H. Acute selenium poisoning by paradise nuts (Lecythis ollaria). Hum. Exp. Toxicol. 2010, 29, 431–434. [Google Scholar] [CrossRef]
- Klapec, T.; Mandić, M.L.; Grgić, J.; Primorac, L.; Ikić, M.; Lovrić, T.; Grgić, Z.; Herceg, Z. Daily dietary intake of selenium in eastern Croatia. Sci. Total Environ. 1998, 217, 127–136. [Google Scholar] [CrossRef]
- Nishina, A.; Kimura, H.; Kozawa, K.; Sommen, G.L.; Nakamura, T.; Heimgartner, H.; Koketsu, M.; Furukawa, S. A superoxide anion-scavenger, 1,3-selenazolidin-4-one suppresses serum deprivation-induced apoptosis in PC12 cells by activating MAP kinase. Toxicol. Appl. Pharmacol. 2011, 257, 388–395. [Google Scholar] [CrossRef] [Green Version]
- Sekiguchi, A.; Nishina, A.; Kimura, H.; Fukumoto, R.; Kogami, M.; Ishihara, H.; Koketsu, M. Bis-(2-amino-5-selenazoyl) ketone as a superoxide anion-scavenger. Pharm. Bull. 2006, 29, 1404–1407. [Google Scholar] [CrossRef]
- Takahashi, H.; Nishina, A.; Kimura, H.; Motoki, K.; Koketsu, M.; Ishihara, H. Tertiary selenoamide compounds are useful superoxide radical scavengers in vitro. Eur. J. Pharm. Sci. 2004, 23, 207–211. [Google Scholar]
- Takahashi, H.; Nishina, A.; Fukumoto, R.; Kimura, H.; Koketsu, M.; Ishihara, H. Selenoureas and thioureas are effective superoxide radical scavengers in vitro. Life Sci. 2005, 76, 2185–2192. [Google Scholar] [CrossRef]
- Takahashi, H.; Nishina, A.; Fukumoto, R.; Kimura, H.; Koketsu, M.; Ishihara, H. Selenocarbamates are effective superoxide anion scavengers in vitro. Eur. J. Pharm. Sci. 2005, 24, 291–295. [Google Scholar] [CrossRef]
- Bijian, K.; Zhang, Z.; Xu, B.; Jie, S.; Chen, B.; Wan, S.; Wu, J.; Jiang, T.; Alaoui-Jamali, M.A. Synthesis and biological activity of novel organoselenium derivatives targeting multiple kinases and capable of inhibiting cancer progression to metastases. Eur. J. Med. Chem. 2012, 48, 143–152. [Google Scholar] [CrossRef]
- Madhunapantula, S.V.; Desai, D.; Sharma, A.; Huh, S.J.; Amin, S.; Robertson, G.P. PBISe, a novel selenium-containing drug for the treatment of malignant melanoma. Mol. Cancer Ther. 2008, 7, 1297–308. [Google Scholar]
- Desai, D.; Madhunapantula, S.V.; Gowdahalli, K.; Sharma, A.; Chandagaludoreswamy, R.; El-Bayoumy, K.; Robertson, G.P.; Amin, S. Synthesis and characterization of a novel iNOS/Akt inhibitor Se,Se0-1,4-phenylenebis(1,2-ethanediyl)bisisoselenourea (PBISe)—against colon cancer. Bioorg. Med. Chem. Lett. 2010, 20, 2038–2043. [Google Scholar] [CrossRef]
- Xing, F.; Li, S.; Ge, X.; Wang, C.; Zeng, H.; Li, D.; Dong, L. The inhibitory effect of a novel organoselenium compound BBSKE on the tongue cancer Tca8113 in vitro and in vivo. Oral Oncol. 2008, 44, 963–969. [Google Scholar] [CrossRef]
- Wang, L.; Yang, Z.; Fu, J.; Yin, H.; Xiong, K.; Tan, Q.; Jin, H.; Li, J.; Wang, T.; Tang, W.; et al. Ethaselen: a potent mammalian thioredoxin reductase 1 inhibitor and novel organoselenium anticancer agent. Free Radic. Biol. Med. 2012, 52, 898–908. [Google Scholar] [CrossRef]
