3. Experimental
3.1. General Techniques
Melting points were determined in open capillary tubes on a Boetius melting point apparatus and are uncorrected. NMR spectra (600/150 MHz) were recorded on a Bruker MSL 600 spectrometer in CDCl3 solvents with tetramethylsilane as internal standard; chemical shifts are reported in ppm (δ) and J values in Hz. Multiplicity is designated as singlet (s), doublet (d), triplet (t), quartet (q), multiplet (m). Mass spectra were recorded under EI conditions on a Finnigan MAT 95 instrument, IR spectra (KBr, pellet) on a IRAffinity-1 Shimadzu spectrophotometer. Elemental C, H analyses were obtained on a Carlo Erba Model 1108 analyzer. TLC was performed on silica gel 60 254F plates (Merck, Darmstadt, Germany) using a mixture of chloroform and ethanol (20:1 or 40:1, v/v) as an eluent. Spots were visualized by spraying with solution of 5% sulfuric acid, followed by heating. Column chromatography was performed on silica gel 60, <63 μm (Merck) using a mixture of chloroform and ethanol (40:1, v/v) as an eluent. Solvents were dried and purified according to usual procedures.
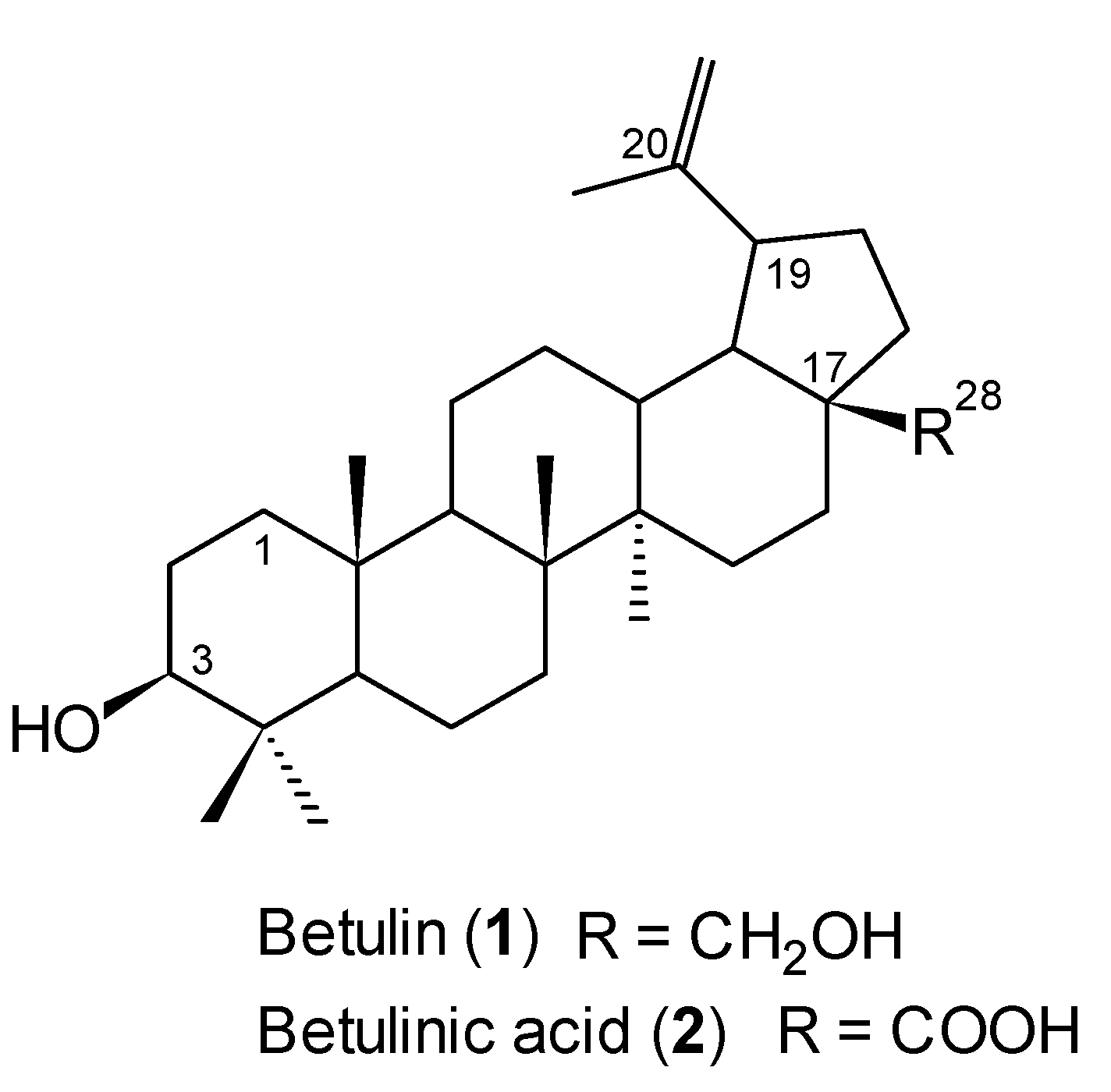
3.2. Isolation of Betulin (1)
External bark (100 g) of the white birch (
Betula verrucosa), collected in Poland, was soaked in dichloromethane (1 L) and refluxed for 8 hours. The solvent was removed under reduced pressure and the crude product was purified by column chromatography (chloroform/ethanol, 20:1, v/v) to give
1 as a white solid (19 g, 19%): m.p. 250–252 °C (lit. m.p. 252–253 °C [
6]; 255–256 °C [
27]); R
f 0.42 (chloroform/ethanol, 20:1, v/v). The
1H- and
13C-NMR spectral data of
1 were in agreement with those published in the literature [
6].
3.3. General Procedure for the Synthesis of Monoesters 3 and Diesters 4
To a mixture of betulin (1, 0.44 g, 1 mmol) and pyridine (2.5 mL) in dry benzene (6 mL) at 0–5 °C, a solution of propargyl chloroformate or 2-butyn-1-yl chloroformate or 3-butyn-1-yl chloroformate or ethyl chloroformate (3.0 mmol) in dry benzene (5 mL) was added. Stirring at this temperature was continued for 4 h. Then the reaction mixture was allowed to warm to room temperature and stirred over night. At the end of the reaction 5 mL of chloroform was added and the reaction mixture was washed with 1 N sulfuric acid, followed by water, then dried with anhydrous magnesium sulfate and concentrated under reduced pressure. The crude product was purified by column chromatography (chloroform/ethanol, 40:1, v/v) to give pure corresponding monoesters 3 and diesters 4.
