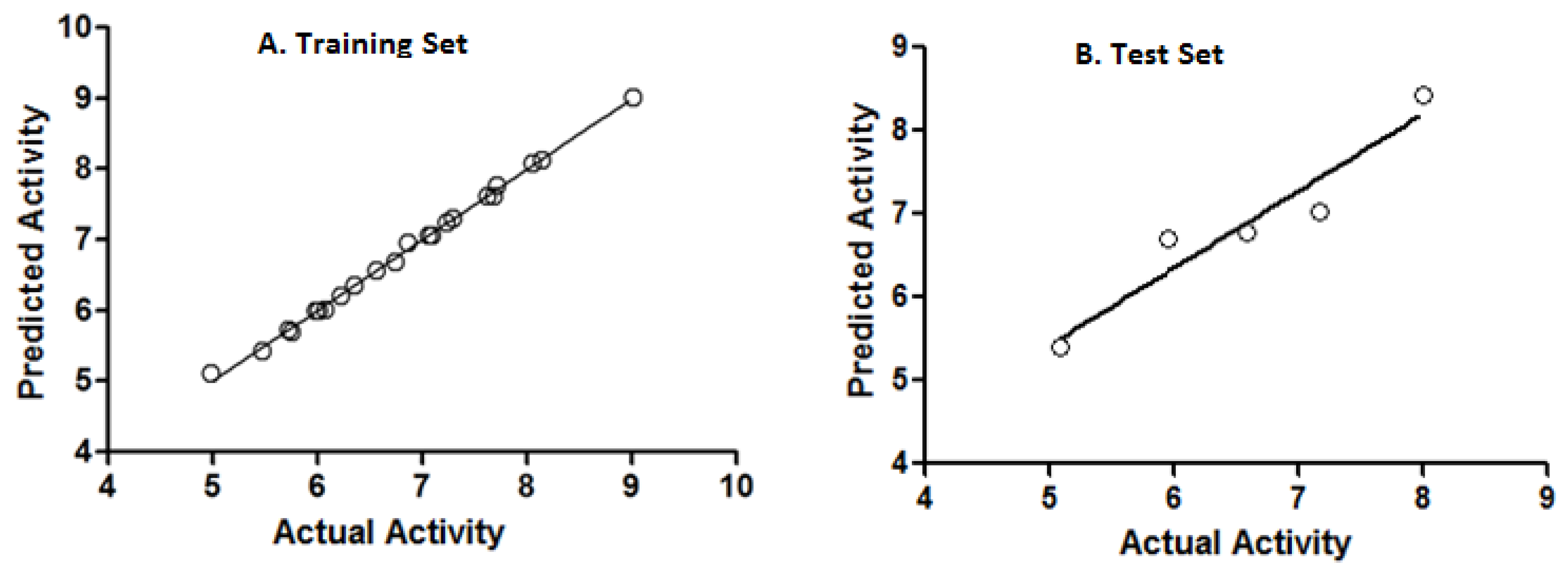
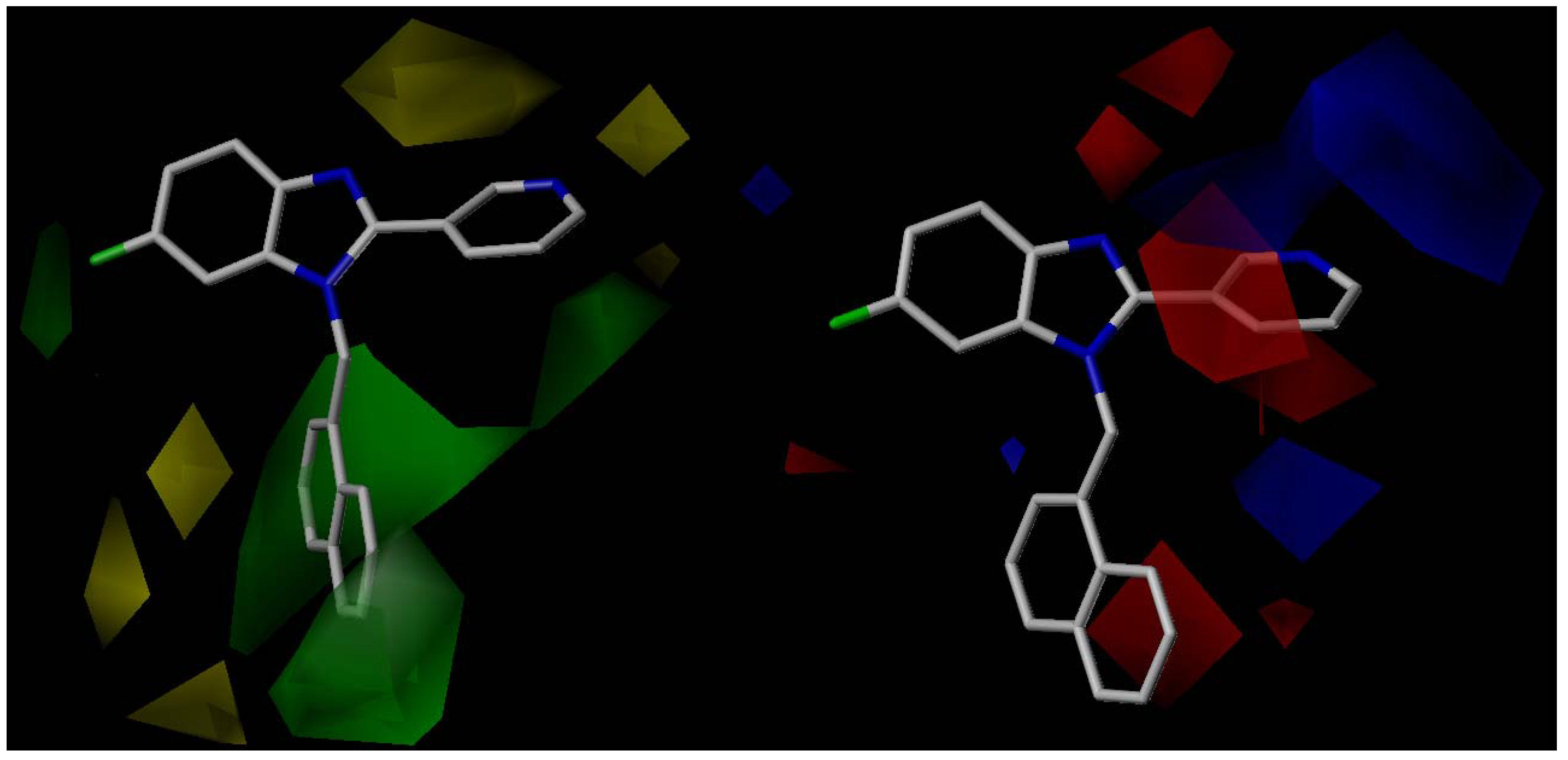
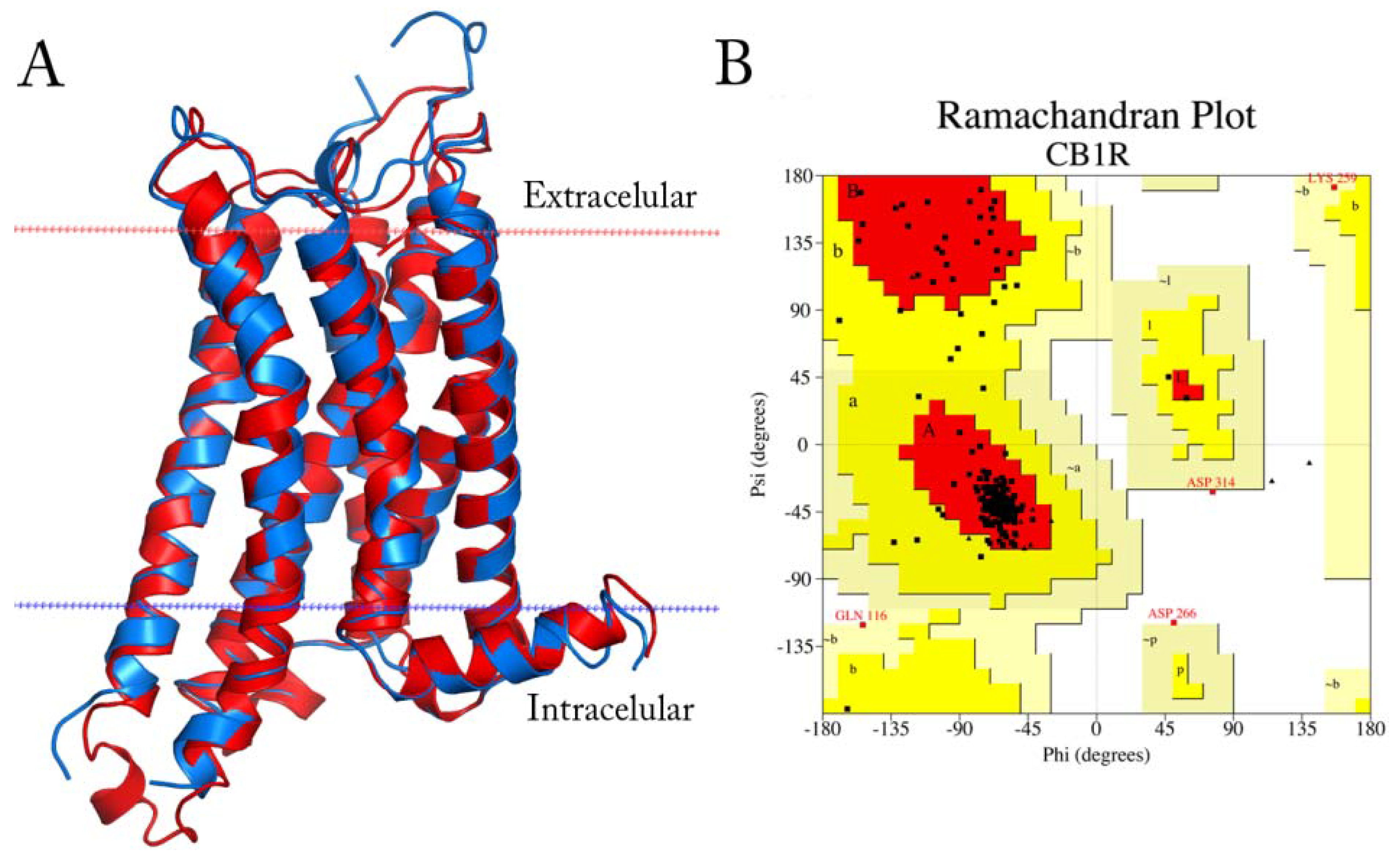
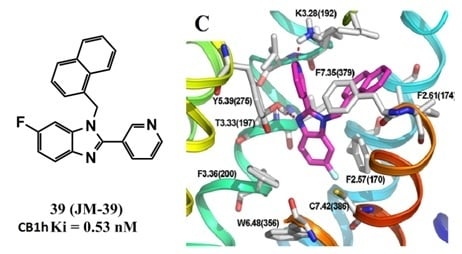
All organic solvents used for the syntheses were analytical grade. Melting points were determined on a Stuart Scientific SMP3 apparatus and are uncorrected. IR spectra were recorded on a Bruker Vector 22 spectrophotometer using KBr discs. 1H and 13C-NMR spectra were obtained on a Bruker AM-400 spectrometer. The chemical shifts are expressed in ppm (δ scale) downfield from TMS, J values are given in Hertz for solutions in CDCl3 unless otherwise indicated. Column chromatography was performed on Merck silica gel 60 (70–230 mesh). Thin layer chromatographic separations were performed on Merck silica gel 60 (70–230 mesh) chromatofoils. Purity of all final derivatives for biological testing was confirmed to be >95% as determined using elemental analysis carried out on a FISONS EA 1108 CHNS-O analyzer.