- Terazawa, R.; Garud, D.R.; Hamada, N.; Fujita, Y.; Itoh, T.; Nozawa, Y.; Nakane, K.; Deguchi, T.; Koketsu, M.; Ito, M. Identification of organoselenium compounds that possess chemopreventive properties in human prostate cancer LNCaP cells. Bioorg. Med. Chem. 2010, 18, 7001–7008. [Google Scholar] [CrossRef]
- Merino-Montiel, P.; Maza, S.; Martos, S.; LOpez, O.; Maya, I.; Fernandez-Bolanos, J.G. Synthesis and antioxidant activity of O-alkyl selenocarbamates, selenoureas and selenohydantoins. Eur. J. Pharm. Sci. 2013, 48, 582–592. [Google Scholar] [CrossRef]
- Barbieri Gerzson, M.F.; Victoria, F.N.; Radatz, C.S.; de Gomes, M.G.; Boeira, S.P.; Jacob, R.G.; Alves, D.; Jesse, C.R.; Savegnago, L. In vitro antioxidant activity and in vivo antidepressant-like effect of α-(phenylselanyl) acetophenone in mice. Pharmacol. Biochem. Behav. 2012, 102, 21–29. [Google Scholar] [CrossRef]
- Acker, C.I.; Brandão, R.; Rodrigues Rosário, A.; Nogueira, C.W. Antioxidant effect of alkynylselenoalcohol compounds on liver and brain of rats in vitro. Environ. Toxicol. Phar. 2009, 28, 280–287. [Google Scholar] [CrossRef]
- Nam, K.N.; Koketsu, M.; Lee, E.H. 5-Chloroacetyl-2-amino-1,3-selenazoles attenuate microglial inflammatory responses through NF-κB inhibition. Eur. J. Pharmacol. 2008, 589, 53–57. [Google Scholar] [CrossRef]
- Letavayova, L.; Vlasakova, D.; Spallholz, J.E.; Brozmanova , J.; Chovanec, M. Toxicity and mutagenicity of selenium compounds in Saccharomyces cerevisiae. Mutat. Res. 2008, 638, 1–10. [Google Scholar] [CrossRef]
- Santos, D.B.; Schiar, V.P.P.; Ribeiro, M.C.P.; Schwab, R.S.; Meinerz, D.F.; Allebrandt, J.; Rocha, J.B.T.; Nogueira, C.W.; Aschner, M.; Barbosa, N.B.V. Genotoxicity of organoselenium compounds in human leukocytes in vitro. Mutat. Res. 2009, 676, 21–26. [Google Scholar] [CrossRef]
- Rosa, R.M.; Picada, J.N.; Saffi, J.; Henriques, J.A.P. Cytotoxic, genotoxic, and mutagenic effects of diphenyl diselenide in Chinese hamster lung fibroblasts. Mutat. Res. 2007, 628, 87–98. [Google Scholar] [CrossRef]
- Tao, H.; Weng, Y.; Zhuo, Z.; Chang, G.; Urbatsch, L.I.; Zhang, Q. Design and synthesis of Selenazole-containing peptides for cocrystallization with P-glycoprotein. ChemBioChem 2011, 12, 868–873. [Google Scholar] [CrossRef]
- Cozma, A.; Vlase, L.; Ignat, A.; Zaharia, V.; Gocan, S.; Marutoiu, C.; Fodor, A. Lipophilicity study of new selenazole derivatives by RP-HPLC. Rev. Chim. (Bucharest) 2012, 63, 651–655. [Google Scholar]
- Cozma, A.; Zaharia, V.; Ignat, A.; Gocan, S.; Grinberg, N. Prediction of the lipophilicity of nine new synthesized selenazoly and three aroyl–hydrazinoselenazoles derivatives by reversed-phase high performance thin-layer chromatography. J. Chromatogr. Sci. 2012, 50, 157–161. [Google Scholar] [CrossRef]
- Zaharia, V.; Ignat, A.; Ngameni, B.; Kuete, V.; Moungang, M.L.; Fokunang, C.N.; Vasilescu, M.; Palibroda, N.; Cristea, C.; Silaghi-Dumitrescu, L.; et al. Heterocycles 23: Synthesis, characterization and anticancer activity of new hydrazinoselenazole derivatives. Med. Chem. Res. 2013, 558. [Google Scholar] [CrossRef]