28-O-Propargyloxycarbonylbetulin (3a). Yield: 69%, m.p. 190–193 °C, Rf 0.49 (chloroform/ethanol, 40:1, v/v); 1H-NMR (CDCl3) δ: 0.76 (s, 3H, CH3), 0.82 (s, 3H, CH3), 0.97 (s, 3H, CH3), 0.98 (s, 3H, CH3), 1.03 (s, 3H, CH3), 1.68 (s, 3H, CH3), 1.06–2.01 (m, 25H, CH, CH2), 2.42 (m, 1H, H-19), 2.53 (t, J = 2.4 Hz, 1H, C≡CH), 3.18 (m, 1H, H-3), 3.95 (d, J = 10.8 Hz, 1H, H-28), 4.38 (d, J = 10.8 Hz, 1H, H-28), 4.59 (s, 1H, H-29), 4.69 (s, 1H, H-29), 4.74 (d, J = 2.4 Hz, 2H, OCH2). 13C-NMR (CDCl3) δ: 14.9, 15.3, 15.4, 15.9, 16.2, 18.3, 19.1, 20.8, 25.2, 26.9, 27.4, 27.9, 28.0, 29.5, 34.2, 34.3, 37.1, 37.6, 38.7, 38.8. 40.8, 42.7, 46.5, 47.6, 48.8, 50.3, 55.2, 55.4, 67.3, 75.6, 78.9, 109.9, 149.9, 155.1. IR (KBr, cm−1) ν: 3421, 3309, 2132, 1749, 1250, 885. EI MS (70 eV) m/z (rel. intensity): 524 (M+, 11), 411 (45), 207 (59), 203 (58), 189 (100), 135 (58); Elemental anal. (%), calcd. for C34H52O4: C, 77.82; H, 9.99; found: C, 77.59; H, 9.84.
28-O-(3-Butynyloxycarbonyl)betulin (3b). Yield: 64%, m.p. 86–88 °C, Rf 0.45 (chloroform/ethanol, 40:1, v/v); 1H-NMR (CDCl3) δ: 0.76 (s, 3H, CH3), 0.82 (s, 3H, CH3), 0.97 (s, 3H, CH3), 0.98 (s, 3H, CH3), 1.03 (s, 3H, CH3), 1.68 (s, 3H, CH3), 1.05–2.02 (m, 25H, CH, CH2), 2.03 (t, J = 2.7 Hz, 1H, C≡CH), 2.43 (m, 1H, H-19), 2.59 (m, 2H, J = 2.7 Hz, J = 7.2 Hz, OCH2CH2), 3.19 (m, 1H, H-3), 3.93 (d, J = 10.8 Hz, 1H, H-28), 4.25 (t, J = 7.2 Hz, 2H, OCH2), 4.36 (d, J = 10.8 Hz, 1H, H-28), 4.59 (s, 1H, H-29), 4.69 (s, 1H, H-29). 13C-NMR (CDCl3) δ: 14.7, 15.3, 15.9, 16.1, 18.2, 19.0, 20.7, 25.1, 26.9, 27.3, 27.9, 29.4, 34.1, 34.3, 37.1, 37.5, 38.6, 38.8, 40.8, 42.6, 46.5, 47.6, 48.7, 50.3, 55.2, 65.3, 66.7, 70.2, 76.7, 78.9, 79.4, 109.9, 150.0, 155.4. IR (KBr, cm−1) ν: 3485, 3310, 2124, 1747, 1248, 884. EI MS (70 eV) m/z (rel. intensity): 538 (M+, 10), 207 (47), 203 (52), 189 (100), 135 (57); Elemental anal. (%), calcd. for C35H54O4: C, 78.02; H, 10.10; found: C, 78.32; H, 9.98.
28-O-(2-Butynyloxycarbonyl)betulin (3c). Yield: 54%, m.p. 112–114 °C, Rf 0.48 (chloroform/ethanol, 40:1, v/v); 1H-NMR (CDCl3) δ: 0.76 (s, 3H, CH3), 0.82 (s, 3H, CH3), 0.96 (s, 3H, CH3), 0.97 (s, 3H, CH3), 1.03 (s, 3H, CH3), 1.68 (s, 3H, CH3), 1.06–2.01 (m, 25H, CH, CH2), 1.87 (t, J = 2.4 Hz, 3H, C≡CCH3), 2.42 (m, 1H, H-19), 3.19 (m, 1H, H-3), 3.94 (d, J = 10.8 Hz, 1H, H-28), 4.37 (d, J = 10.8 Hz, 1H, H-28), 4.59 (s, 1H, H-29), 4.69 (s, 1H, H-29), 4.70 (q, 2H, J = 2.4 Hz, OCH2). 13C-NMR (CDCl3) δ: 3.7, 14.7, 15.3, 15.9, 16.1, 18.2, 19.0, 20.7, 25.1, 26.9, 27.3, 27.9, 29.4, 29.4, 34.1, 34.3, 37.1, 37.5, 38.6, 38.8, 40.8, 42.6, 46.5, 47.6, 48.7, 50.2, 55.2, 56.1, 66.9, 72.5, 76.7, 78.9, 109.9, 150.0, 155.2. IR (KBr, cm−1) ν: 3462, 2942, 2248, 1748, 1248, 883. EI MS (70 eV) m/z (rel. intensity): 538 (M+, 14), 207 (48), 203 (53), 189 (100), 135 (60); Elemental anal. (%), calcd. for C35H54O4: C, 78.02; H, 10.10; found: C, 77.84; H, 10.22.