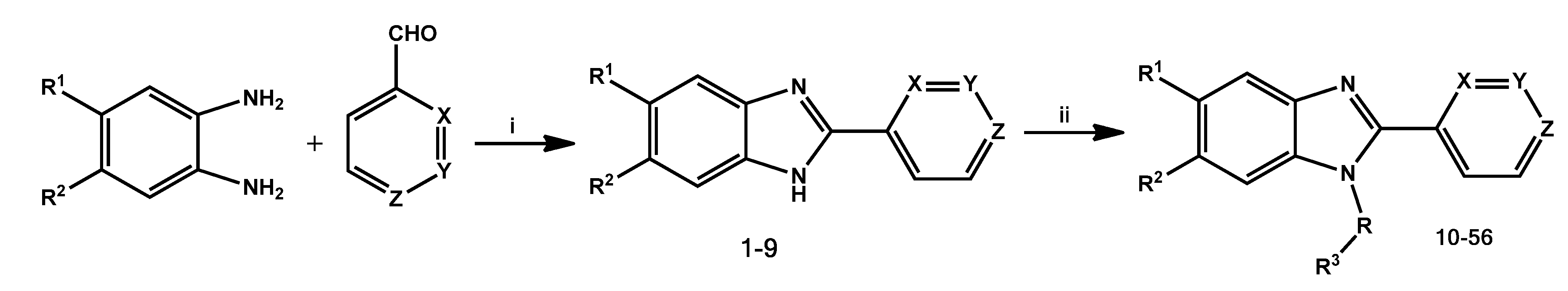
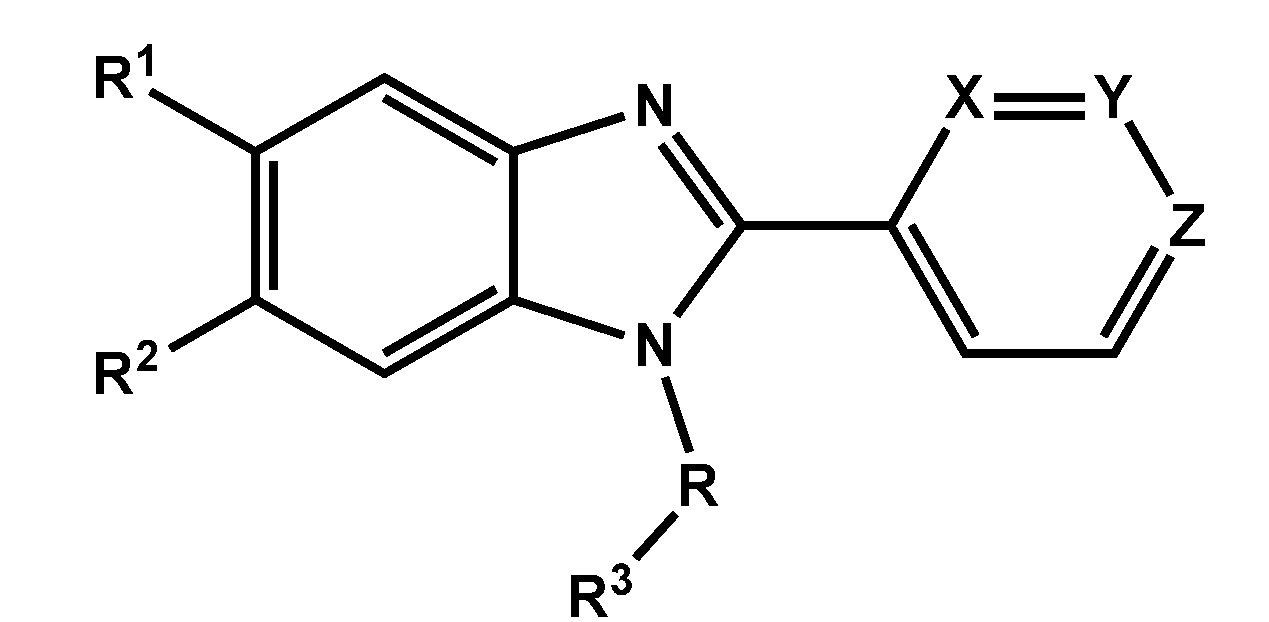
General Method for the Synthesis of (2-Pyridinyl-1H-benzo[d]imidazol-1-yl)(naphthalenyl)methanones 10–32 and 2-Pyridinyl-1-(naphthylmethyl)-1H-benzo[d]imidazoles 33–56
A solution of 2-pyridinyl-1H-benzo[d]imidazole (1 mmol) in anhydrous THF (50 mL) containing NaH (1 mmol) and the corresponding 1 or 2-naphthoyl chloride (1 mmol), or 1 or 2-(bromomethyl)naphthalene (1 mmol) was stirred at room temperature for 20 min under a nitrogen atmosphere. The solvent was evaporated under reduced pressure, and the residue was purified by column chromatography on silica gel with dichloromethane as eluent to yield target compounds 10–56 as pale yellow solids.
Naphthalen-1-yl(2-(pyridin-2-yl)-1H-benzo[d]imidazol-1-yl)methanone (10). Yield = 77%. Mp. 138.0–138.1 °C. 1H-NMR (CDCl3) δ 8.80 (d, J = 8.6 Hz, 1H), 8.06 (d, J = 7.9 Hz, 1H), 7.93 (d, J = 7.1 Hz, 1H), 7.88–7.79 (m, 3H), 7.78–7.70 (m, 2H), 7.64–7.52 (m, 2H), 7.42 (pd, J1 = 7.3 Hz, J2 = 1.4 Hz, 2H), 7.20–7.08 (m, 2H), 6.88 (ddd, J1 = 7.5 Hz, J2 = 4.8 Hz, J3 = 1.2 Hz, 1H). 13C-NMR (CDCl3) δ 169.0, 152.3, 148.4, 148.2, 142.8, 136.4, 135.6, 133.6, 133.3, 132.9, 131.3, 128.3, 128.3, 126.8, 125.9, 125.5, 124.5, 124.0, 123.5, 123.1(2C), 120.5, 112.9. IR (KBr) 1720 cm−1. Anal. Calcd. For C23H15N3O (MW 349.38): C, 79.07%; H, 4.33%; N, 12.03%. Found: C, 79.51%; H, 4.35%; N, 12.02%.