- Gaina, L.; Gal, E.; Mataranga-Popa, L.; Porumb, D.; Nicolescu, A.; Cristea, C.; Silaghi-Dumitrescu, L. Synthesis, structural investigations, and DFT calculations on novel 3-(1,3-dioxan-2-yl)-10-methyl-10H-phenothiazine derivatives with fluorescence properties. Tetrahedron 2011, 68, 2465–2470. [Google Scholar]
- Gaina, L.; Porumb, D.; Silaghi-Dumitrescu, L.; Cristea, C.; Silaghi-Dumitrescu, L. On the microwave-assisted synthesis of acylphenothiazine derivatives-Experiment versus theory synergism. Can. J. Chem. 2010, 88, 42–49. [Google Scholar] [CrossRef]
- Gaina, L.; Cristea, C.; Moldovan, C.; Porumb, D.; Surducan, E.; Deleanu, C.; Mahamoud, A.; Barbe, J.; Silberg, I.A. Microwave-assisted synthesis of phenothiazine and quinoline derivatives. Int. J. Mol. Sci. 2007, 8, 70–80. [Google Scholar] [CrossRef]
- Vaibhav, P.M.; Van der Eycken, E.V. Microwave-assisted C-C bond forming cross-coupling reactions: an overview. Chem. Soc. Rev. 2011, 40, 4925–4936. [Google Scholar] [CrossRef]
- Audi, G.; Wapstra, A.H. The 1993 atomic mass evaluation. Nucl. Phys. A 1993, 565, 1–65. [Google Scholar] [CrossRef]
- Audi, G.; Wapstra, A.H. The 1995 update to the atomic mass evaluation. Nucl. Phys. A 1995, 595, 409–480. [Google Scholar] [CrossRef]
- Brahemi, G.; Kona, F.R.; Fiasella, A.; Buac, D.; Soukupov, J.; Brancale, A.; Burger, A.M.; Westwell, A.D. Exploring the structural requirements for inhibition of the ubiquitin E3 ligase breast cancer associated protein 2 (BCA2) as a treatment for breast cancer. J. Med. Chem. 2010, 53, 2757–2765. [Google Scholar] [CrossRef]
- Wiles, D.M.; Suprunchuk, T. The C==S stretching vibration in the infrared spectra of some thiosemicarbazones. II. Aldehyde thiosemicarbazones containing aromatic groups. Can. J. Chem. 1967, 45, 2258–2263. [Google Scholar]
- Collard-Charon, C.; Renson, M. Synthèse des Sélénosemicarbazides Substituées III. Synthèse des sélénosemicarbazides substituées en 1. Bull. Soc. Chim. Belg. 1962, 71, 554–562. [Google Scholar] [CrossRef]
- O’Brien, J.; Wilson, I.; Orton, T.; Pognan, F. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur. J. Biochem. 2000, 267, 5421–5426. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 1, 2, 3a–e, 4, 5a–c are available from the author A. Ignat (Grozav).
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Grozav Ignat, A.; Gaina, L.; Kuete, V.; Silaghi-Dumitrescu, L.; Efferth, T.; Zaharia, V. Microwave-Assisted Synthesis of New Selenazole Derivatives with Antiproliferative Activity. Molecules 2013, 18, 4679-4688. https://doi.org/10.3390/molecules18044679
Grozav Ignat A, Gaina L, Kuete V, Silaghi-Dumitrescu L, Efferth T, Zaharia V. Microwave-Assisted Synthesis of New Selenazole Derivatives with Antiproliferative Activity. Molecules. 2013; 18(4):4679-4688. https://doi.org/10.3390/molecules18044679
Chicago/Turabian StyleGrozav Ignat, Adriana, Luiza Gaina, Victor Kuete, Luminita Silaghi-Dumitrescu, Thomas Efferth, and Valentin Zaharia. 2013. "Microwave-Assisted Synthesis of New Selenazole Derivatives with Antiproliferative Activity" Molecules 18, no. 4: 4679-4688. https://doi.org/10.3390/molecules18044679