28-O-Ethoxycarbonylbetulin (3d). Yield: 68%, m.p. 94–96 °C, Rf 0.47 (chloroform/ethanol, 40:1, v/v); 1H-NMR (CDCl3) δ: 0.75 (s, 3H, CH3), 0.81 (s, 3H, CH3), 0.96 (s, 3H, CH3), 0.97 (s, 3H, CH3), 1.03 (s, 3H, CH3), 1.31 (t, J = 7.2 Hz, 3H, OCH2CH3), 1.67 (s, 3H, CH3), 1.05–2.02 (m, 25H, CH, CH2), 2.42 (m, 1H, H-19), 3.18 (m, 1H, H-3), 3.90 (d, J = 10.8 Hz, 1H, H-28), 4.20 (q, J = 7.2 Hz, 2H, OCH2), 4.32 (d, J = 10.8 Hz, 1H, H-28), 4.58 (s, 1H, H-29), 4.68 (s, 1H, H-29). 13C-NMR (CDCl3) δ: 14.2, 14.7, 15.3, 15.9, 16.0, 18.2, 19.0, 20.7, 25.1, 26.9, 27.3, 27.9, 29.5, 34.1, 34.3, 37.1, 37.5, 38.6, 38.8, 40.8, 42.6, 46.4, 47.6, 48.7, 50.3, 55.2, 63.9. 66.3, 78.9, 109.8, 150.0, 155.7. IR (KBr, cm−1) ν: 3486, 2942, 1744, 1249, 1013, 882. EI MS (70 eV) m/z (rel. intensity): 514 (M+, 18), 424 (40), 203 (54), 189 (100), 135 (56); Elemental anal. (%), calcd. For C33H54O4: C, 77.00; H, 10.57; found: C, 77.28; H, 10.43.
3,28-O,O'-Di(propargyloxycarbonyl)betulin (4a). Yield: 27%, m.p. 125–127 °C, Rf 0.77 (chloroform/ethanol, 40:1, v/v); 1H-NMR (CDCl3) δ: 0.84 (s, 3H, CH3), 0.85 (s, 3H, CH3), 0.91 (s, 3H, CH3), 0.96 (s, 3H, CH3), 1.02 (s, 3H, CH3), 1.67 (s, 3H, CH3), 1.07–2.02 (m, 25H, CH, CH2), 2.42 (m, 1H, H-19), 2.51 (t, J = 2.4 Hz, 1H, C≡CH), 2.53 (t, J = 2.4 Hz, 1H, C≡CH), 3.94 (d, J = 10.8 Hz, 1H, H-28), 4.33 (m, 1H, H-3), 4.37 (d, J = 10.8 Hz, 1H, H-28), 4.59 (s, 1H, H-29), 4.68 (s, 1H, H-29), 4.73 (d, J = 2.4 Hz, 2H, OCH2), 4.76 (d, J = 2.4, Hz, 2H, OCH2). 13C-NMR (CDCl3) δ: 15.9, 16.0, 16.1, 16.2, 16.3, 18.0, 19.0, 20.7, 23.5, 25.1, 27.0, 27.7, 27.9, 29.4, 34.0, 34.3, 37.0, 37.6, 38.0, 38.3, 40.8, 42.7, 46.5, 47.6, 48.7, 50.2, 54.9, 55.2, 55.6, 67.2, 75.6, 75.9, 77.0, 86.2, 109.9, 149.9, 153.9, 154.5. IR (KBr, cm−1) ν: 3305, 2941, 2133, 1730, 1442, 1253. EI MS (70 eV) m/z (rel. intensity): 606 ((M+, 7), 506 (41), 203 (52), 189 (100), 121 (42); Elemental anal. (%), calcd. for C38H54O6: C, 75.21; H, 8.97; found: C, 75.03; H 9.12.
3,28-O,O'-Di(3-butynyloxycarbonyl)betulin (4b). Yield: 23%, m.p. 140–142 °C, Rf 0.75 (chloroform/ ethanol, 40:1, v/v); 1H-NMR (CDCl3) δ: 0.84 (s, 3H, CH3), 0.85 (s, 3H, CH3), 0.91 (s, 3H, CH3), 0.97 (s, 3H, CH3), 1.03 (s, 3H, CH3), 1.68 (s, 3H, CH3), 1.05–2.02 (m, 25H, CH, CH2), 2.01 (t, J = 2.4 Hz, 1H, C≡CH), 2.03 (t, J = 2.4 Hz, 1H, C≡CH), 2.43 (m, 1H, H-19), 2.59 (m, 4H, 2 × OCH2CH2), 3.94 (d, J = 10.8 Hz, 1H, H-28), 4.24 (m, 4H, 2 × OCH2), 4.31 (m, 1H, H-3), 4.36 (d, J = 10.8 Hz, 1H, H-28), 4.59 (s, 1H, H-29), 4.69 (s, 1H, H-29). 13C-NMR (CDCl3) δ: 14.6, 15.9, 16.1, 16.3, 18.0, 19.0, 20.7, 23.5, 25.0, 26.9, 27.8, 29.4, 33.9, 34.3, 36.9, 37.5, 38.0, 38.2, 40.8, 42.6, 46.4, 47.6, 48.7, 50.1, 55.3, 64.9, 65.3, 66.7, 70.1, 70.2, 76.7, 79.4, 79.5, 79.4, 85.6, 109.9, 149.9, 154.9, 155.4. IR (KBr, cm−1) ν: 3312, 2951, 2128, 1745, 1249, 884. EI MS (70 eV) m/z (rel. intensity): 634 (M+, 3), 203 (40), 189 (100), 135 (48), 121 (46); Elemental anal. (%), calcd. for C40H58O6: C, 75.67; H, 9.21; found: C, 75.89; H, 9.14.