(5-Fluoro-2-(pyridin-2-yl)-1H-benzo[d]imidazol-1-yl)(naphthalen-1-yl)methanone (11). Yield = 70%. Mp. 167.2–167.9 °C. 1H-NMR (CDCl3) δ 8.76 (d, J = 8.5 Hz, 1H), 8.03 (t, J = 7.0 Hz, 1H), 7.85–7.79 (m, 3H), 7.77–7.69 (m, 2H), 7.64–7.54 (m, 3H), 7.19 (dt, J1 = 9.1, J2 = 2.5 Hz, 1H), 7.16–7.10 (m, 2H), 6.88 (dd, J1 = 7.7 Hz, J2 = 4.1 Hz, 1H). 13C-NMR (CDCl3) δ 168.9, 160.6 (d, J = 241.0 Hz), 153.8, 148.4, 148.2, 136.7, 133.7 (d, J = 7.8 Hz), 132.9, 132.2, 131.5, 128.5, 128.4, 127.1, 126.0, 124.1, 123.9, 123.4, 121.6 (d, J = 10.4 Hz), 114.0, 113.8, 113.7, 106.6 (d, J = 24.0 Hz), 100.2 (d, J = 28.9 Hz). IR (KBr) 1735 cm−1. Anal. Calcd. For C23H14FN3O (MW 367.38): C, 75.19%; H, 3.84%; N, 11.44%. Found: C, 75.59%; H, 4.30%; N, 11.04%.
(6-Fluoro-2-(pyridin-2-yl)-1H-benzo[d]imidazol-1-yl)(naphthalen-1-yl)methanone (12). Yield = 64%. Mp. 177.2–178.0 °C. 1H-NMR (CDCl3) δ 8.76 (d, J = 8.6 Hz, 1H), 8.02 (d, J = 7.6 Hz, 1H), 7.86 (dd, J1 = 9.0 Hz, J2 = 5.1 Hz 1H), 7.84–7.79 (m, 3H), 7.74 (t, J = 7.7 Hz, 1H), 7.64–7.52 (m, 2H), 7.48 (dd, J1 = 8.8 Hz, J2 = 2.4 Hz, 1H), 7.19 (dt, J1 = 9.2 Hz, J2 = 2.5 Hz, 1H), 7.17–7.12 (m, 2H), 6.89 (J1 = 7.0 Hz, J2 = 4.7 Hz, 1H). 13C-NMR (CDCl3) δ 168.7, 161.1 (d, J = 243.5 Hz), 152.8, 148.2 (d, J = 19.1 Hz), 139.1, 136.5, 133.6, 133.6 (d, J = 5.5 Hz), 131.3, 128.4, 128.2, 126.9, 125.8, 123.9, 123.6, 122.9, 121.4, 121.3,113.8, 113.6, 113.0 (d, J = 25.3 Hz), 110.0, 100.0 (d, J = 29.2 Hz). IR (KBr) 1740 cm−1. Anal. Calcd. For C23H14FN3O (MW 367.38): C, 75.19%; H, 3.84%; N, 11.44%. Found: C, 75.49%; H, 4.35%; N, 11.94%.
(5,6-Dimethyl-2-(pyridin-2-yl)-1H-benzo[d]imidazol-1-yl)(naphthalen-1-yl)methanone (13). Yield = 93%. Mp. 167.2–168.0 °C. 1H-NMR (CDCl3) δ 8.63 (d, J = 4.5 Hz, 1H), 8.57 (d, J = 4.3 Hz, 1H), 8.47 (d, J = 8.0 Hz, 1H), 7.84 (td, J1 = 7.7 Hz, J2 = 1.8 Hz, 1H), 7.65 (s broad, 1H), 7.51 (td, J1 = 7.7 Hz, J2 = 1.8 Hz, 1H), 7.36–7.26 (m, 2H), 7.22–7.11 (m, 2H), 6.87 (d, J = 7.9 Hz, 1H), 6.28 (s, 2H), 2.41 (s, 3H, CH3), 2.37 (s, 3H, CH3). 13C-NMR (CDCl3) δ 158.0, 150.9, 149.3, 148.8, 144.0, 141.6, 139.6, 137.1, 137.0, 135.7, 133.4, 132.2, 131.6(2C), 128.7, 126.5, 125.7, 125.0, 124.5, 123.8, 114.7, 113.1, 110.9, 18.8(2C). IR (KBr) 1635 cm−1. Anal. Calcd. For C25H19N3O (MW 377.44): C, 79.55%; H, 5.07%; N, 11.13%; Found: C, 79.49%; H, 5.35%; N, 11.54%.
Naphthalen-1-yl-(2-(pyridin-3-yl)-1H-benzo[d]imidazol-1-yl)methanone (14). Yield = 79%. Mp. 148.0–148.1 °C. 1H-NMR (CDCl3) δ 8.67 (s, 1H), 8.33 (s, 1H), 7.98 (d, J = 7.97 Hz, 1H), 7.88 (d, J = 3.65 Hz, 1H), 7.86 (d, J = 3.98 Hz, 1H), 7.80 (d, J = 7.20 Hz, 1H), 7.66 (d, J = 8.10 Hz, 1H), 7.60 (d, J = 7.27 Hz, 2H), 7.54 (t, J1 = 7.08 Hz, J2 = 7.08 Hz, 2H), 7.42 (t, J1 = 7.58 Hz, J2 = 7.58 Hz, 1H), 7.38–7.28 (m, 2H), 6.90 (s, 1H). 13C-NMR (CDCl3) δ 151.4, 150.1, 149.2, 142.9, 135.7, 133.9 (2C), 133.5, 130.7 (2C), 130.1 (2C), 128.7, 127.1, 125.7, 125.2, 124.5, 124.2 (2C), 122.17, 120.6, 114.2 (2C). IR (KBr) 1708 cm−1. Anal. Calcd. For C23H15N3O (MW 349.38): C, 79.07%; H, 4.33%; N, 12.03%. Found: C, 79.64%; H, 4.34%; N, 12.12%.
(5-Fluoro-2-(pyridin-3-yl)-1H-benzo[d]imidazol-1-yl)(naphthalen-1-yl)methanone (15). Yield = 60%. Mp. 169.7–170.7 °C. 1H-NMR (CDCl3) δ 8.60 (d, J = 1.3 Hz, 1H), 8.30 (dd, J1 = 1.3 Hz, J2 = 4.9 Hz 1H), 7.99 (d, J = 8.2 Hz, 1H), 7.87 (d, J = 8.2 Hz, 1H), 7.81 (d, J = 7.6 Hz, 1H), 7.71 (dd, J1 = 9.0 Hz, J2 = 4.7 Hz, 1H), 7.63–7.52 (m, 5H), 7.31 (t, J = 7.7 Hz, 1H), 7.13 (dt, J1 = 9.1 Hz, J2 = 2.4 Hz, 1H), 6.87 (dd, J1 = 7.7 Hz, J2 = 4.9 Hz, 1H).13C-NMR (CDCl3) δ 168.2, 160.8 (d, J = 242.1 Hz), 152.9, 150.4, 149.3, 143.7 (d, J = 12.4 Hz), 139.3, 135.8, 134.2, 133.6, 131.2, 130.7 (d, J = 21.6 Hz), 130.2, 128.97, 129.0, 128.3, 126.9, 124.6, 124.2, 122.3, 115.2 (d, J = 9.5 Hz), 113.9 (d, J = 25.5 Hz), 106.7 (d, J = 24.4 Hz). IR (KBr) 1735 cm−1. Anal. Calcd. For C23H14FN3O (MW 367.38): C, 75.19%; H, 3.84%; N, 11.44%. Found: C, 75.19%; H, 3.35%; N, 11.34%.