3,28-O,O'-Di(2-butynyloxycarbonyl)betulin (4c). Yield: 28%, m.p. 70–73 °C, Rf 0.78 (chloroform/ ethanol, 40:1, v/v); 1H-NMR (CDCl3) δ: 0.84 (s, 3H, CH3), 0.85 (s, 3H, CH3), 0.91 (s, 3H, CH3), 0.97 (s, 3H, CH3), 1.03 (s, 3H, CH3), 1.68 (s, 3H, CH3), 1.05–2.02 (m, 25H, CH, CH2), 1.86 (t, J = 2.4 Hz, 3H, C≡CCH3), 1.87 (t, J = 2.4 Hz, 3H, C≡CCH3), 2.42 (m, 1H, H-19), 3.94 (d, J = 10.8 Hz, 1H, H-28), 4.32 (m, 1H, H-3), 4.37 (d, J = 10.8 Hz, 1H, H-28), 4.59 (s, 1H, H-29), 4.69 (s, 1H, H-29), 4.71 (m, 4H, 2 × OCH2). 13C-NMR (CDCl3) δ: 5.1, 5.3, 15.9, 16.1, 16.3, 18.0, 19.0, 20.7, 23.5, 25.0, 26.9, 27.8, 29.4, 33.9, 34.2, 36.9, 37.5, 37.9, 38.2, 40.8, 42.6, 46.4, 47.6, 48.7, 50.1, 55.3, 55.8, 56.1, 56.4, 66.9, 72.5, 72.7, 83.9, 84.1, 84.2, 85.8, 109.9, 149.9, 154.7, 155.2. IR (KBr, cm−1) ν: 2948, 2241, 1747, 1456, 1265, 884. EI MS (70 eV) m/z (rel. intensity): 634 (M+, 6), 520 (94), 203 (42), 189 (100), 135 (41); Elemental anal. (%), calcd. for C40H58O6: C, 75.67; H, 9.21; found: C, 75.42; H, 9.33.
3,28-O,O'-Di(ethoxycarbonyl)betulin (4d). Yield: 24%, m.p. 84–86 °C, Rf 0.76 (chloroform/ethanol, 40:1, v/v); 1H-NMR (CDCl3) δ: 0.86 (s, 3H, CH3), 0.87 (s, 3H, CH3), 0.93 (s, 3H, CH3), 0.99 (s, 3H, CH3), 1.05 (s, 3H, CH3), 1.32 (m, 6H, 2 × OCH2CH3), 1.69 (s, 3H, CH3), 0.81–2.05 (m, 25H, CH, CH2), 2.44 (m, 1H, H-19), 3.92 (d, J = 10.8 Hz, 1H, H-28), 4.22 (m, 4H, 2 × OCH2), 4.31 (m, 1H, H-3) 4.35 (d, J = 10.8 Hz, 1H, H-28), 4.60 (s, 1H, H-29), 4.70 (s, 1H, H-29). 13C-NMR (CDCl3) δ: 14.2, 14.7, 16.0, 16.1, 16.4, 18.1, 19.1, 20.8, 23.6, 25.1, 27.0, 27.8, 29.5, 34.1, 34.3, 37.0, 37.6, 38.0, 38.3, 40.9, 42.7, 46.5, 47.6, 48.8, 50.2, 55.4, 63.6, 63.9, 66.4, 85.1, 109.9, 150.0, 155.2, 155.7. IR (KBr, cm−1) ν: 2948, 1741, 1466, 1265, 1009, 885. EI MS (70 eV) m/z (rel. intensity): 586 (M+, 4), 496 (70), 203 (42), 189 (100), 135 (41). Elemental anal. (%), calcd. for C36H58O6: C, 73.68; H, 9.96; found: C, 73.56; H, 9.85.
3.4. General Procedure for the Synthesis of Monoesters 5 and Diesters 6
To a mixture of betulin (1, 0.44 g, 1 mmol) and propynoic acid (0.08 g, 1.10 mmol), phenylpropynoic acid (0.16 g, 1.10 mmol) or propanoic acid (0.08 g, 1.10 mmol) in dry dichloromethane (5 mL) under argon at −10 °C, a solution of DCC (0.23 g, 1.12 mmol) and DMAP (0.01 g, 0.08 mmol) in dry dichloromethane (1 mL) was added. Stirring at this temperature was continued for 5 h. Then the reaction mixture was allowed to warm to room temperature and stirred over night. After the reaction was complete, the reaction mixture was filtered and the solvent was removed under reduced pressure. The crude product was purified by column chromatography (chloroform/ethanol 40:1, v/v) to give pure corresponding monoesters 5 and diesters 6.
28-O-Propynoylbetulin (5a). Yield: 60%, m.p. 133–135 °C, Rf 0.46 (chloroform/ethanol, 20:1, v/v); 1H-NMR (CDCl3) δ: 0.76 (s, 3H, CH3), 0.82 (s, 3H, CH3), 0.97 (s, 3H, CH3), 0.98 (s, 3H, CH3), 1.03 (s, 3H, CH3), 1.68 (s, 3H, CH3), 1.06–1.98 (m, 25H, CH, CH2), 2.43 (m, 1H, H-19), 2.89 (s, 1H, C≡CH), 3.18 (m, 1H, H-3), 3.99 (d, J = 10.8 Hz, 1H, H-28), 4.38 (d, J = 10.8 Hz, 1H, H-28), 4.59 (s, 1H, H-29), 4.69 (s, 1H, H-29). 13C-NMR (CDCl3) δ: 14.7, 15.3, 15.9, 16.0, 18.2, 19.1, 20.7, 25.1, 26.9, 27.3, 27.9, 29.4, 29.5, 34.1, 34.4, 37.1, 37.6, 38.6, 38.8, 40.8, 42.6, 46.3, 47.6, 48.7, 50.3, 55.2, 64.8, 74.6, 74.6, 78.9, 109.9, 149.8, 153.2. IR (KBr, cm−1) ν: 3419, 3302, 2994, 2120, 1715, 1227. EI MS (70 eV) m/z (rel. intensity): 494 (M+, 32), 207 (66), 203 (50), 189 (100), 135 (59); Elemental anal. (%), calcd. for C33H50O3: C, 80.11; H, 10.19; found: C, 80.39; H, 10.02.
28-O-Phenylpropynoylbetulin (5b). Yield: 70%, m.p. 204–206 °C, Rf 0.47 (chloroform/ethanol, 20:1, v/v); 1H-NMR (CDCl3) δ: 0.76 (s, 3H, CH3), 0.83 (s, 3H, CH3), 0.97 (s, 3H, CH3), 0.99 (s, 3H, CH3), 1.05 (s, 3H, CH3), 1.69 (s, 3H, CH3), 1.07–2.05 (m, 25H, CH, CH2), 2.46 (m, 1H, H-19), 3.18 (m, 1H, H-3), 4.04 (d, J = 10.8 Hz, 1H, H-28), 4.42 (d, J = 10.8 Hz, 1H, H-28), 4.60 (s, 1H, H-29), 4.70 (s, 1H, H-29), 7.36–7.60 (m, 5H, Ar-H). 13C-NMR (CDCl3) δ: 14.7, 15.3, 16.0, 16.1, 18.2, 19.1, 20.7, 25.1, 27.0, 27.3, 27.9, 29.5, 29.6, 34.1, 34.5, 37.1, 37.6, 38.6, 38.8, 40.8, 42.7, 46.4, 47.6, 48.8, 50.3, 55.2, 64.5, 78.9, 80.7, 86.2, 109.9, 119.6, 128.5, 128.5, 130.5, 132.9, 132.9, 149.9, 154.7. IR (KBr, cm−1) ν: 3587, 2993, 2220, 1691, 1287, 1195. EI MS (70 eV) m/z (rel. intensity): 570 (M+, 15), 189 (71), 135 (42), 129 (100), 95 (41); Elemental anal. (%), calcd. for C39H54O3: C, 82.06; H, 9.53; found: C, 82.31; H, 9.48.
28-O-Propanoylbetulin (5c). Yield: 86%, m.p. 160–162 °C, Rf 0.46 (chloroform/ethanol, 20:1, v/v); 1H-NMR (CDCl3) δ: 0.75 (s, 3H, CH3), 0.81 (s, 3H, CH3), 0.96 (s, 3H, CH3), 0.97 (s, 3H, CH3), 1.02 (s, 3H, CH3), 1.15 (t, J = 7.2 Hz, 3H, CH2CH3), 1.67 (s, 3H, CH3), 0.88–1.93 (m, 25H, CH, CH2), 2.34 (q, J = 7.2 Hz, 2H, CH2CH3), 2.43 (m, 1H, H-19), 3.18 (m, 1H, H-3), 3.84 (d, J = 10.8 Hz, 1H, H-28), 4.25 (d, J = 10.8 Hz, 1H, H-28), 4.58 (s, 1H, H-29), 4.68 (s, 1H, H-29). 13C-NMR (CDCl3) δ: 9.2, 14.7, 15.3, 15.9, 16.0, 18.2, 19.1, 20.7, 25.1, 27.0, 27.3, 27.7, 27.9, 29.5, 29.7, 34.1, 34.5, 37.1, 37.5, 38.6, 38.8, 40.8, 42.6, 46.3, 47.6, 48.7, 50.3, 55.2, 62.5, 78.9, 109.8, 150.1, 174.9. IR (KBr, cm−1) ν: 3357, 2940, 1735, 1276, 1186, 882. EI MS (70 eV) m/z (rel. intensity): 498 (M+, 17), 207 (52), 203 (54), 189 (100), 135 (58); Elemental anal. (%), calcd. for C33H54O3: C, 79.46; H, 10.91; found: C, 79.34; H, 10.82.
3,28-O,O'-Dipropynoylbetulin (6a). Yield: 12%, m.p. 102–104 °C, Rf 0.73 (chloroform/ethanol, 20:1, v/v); 1H-NMR (CDCl3) δ: 0.86 (s, 3H, CH3), 0.87 (s, 3H, CH3), 0.89 (s, 3H, CH3), 0.98 (s, 3H, CH3), 1.03 (s, 3H, CH3), 1.68 (s, 3H, CH3), 1.07–2.01 (m, 25H, CH, CH2), 2.43 (m, 1H, H-19), 2.85 (s, 1H, C≡CH), 2.89 (s, 1H, C≡CH), 3.99 (d, J = 10.8 Hz, 1H, H-28), 4.39 (d, J = 10.8 Hz, 1H, H-28), 4.59 (m, 1H, H-3), 4.60 (s, 1H, H-29), 4.69 (s, 1H, H-29). 13C-NMR (CDCl3) δ: 14.7, 15.9, 16.1, 16.4, 18.1, 19.1, 20.7, 23.4, 25.1, 26.9, 27.8, 29.4, 29.5, 34.0, 34.4, 37.0, 37.6, 37.9, 38.3, 40.8, 42.6, 46.3, 47.6, 48.7, 50.2, 55.3, 64.8, 74.1, 74.6, 74.7, 75.1, 83.6, 110.0, 149.8, 152.7, 153.2. IR (KBr, cm−1) ν: 3301, 2945, 2119, 1714, 1234, 886. EI MS (70 eV) m/z (rel. intensity): 546 (M+, 9), 195 (46), 189 (53), 113 (41), 55 (100); Elemental anal. (%), calcd. for C36H50O4: C, 79.08; H, 9.22; found: C, 79.16; H, 9.28.
3,28-O,O'-Di(phenylpropynoyl)betulin (6b). Yield: 27%, m.p. 91–92 °C, Rf 0.79 (chloroform/ethanol, 20:1, v/v); 1H-NMR (CDCl3) δ: 0.88 (s, 3H, CH3), 0.92 (s, 3H, CH3), 0.93 (s, 3H, CH3), 0.99 (s, 3H, CH3), 1.06 (s, 3H, CH3), 1.70 (s, 3H, CH3), 1.06–2.05 (m, 25H, CH, CH2), 2.46 (m, 1H, H-19), 4.04 (d, J = 10.8 Hz, 1H, H-28), 4.42 (d, J = 10.8 Hz, 1H, H-28), 4.61 (s, 1H, H-29), 4.66 (m, 1H, H-3), 4.71 (s, 1H, H-29), 7.36–7.60 (m, 10H, 2 × Ar-H). 13C-NMR (CDCl3) δ: 14.7, 16.0, 16.1, 16.5, 18.1, 19.1, 20.8, 23.6, 25.1, 25.8, 25.9, 27.0, 27.9, 29.4, 29.5, 29.6, 34.1, 34.5, 37.0, 37.6, 38.0, 38.4, 40.9, 42.7, 46.4, 47.6, 48.8, 50.2, 55.4, 64.5, 80.7, 81.0, 83.2, 85.7, 86.3, 109.9, 119.6, 119.8, 128.4, 128.5, 130.4, 130.5, 132.9, 132.9, 149.9, 154.2, 154.7. IR (KBr, cm−1) ν: 2945, 2223, 1706, 1490, 1285, 1187. EI MS (70 eV) m/z (rel. intensity): 698 (M+, 6), 552 (30), 189 (33), 135 (15), 129 (100); Elemental anal. (%), calcd. for C48H58O4: C, 82.48; H, 8.36; found: C, 82.35; H, 8.27.