(6-Fluoro-2-(pyridin-3-yl)-1H-benzo[d]imidazol-1-yl)(naphthalen-1-yl)methanone (16). Yield = 55%. Mp. 168.1–168.7 °C. 1H-NMR (CDCl3) δ 8.60 (d, J = 1.6 Hz, 1H), 8.29 (dd, J1 = 4.9 Hz, J2 = 1.7 Hz, 1H), 7.99 (d, J = 8.5 Hz, 1H), 7.88 (d, J = 8.2 Hz, 1H), 7.85–7.79 (m, 2H) 7.63–7.53 (m, 4H), 7.49 (dd, J1 = 9.0 Hz, J2 = 2.4 Hz, 1H), 7.33 (dd, J1 = 8.2 Hz, J2 = 7.3 Hz, 1H), 7.20 (td, J2 = 9.0 Hz, J2 = 2.5 Hz, 1H), 6.87 (dd, J1 = 7.8 Hz, J2 = 4.9 Hz, 1H).13C-NMR (CDCl3) δ 168.3, 161.2 (d, J = 243.6 Hz), 151.9, 151.9, 150.2, 149.2, 139.4, 135.8, 135.0 (d, J = 13.8 Hz), 134.3, 133.6, 130.8, 130.5, 130.3, 129.0 (d, J = 3.1 Hz), 127.4, 127.0, 124.61, 124.2, 122.4, 121.5 (d, J = 10.1 Hz), 113.7 (d, J = 24.9 Hz), 101.9 (d, J = 29.4 Hz). IR (KBr) 1740 cm−1 Anal. Calcd. For C23H14FN3O (MW 367.38): C, 75.19%; H, 3.84%; N, 11.44%. Found: C, 75.20%; H, 3.95%; N, 11.92%.
(5,6-Dimethyl-2-(pyridin-3-yl)-1H-benzo[d]imidazol-1-yl)(naphthalen-1-yl)methanone (17). Yield = 73%. Mp. 177.2–178.0 °C. 1H-NMR (CDCl3/DMSO-d6) δ 8.51 (s, 1H), 8.25 (s, 1H), 8.03–7.95 (m, 1H), 7.95–7.88 (m, 2H), 7.74–7.66 (m, 2H), 7.65–7.51 (m, 3H), 7.50–7.45 (m, 1H), 7.39 (t, J = 7.72 Hz, 1H), 7.02–6.89 (m, 1H), 2.39 (s, 3H, CH3), 2.32 (s, 3H, CH3). 13C-NMR (CDCl3/DMSO-d6) δ 188.8, 168.3, 150.7, 150.0, 149.0, 141.5, 136.2, 134.7, 134.0, 133.6, 133.3, 133.0, 131.0, 130.3, 129.0, 128.7, 127.4, 127.2, 125.0, 124.3, 122.5, 120.5, 114.6, 20.7, 20.3. IR (KBr) 1637 cm−1. Anal. Calcd. For C25H19N3O (MW 377.44): C, 79.55%; H, 5.07%; N, 11.13%; Found: C, 80.00%; H, 5.57%; N, 11.62%.
Naphthalen-1-yl-(2-(pyridin-4-yl)-1H-benzo[d]imidazol-1-yl)methanone (18). Yield = 65%. Mp. 155.0–156.1 °C. 1H-NMR (DMSO-d6) δ 7.55–7.43 (m, 3H), 7.22–7.07 (m, 3H), 6.97–6.90 (m, 2H), 6.88–6.83 (m, 1H), 6.82–6.76 (m, 1H), 6.72–6.65 (m, 1H), 6.65–6.53 (m, 2H), 6.52–6.46 (m, 2H). 13C-NMR (CDCl3) δ 166.6, 150.7(2C), 148.2, 142.3, 137.1, 134.3, 133.0, 132.7, 130.5, 130.4, 129.3, 128.0, 127.8, 126.2, 124.8, 124.1, 124.0, 123.4, 121.8(2C), 120.2, 113.3. IR (KBr) 1720 cm−1. Anal. Calcd. For C23H15N3O (MW 349.38): C, 79.07%; H, 4.33%; N, 12.03%. Found: C, 79.04%; H, 4.84%; N, 12.10%.
(5-Fluoro-2-(pyridin-4-yl)-1H-benzo[d]imidazol-1-yl)(naphthalen-1-yl)methanone (19). Yield = 62%. Mp. 180.7–181.7°C. 1H-NMR (CDCl3) δ 8.30–8.26 (m, 2H), 8.11 (d, J = 8.3 Hz, 1H), 7.90 (d, J = 8.2 Hz, 1H), 7.83 (d, J = 7.7 Hz, 1H), 7.69 (dd, J1 = 9.0 Hz, J2 = 4.7 Hz, 1H), 7.63 (dt, J1 = 8.5 Hz, J2 = 1.6 Hz, 1H), 7.60–7.55 (m, 2H), 7.54 (d, J = 7.0 Hz, 1H), 7.29 (dd, J1 = 7.6 Hz, J2 = 7.9 Hz 1H), 7.26–7.23 (m, 2H), 7.15 (td, J1 = 9.1 Hz, J2 = 2.5 Hz, 1H). 13C-NMR (CDCl3) δ 168.0, 160.8 (d, J = 242.8 Hz), 153.1, 149.1, 143.5 (d, J = 12.4 Hz), 138.3, 134.6, 133.6, 133.5, 131.3, 131.0, 130.5, 130.4, 129.1(2C), 127.8, 126.3, 124.3 (d, J = 12.1 Hz), 122.9(2C), 115.2 (d, J = 9.6 Hz), 114.4 (d, J = 25.4 Hz), 107.0 (d, J = 24.5 Hz). IR (KBr) 1710 cm−1. Anal. Calcd. For C23H14FN3O (MW 367.38): C, 75.19%; H, 3.84%; N, 11.44%. Found: C, 75.69%; H, 4.25%; N, 11.92%.
(6-Fluoro-2-(pyridin-4-yl)-1H-benzo[d]imidazol-1-yl)(naphthalen-1-yl)methanone (20). Yield = 63%. Mp. 190.4–190.9 °C. 1H-NMR (CDCl3) δ 8.29–8.24 (m, 2H), 8.12 (d, J = 8.4 Hz, 1H), 7.91 (d, J = 8.5 Hz, 1H), 7.87–7.81 (m, 2H), 7.67–7.57 (m, 2H), 7.54 (dd, J1 = 7.2 Hz, J2 = 1.3 Hz, 1H) 7.47 (dd, J1 = 8.9 Hz, J2 = 2.5 Hz, 1H), 7.30 (dd, J1 = 8.3 Hz, J2 = 7.2 Hz, 1H), 7.25–7.21 (m, 2H), 7.19 (dd, J1 = 9.0 Hz, J2 = 2.5 Hz, 1H).13C-NMR (CDCl3) δ 168.0, 161.4 (d, J = 244.4 Hz), 152.2 (d, J = 3.6 Hz), 150.5, 149.2(2C), 139.3, 138.2, 135.2, 135.1, 134.7, 133.6, 131.0, 130.4, 130.4, 129.1, 127.4, 124.3 (d, J = 12.4 Hz), 122.8(2C), 121.8 (d, J = 10.2 Hz), 113.9 (d, J = 25.0 Hz), 101.8 (d, J = 29.4 Hz). IR (KBr) 1733 cm−1. Anal. Calcd. For C23H14FN3O (MW 367.38): C, 75.19%; H, 3.84%; N, 11.44%. Found: C, 74.98%; H, 4.33%; N, 11.01%.
(5,6-Dimethyl-2-(pyridin-4-yl)-1H-benzo[d]imidazol-1-yl)(naphthalen-1-yl)methanone (21). Yield = 77%. Mp. 167.2–168.0 °C. 1H-NMR (CDCl3) δ 8.20 (d, J = 5.9 Hz, 2H), 8.07 (d, J = 8.3 Hz, 1H), 7.86 (d, J = 8.2 Hz, 1H), 7.80 (d, J = 7.6 Hz, 1H), 7.64 (s, 1H), 7.62–7.48 (m, 4H), 7.30–7.23 (m, 1H), 7.17 (d, J = 5.9 Hz, 2H), 2.41 (s, 3H, CH3), 2.36 (s, 3H, CH3).13C-NMR (CDCl3) δ 168.5, 151.1, 149.3(2C), 141.5, 138.6, 135.8, 134.6, 134.2, 133.6, 133.4, 131.2, 131.1, 130.3, 129.0, 128.8, 127.2, 124.5, 124.4, 122.8(2C), 120.9, 114.7, 20.9, 20.5. IR (KBr) 1617 cm−1. Anal. Calcd. For C25H19N3O (MW 377.44): C, 79.55%; H, 5.07%; N, 11.13%; Found: C, 79.05%; H, 5.52%; N, 10.98%.
Naphthalen-2-yl(2-(pyridin-2-yl)-1H-benzo[d]imidazol-1-yl)methanone (22). Yield = 82%. Mp. 145.0–146.0 °C. 1H-NMR (CDCl3) δ 8.29 (d, J = 8.0 Hz, 1H), 8.22 (d, J = 4.7 Hz, 1H), 8.05 (s, 1H), 7.97–7.91 (m, 2H), 7.86–7.81 (m, 2H), 7.74 (d, J = 8.2 Hz, 1H), 7.69 (td, J1 = 7.7 Hz, J2 = 1.7 Hz, 1H), 7.56 (td, J1 = 8.1 Hz, J2 = 1.2 Hz, 1H), 7.53–7.46 (m, 2H), 7.41 (td, J1 = 8.1 Hz, J2 = 7.7 Hz, J3 = 1.3 Hz, 1H), 7.35 (d, J = 8.2 Hz, 1H), 7.05 (ddd, J1 = 7.6 Hz, J2 = 4.8 Hz, J3 = 1.2 Hz, 1H). 13C-NMR (CDCl3) δ 169.4, 152.0, 148.5, 148.1, 143.0, 136.7, 135.6, 135.5, 132.2, 131.8, 131.5, 129.5, 128.9, 128.8, 127.8, 127.0, 125.3, 125.1, 124.2, 124.0, 123.3, 120.5, 112.3. IR (KBr) 1715 cm−1. Anal. Calcd. For C23H15N3O (MW 349.38): C, 79.07%; H, 4.33%; N, 12.03%. Found: C, 79..57%; H, 4.83%; N, 12.01%.
(6-Fluoro-2-(pyridin-2-yl)-1H-benzo[d]imidazol-1-yl)(naphthalen-2-yl)methanone (23). Yield = 66%. Mp. 195.7–195.9 °C. 1H-NMR (CDCl3) δ 8.78 (s, 1H), 8.61 (d, J = 4.8 Hz, 1H), 8.54 (d, J = 7.9 Hz, 1H), 8.21 (d, J = 8.6 Hz, 1H), 8.00 (d, J = 8.1 Hz, 1H), 7.92 (t, J = 9.0 Hz, 3H), 7.66 (dd, J1 = 8.7 Hz, J2 = 4.7 Hz, 1H),7.63–7.53 (m, 2H), 7.45–7.34 (m, 2H), 7.06 (td, J1 = 9.2 Hz, J2 = 2.4 Hz, 1H). 13C-NMR (CDCl3) δ 171.0, 160.1 (d, J = 239.6 Hz), 151.3, 148.6, 147.8, 139.0, 138.1, 135.8, 132.6, 131.8, 129.5, 128.4 (d, J = 18.9 Hz), 127.8, 127.6, 127.4, 126.7, 125.7, 124.9, 123.2, 122.6, 116.8, 112.1 (d, J = 25.5 Hz), 101.6 (d, J = 40.1 Hz). IR (KBr) 1723 cm−1. Anal. Calcd. For C23H14FN3O (MW 367,38): C, 75.19%; H, 3.84%; N, 11.44%. Found: C, 75.20%; H, 4.26%; N, 11.00%.