3,28-O,O'-Dipropanoylbetulin (6c). Yield: 11%, m.p. 142–144 °C, Rf 0.71 (chloroform/ethanol, 20:1, v/v); 1H-NMR (CDCl3) δ: 0.83 (s, 3H, CH3), 0.83 (s, 3H, CH3), 0.84 (s, 3H, CH3), 0.96 (s, 3H, CH3), 1.02 (s, 3H, CH3), 1.15 (m, 6H, 2 × CH2CH3), 1.68 (s, 3H, CH3), 1.06–1.96 (m, 25H, CH, CH2), 2.34 (m, 4H, 2 × CH2CH3), 2.43 (m, 1H, H-19), 3.83 (d, J = 10.8 Hz, 1H, H-28), 4.26 (d, J = 10.8 Hz, 1H, H-28), 4.65 (m, 1H, H-3), 4.58 (s, 1H, H-29), 4.68 (s, 1H, H-29). 13C-NMR (CDCl3) δ: 9.2, 9.3, 14.7, 15.9, 16.1, 16.5, 18.1, 19.1, 20.7, 23.6, 25.1, 27.0, 27.7, 27.9, 28.0, 29.5, 29.7, 34.0, 34.5, 37.0, 37.4, 37.8, 38.3, 40.8, 42.6, 46.3, 47.6, 48.7, 50.2, 55.3, 62.5, 80.5, 109.8, 150.1, 174.3, 174.9. IR (KBr, cm−1) ν: 2960, 1736, 1461, 1261, 1185, 891. EI MS (70 eV) m/z (rel. intensity): 554 (M+, 7), 480 (100), 203 (45), 189 (98), 135 (37); Elemental anal. (%), calcd. for C36H58O4: C, 77.93; H, 10.54; found: C, 77.85; H, 10.62.
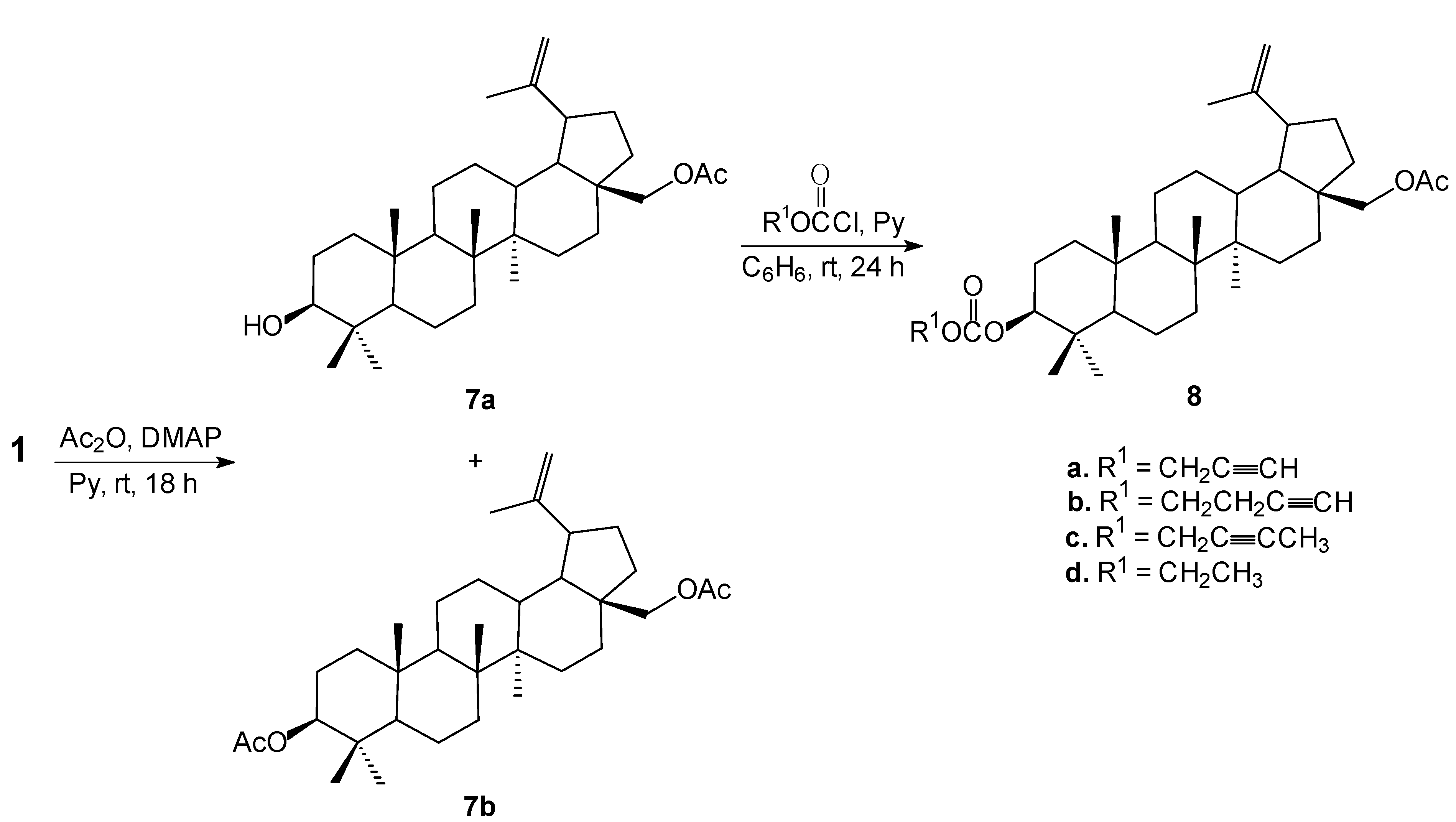
3.5. Synthesis of 28-O-Acetylbetulin (7a) and 3,28-O,O'-Diacetylbetulin (7b)
To a mixture of betulin (
1, 1.0 g, 2.26 mmol), DMAP (0.01 g, 0.07 mmol) and pyridine (1.5 mL) in dry dichloromethane (9 mL) was added dropwise acetic anhydride (0.24 g, 2.26 mmol) at 0 °C, and the reaction mixture was stirred at room temperature. After being stirred for 18 h, the mixture was washed with 10% aqueous hydrochloric acid (30 mL), water (15 mL), saturated aqueous NaHCO
3 solution and then brine (20 mL). After separation of phases, the organic layer was dried with anhydrous magnesium sulfate and concentrated under reduced pressure. The crude product was purified by column chromatography (chloroform/ethanol, 40:1, v/v) to give 28-
O-acetylbetulin (
7a, 0.77 g, 71%): m.p. 214–215 °C (lit. m.p. 210–212 °C [
28]; 217–219 °C [
34]); R
f 0.44 (chloroform/ethanol, 20:1, v/v) and 3,28-
O,O'-diacetylbetulin (
7b, 0.21 g, 18%): m.p. 220–222 °C (lit. m.p. 221–223 °C [
34]).
1H and
13C-NMR spectral data of
7a and
7b were in agreement with those published in the literature [
28,
34].
3.6. Synthesis of 28-O-acetyl-3-O'-alkynyloxycarbonylbetulins (8)
To a mixture of 28-O-acetylbetulin (7a, 0.48 g, 1 mmol) and pyridine (2.5 mL) in dry benzene (6 mL) at 0–5 °C, a solution of propargyl chloroformate or 2-butyn-1-yl chloroformate or 3-butyn-1-yl chloroformate or ethyl chloroformate (2.0 mmol) in dry benzene (5 mL) was added. Stirring at this temperature was continued for 4 h. Then the reaction mixture was allowed to warm to room temperature and stirred over night. At the end of the reaction 5 mL of chloroform was added and the reaction mixture was washed with 1 N sulfuric acid, followed by water, then dried with anhydrous magnesium sulfate and concentrated under reduced pressure. The crude product was purified by column chromatography using chloroform/ethanol (40:1, v/v) to give pure corresponding esters 8.
28-O-Acetyl-3-O'-propargyloxycarbonylbetulin (8a). Yield: 89%, m.p. 188–189 °C, Rf 0.73 (chloroform/ ethanol, 20:1, v/v); 1H-NMR (CDCl3) δ: 0.84 (s, 3H, CH3), 0.85 (s, 3H, CH3), 0.92 (s, 3H, CH3), 0.97 (s, 3H, CH3), 1.03 (s, 3H, CH3), 1.68 (s, 3H, CH3), 1.06–1.99 (m, 25H, CH, CH2), 2.07 (s, 3H, COCH3), 2.44 (m, 1H, H-19), 2.51 (t, 1H, J = 2.4 Hz, C≡CH), 3.85 (d, J = 10.8 Hz, 1H, H-28), 4.24 (d, J = 10.8 Hz, 1H, H-28), 4.33 (m, 1H, H-3), 4.58 (s, 1H, H-29), 4.68 (s, 1H, H-29), 4.71 (d, 2H, J = 2.4 Hz, OCH2). 13C-NMR (CDCl3) δ: 14.6, 16.0, 16.1, 18.1, 19.1, 20.8, 21.0, 23.5, 25.1, 27.0, 27.7, 27.8, 29.5, 29.7, 34.1, 34.5, 37.0, 37.5, 38.0, 40.8, 42.6, 46.3, 47.7, 48.7, 50.2, 54.9, 55.6, 62.7, 75.4, 75.9, 77.2, 86.2, 109.8, 150.1, 154.5, 171.6. IR (KBr, cm−1) ν: 3298, 2997, 2140, 1735, 1243, 889. EI MS (70 eV) m/z (rel. intensity): 566 (M+, 7), 466 (76), 203 (46), 189 (100), 135 (40); Elemental anal. (%), calcd. for C36H54O5: C, 76.28; H, 9.60; found: C, 76.49; H, 9.52.
28-O-Acetyl-3-O'-(3-butynyloxycarbonyl)betulin (8b). Yield: 63%, m.p. 118–121 °C, Rf 0.72 (chloroform/ethanol, 20:1, v/v); 1H-NMR (CDCl3) δ: 0.83 (s, 3H, CH3), 0.84 (s, 3H, CH3), 0.90 (s, 3H, CH3), 0.96 (s, 3H, CH3), 1.02 (s, 3H, CH3), 1.67 (s, 3H, CH3), 1.06–1.99 (m, 25H, CH, CH2), 2.00 (t, J = 2.7 Hz, 1H, C≡CH), 2.06 (s, 3H, COCH3), 2.44 (m, 1H, H-19), 2.57 (m, J = 2.7 Hz, J = 7.2 Hz 2H, OCH2CH2), 3.84 (d, J = 10.8 Hz, 1H, H-28), 4.21 (t, J = 7.2 Hz, 2H, OCH2), 4.25 (d, J = 10.8 Hz, 1H, H-28), 4.32 (m, 1H, H-3), 4.58 (s, 1H, H-29), 4.68 (s, 1H, H-29). 13C-NMR (CDCl3) δ: 14.7, 15.9, 16.1, 16.3, 18.0, 19.0, 20.7, 21.0, 23.5, 25.0, 26.9, 27.8, 29.5, 29.6, 34.0, 34.5, 36.9, 37.4, 38.0, 38.2, 40.8, 42.6, 46.2, 47.6, 48.7, 50.2, 55.3, 62.7, 65.0, 65.5, 70.1, 79.4, 85.6, 109.8, 150.1, 154.9, 171.6. IR (KBr, cm−1) ν: 3284, 2944, 2212, 1741, 1249, 889. EI MS (70 eV) m/z (rel. intensity): 580 (M+, 7), 466 (66), 203 (42), 189 (100), 135 (46); Elemental anal. (%), calcd. for C37H56O5: C, 76.51; H, 9.72; found: C, 76.79; H, 9.59.