(5,6-Dimethyl-2-(pyridin-2-yl)-1H-benzo[d]imidazol-1-yl)(naphthalen-2-yl)methanone (24). Yield = 85%. Mp. 168.7–169.6 °C. 1H-NMR (CDCl3) δ 8.59 (d, J = 4.9 Hz, 1H), 8.53 (d, J = 5.0 Hz, 1H), 8.45 (d, J = 8.0 Hz, 1H), 7.81 (td, J1 = 7.7 Hz, J2 = 1.8 Hz, 1H), 7.62 (s, 1H), 7.48 (td, J1 = 7.7 Hz, J2 = 1.9 Hz, 1H), 7.31–7.22 (m, 2H), 7.19–7.04 (m, 2H), 6.84 (d, J = 7.8 Hz, 1H), 6.25 (s, 2H), 2.38 (s, 3H, CH3 ), 2.33 (s, 3H, CH3). 13C-NMR (CDCl3) δ 157.8, 153.1, 150.6, 149.1, 148.6, 148.5, 147.9, 147.8, 141.4, 139.0, 136.9, 136.8, 135.4, 133.2, 132.0, 124.3, 123.5, 122.2, 120.8, 120.1, 119.0, 118.4, 110.7, 20.6, 20.3. IR (KBr) 1629 cm−1. Anal. Calcd. For C25H19N3O (MW 377.44): C, 79.55%; H, 5.07%; N, 11.13%; Found: C, 80.04%; H, 5.57%; N, 10.84%.
Naphthalen-2-yl(2-(pyridin-3-yl)-1H-benzo[d]imidazol-1-yl)methanone (25). Yield = 90%. Mp. 148.0–148.6 °C. 1H-NMR (CDCl3) δ 8.92 (s, 1H), 8.46 (d, J = 3.3 Hz, 1H), 8.27 (s, 1H), 7.97 (dt, J1 = 7.9 Hz, J2 = 1.8 Hz, 1H), 7.93 (d, J = 8.0 Hz, 1H), 7.90–7.86 (m, 2H), 7.85–7.80 (m, 2H), 7.65 (dt, J1 = 7.6 Hz, J2 = 1.1 Hz, 1H), 7.56 (dt, J1 = 7.5 Hz, J2 = 1.0 Hz, 1H), 7.41 (dt, J1 = 7.6 Hz, J2 = 1.2 Hz, 1H), 7.36 (d, J = 7.9 Hz, 1H), 7.31–7.26 (m, 1H), 7.19 (dd, J1 = 7.7 Hz, J2 = 4.9 Hz, 1H). 13C-NMR (CDCl3) δ 168.7, 151.4, 150.5, 149.7, 143.0, 136.2, 136.0, 135.0, 133.1, 132.2, 129.8, 129.6, 129.5, 129.3, 128.0, 127.5, 127.0, 125.3, 125.2, 124.8, 123.0, 120.6, 113.3. IR (KBr) 1755 cm−1. Anal. Calcd. For C23H15N3O (MW 349.38): C, 79.07%; H, 4.33%; N, 12.03%. Found: C, 78..61%; H, 4.87%; N, 12.02%.
(5-Fluoro-2-(pyridin-3-yl)-1H-benzo[d]imidazol-1-yl)(naphthalen-2-yl)methanone (26). Yield = 50%. Mp. 177.7–118.6 °C. 1H-NMR (CDCl3) δ 8.63 (s, 1H), 8.51 (d, J = 8.1 Hz, 1H), 8.25 (s, 1H), 8.09 (d, J = 8.6 Hz, 1H), 7.95 (d, J = 8.0 Hz, 1H), 7.91–7.82 (m, 3H), 7.79 (dd, J1 = 8.6 Hz, J2 = 1.8 Hz, 1H), 7.63–7.51 (m, 3H), 7.35 (dd, J1 = 9.0 Hz, J2 = 4.6 Hz, 1H), 7.07 (dt, J1 = 9.1 Hz, J2 = 2.5 Hz, 1H). 13C-NMR (CDCl3) δ 168.3, 160.2 (d, J = 241.6 Hz), 152.7, 150.5, 150.4, 149.5, 147.9, 143.5 (d, J = 12.1 Hz), 136.2, 135.9, 135.4, 133.0, 132.0, 131.4, 129.7, 129.4 (d, J = 3.8 Hz), 128.0, 127.6, 126.5, 125.0, 114.0 (d, J = 9.7 Hz), 113.2 (d, J = 26.0 Hz), 106.4 (d, J = 23.6 Hz). IR (KBr) 1715 cm−1. Anal. Calcd. For C23H14FN3O (MW 367.38): C, 75.19%; H, 3.84%; N, 11.44%. Found: C, 74.89%; H, 4.27%; N, 11.42%.
(6-Fluoro-2-(pyridin-3-yl)-1H-benzo[d]imidazol-1-yl)(naphthalen-2-yl)methanone (27). Yield = 50%. Mp. 167.2–167.7 °C. 1H-NMR (CDCl3) δ 8.74 (s, 1H), 8.46 (d, J = 8.5 Hz, 1H), 8.26 (s, 1H), 8.17 (d, J = 8.6 Hz, 1H), 7.98 (d, J = 8.4 Hz, 1H), 7.95–7.76 (m, 3H), 7.70–7.54 (m, 2H), 7.49 (dd, J1 = 8.8 Hz, J2 = 4.6 Hz, 1H), 7.24–7.11 (m, 2H), 6.96 (t, J = 8.3 Hz, 1H). 13C-NMR (CDCl3) δ 168.4, 160.4 (d, J = 158.9 Hz), 151.6, 150.3, 149.4, 139.3, 136.4, 136.0, 135.7, 135.3, 134.9, 132.1, 129.4 (d, J = 10.9 Hz), 129.3, 128.4, 128.0, 127.8, 127.7, 126.7, 125.1, 121.4 (d, J = 10.2 Hz), 113.2 (d, J = 25.4 Hz), 111.9 (d, J = 25.4 Hz). IR (KBr) 1739 cm−1. Anal. Calcd. For C23H14FN3O (MW 367.38): C, 75.19%; H, 3.84%; N, 11.44%. Found: C, 74.70%; H, 4.34%; N, 11.93%.