28-O-Acetyl-3-O'-(2-butynyloxycarbonyl)betulin (8c). Yield: 67%, m.p. 126–129 °C, Rf 0.75 (chloroform/ethanol, 20:1, v/v); 1H-NMR (CDCl3) δ: 0.84 (s, 3H, CH3), 0.85 (s, 3H, CH3), 0.91 (s, 3H, CH3), 0.97 (s, 3H, CH3), 1.03 (s, 3H, CH3), 1.68 (s, 3H, CH3), 1.06–1.99 (m, 25H, CH, CH2), 1.86 (t, J = 2.4 Hz, 3H, C≡CCH3), 2.07 (s, 3H, COCH3), 2.44 (m, 1H, H-19), 3.84 (d, J = 10.8 Hz, 1H, H-28), 4.24 (d, J = 10.8 Hz, 1H, H-28), 4.32 (m, 1H, H-3), 4.59 (s, 1H, H-29), 4.68 (s, 1H, H-29), 4.71 (q, J = 2.4 Hz, 2H, OCH2). 13C-NMR (CDCl3) δ: 3.6, 14.7, 16.1, 16.3, 18.0, 19.0, 20.7, 21.0, 23.5, 25.0, 26.9, 27.8, 29.5, 29.6, 34.0, 34.5, 36.9, 37.4, 38.0, 38.2, 40.8, 42.6, 46.2, 47.6, 48.7, 50.2, 55.3, 56.4, 62.7, 72.7, 83.8, 84.2, 85.9, 109.8, 150.1, 154.7, 171.6. IR (KBr, cm−1) ν: 2956, 2233, 1742, 1457, 1256, 920. EI MS (70 eV) m/z (rel. intensity): 580 (M+, 3), 466 (64), 203 (37), 189 (100), 187 (47), 135 (40); Elemental anal. (%), calcd. for C37H56O5: C, 76.51; H, 9.72; found: C, 76.63; H, 9.87.
28-O-Acetyl-3-O'-(ethoxycarbonyl)betulin (8d). Yield: 84%, m.p. 200–202 °C, Rf 0.73 (chloroform/ ethanol, 20:1, v/v); 1H-NMR (CDCl3) δ: 0.84 (s, 3H, CH3), 0.85 (s, 3H, CH3), 0.91 (s, 3H, CH3), 0.97 (s, 3H, CH3), 1.03 (s, 3H, CH3), 1.30 (t, J = 7.2 Hz, 3H, CH2CH3), 1.68 (s, 3H, CH3), 1.05–2.00 (m, 25H, CH, CH2), 2.07 (s, 3H, COCH3), 2.44 (m, 1H, H-19), 3.85 (d, J = 10.8 Hz, 1H, H-28), 4.18 (q, J = 7.2 Hz, 2H, OCH2), 4.24 (d, J = 10.8 Hz, 1H, H-28), 4.30 (m, 1H, H-3), 4.59 (s, 1H, H-29), 4.69 (s, 1H, H-29). 13C-NMR (CDCl3) δ: 14.3, 14.7, 16.0, 16.1, 16.4, 18.1, 19.1, 20.8, 21.0, 23.6, 25.1, 27.0, 27.8, 29.6, 34.1, 34.5, 37.0, 37.5, 38.0, 38.3, 40.9, 42.7, 46.3, 47.7, 48.7, 50.2, 55.4, 62.8, 63.6, 85.1, 109.8, 150.1, 155.2, 171.5. IR (KBr, cm−1) ν: 2941, 1741, 1446, 1253, 1012, 890. EI MS (70 eV) m/z (rel. intensity): 556 (M+, 6), 466 (73), 203 (38), 189 (100), 135 (41); Elemental anal. (%), calcd. for C35H56O5: C, 75.50; H, 10.14; found: C, 75.68; H, 10.04.
3.7. Crystal Structure Determination
X-ray diffraction measurement was performed at 100(1) K. The colorless single crystal was preselected under polarization microscope. The crystal was mounted on a glass capillary and cooled down by a cold, dry nitrogen gas stream (Oxford Cryosystem Equipment). The data were collected using SuperNova kappa diffractometer with Atlas CCD detector and CuKα radiation. Accurate cell parameters were determined and refined with CrysAlis Pro program [
35]. Also for the integration of the collected data program CrysAlis Pro was used. The structure was solved using direct methods with SHELXS97 program and then refined using SHELXL-97 program [
36]. Nonhydrogen atoms were refined with anisotropic displacement parameters. The hydrogen atoms were fixed geometrically at calculated distances and allowed to ride on the parent carbon or oxygen atoms.
3.8. Antiproliferative Assay in Vitro
3.8.1. Cells
The following established in vitro cell lines were applied: SW707 (human colorectal adenocarcinoma), CCRF/CEM (human leukemia), T47D (human breast cancer), P388 (mouse leukemia) and Balb3T3 (normal mouse fibroblasts). All lines were obtained from the American Type Culture Collection (Rockville, MD, USA) and maintained at the Cell Culture Collection of the Institute of Immunology and Experimental Therapy (Wroclaw, Poland).
Twenty-four hours before addition of the tested agents, the cells were plated in 96-well plates (Sarstedt, Numbrecht, Germany) at a density of 104 cells per well in 100 μL of culture medium. The cells were cultured in the opti-MEM medium supplemented with 2 mM glutamine (Gibco, Grand Island, NY, USA), streptomycin (50 μg/mL), penicillin (50 U/mL) (both antibiotics from Polfa, Tarchomin, Poland) and 5% fetal calf serum (Gibco). The cell cultures were maintained at 37 °C in humid atmosphere saturated with 5% CO2.
3.8.2. Compounds
The compounds were dissolved in DMSO and culture medium (1:9) to the concentration of 1 mg/mL, and subsequently diluted in culture medium to reach the required concentrations (ranging from 1 to 100 mg/mL).
3.9. Antiproliferative Assay
The
in vitro cytotoxic effect of all agents was examined using the SRB assay for adherent cells and MTT assay for leukemia cells as described by Wietrzyk
et al. [
37]. Each compound in given concentration was tested in triplicates in each experiment, which was repeated 3–5 times. The results of cytotoxic activity
in vitro were expressed as an IC
50 – the concentration of compound (in μg/mL) that inhibits proliferation rate of the tumor cells by 50% as compared to the control untreated cells.