(5,6-Dimethyl-2-(pyridin-3-yl)-1H-benzo[d]imidazol-1-yl)(naphthalen-2-yl)methanone (28). Yield = 75%. Mp. 166.7–166.9 °C. 1H-NMR (CDCl3) δ 8.98 (s broad, 1H), 8.51 (s broad, 1H), 8.32 (s, 1H), 8.02 (dt, J1 = 7.9 Hz, J2 = 1.9 Hz, 1H), 7.96 (d, J = 8.5 Hz, 2H), 7.94–7.87 (m, 2H), 7.77 (s, 1H), 7.73 (td, J1 = 7.6 Hz, J2 = 1.4 Hz, 1H), 7.65 (td, J1 = 7.5 Hz, J2 = 1.2 Hz, 1H), 7.34 (s, 1H), 7.24 (d, J = 5.2 Hz, 1H), 2.51 (s, 3H, CH3 ), 2.40 (s, 3H, CH3). 13C-NMR (CDCl3) δ 169.1, 150.3, 149.8, 148.1, 144.0, 141.7, 136.2, 136.0, 134.9, 134.0, 133.7, 133.2, 132.3, 130.2, 129.6(2C), 129.3, 128.1, 127.6, 125.5, 123.1, 120.7, 113.7, 20.8, 20.5. IR (KBr) 1639 cm−1. Anal. Calcd. For C25H19N3O (MW 377.44): C, 79.55%; H, 5.07%; N, 11.13%; Found: C, 80.04%; H, 5.56%; N, 10.64%.
Naphthalen-2-yl(2-(pyridin-4-yl)-1H-benzo[d]imidazol-1-yl)methanone (29). Yield = 75%. Mp. 145.0–146.1 °C. 1H-NMR (CDCl3) δ 8.57 (s broad, 2H), 8.27 (s, 1H), 7.93 (d, J = 8.3 Hz, 1H), 7.90 (s broad, 1H), 7.88-–7.86 (m, 1H), 7.83 (d, J = 8.1 Hz, 1H), 7.66 (t, J = 7.2 Hz, 1H), 7.61–7.53 (m, 3H), 7.42 (t, J = 7.4 Hz, 1H), 7.35–7.26 (m, 2H), 7.88–7.86 (m, 1H). 13C-NMR (CDCl3) δ 168.7, 151.8, 150.1, 143.1, 138.2, 136.2, 135.3, 133.3, 132.3, 129.9, 129.9, 129.6, 129.5, 128.2, 127.8(2C), 125.7, 125.4(2C), 125.0, 123.2, 121.0, 113.4. IR (KBr) 1731 cm−1. Anal. Calcd. For C23H15N3O (MW 349.38): C, 79.07%; H, 4.33%; N, 12.03%. Found: C, 79.57%; H, 4.33%; N, 12.01%.
(5-Fluoro-2-(pyridin-4-yl)-1H-benzo[d]imidazol-1-yl)(naphthalen-2-yl)methanone (30). Yield = 40%. Mp. 195.7–200.6 °C. 1H-NMR (CDCl3) δ 8.52 (d, J = 5.5 Hz, 2H), 8.22 (s, 1H), 7.88 (t, J = 8.4 Hz, 2H), 7.84–7.79 (m, 2H), 7.65 (t, J = 7.5 Hz, 1H), 7.59 (d, J1 = 8.35 Hz, J2 = 2.5 Hz, 1H), 7.57–7.55 (m, 1H), 7.53 (d, J = 5.5 Hz, 2H), 7.31 (dd, J1 = 9.0 Hz, J2 = 4.6 Hz, 1H), 7.05 (td, J1 = 9.1 Hz, J2 = 2.5 Hz, 1H). 13C-NMR (CDCl3) δ 168.4, 160.5 (d, J = 242.0 Hz), 153.1, 150.0(2C), 143.6 (d, J = 12.6 Hz), 137.9, 136.2, 133.2, 132.1, 131.7, 130.0, 129.5(3C), 128.1, 127.8, 125.2, 123.1(2C), 114.1, 113.9 (d, J = 26.2 Hz), 106.8 (d, J = 24.4 Hz). IR (KBr) 1755 cm−1. Anal. Calcd. For C23H14FN3O (MW 367.38): C, 75.19%; H, 3.84%; N, 11.44%. Found: C, 75.69%; H, 3.37%; N, 11.40%.
(6-Fluoro-2-(pyridin-4-yl)-1H-benzo[d]imidazol-1-yl)(naphthalen-2-yl)methanone (31). Yield = 55%. Mp. 187.3–188.2°C. 1H-NMR (CDCl3) δ 8.52 (d, J = 6.1 Hz, 2H), 8.23 (s, 1H), 7.95–7.80 (m, 5H), 7.67 (t, J = 7.6 Hz, 1H), 7.59 (d, J = 7.6 Hz, 1H), 7.53 (d, J = 6.1 Hz, 2H), 7.18 (td, J1 = 9.0 Hz, J2 = 2.4 Hz, 1H), 7.12 (dd, J1 = 9.0 Hz, J2 = 2.5 Hz, 1H).13C-NMR (CDCl3) δ 170.3, 161.6 (d, J = 245.0 Hz), 159.3, 152.2, 150.0 (2C), 143.7 (d, J = 11.9 Hz), 143.0, 138.0, 136.0, 133.2, 132.2, 131.7, 130.1, 129.5, 128.0, 127.8, 125.2, 123.0(2C), 114.0, 113.8 (d, J = 26.2 Hz), 106.7 (d, J = 24.3 Hz). IR (KBr) 1754 cm−1. Anal. Calcd. For C23H14FN3O (MW 367.38): C, 75.19%; H, 3.84%; N, 11.44%. Found: C, 75.01%; H, 3.94%; N, 11.43%.
(5,6-Dimethyl-2-(pyridin-4-yl)-1H-benzo[d]imidazol-1-yl)(naphthalen-2-yl)methanone (32). Yield = 85%. Mp. 160.4–160.9 °C. 1H-NMR (CDCl3) δ 8.49 (s broad, 1H), 8.21 (s, 1H), 7.94–7.77 (m, 4H), 7.70–7.64 (m, 2H), 7.60 (dd, J1 = 3.8 Hz, J2 = 1.4 Hz, 1H), 7.57–7.48 (m, 3H), 7.20 (s, 1H), 2.41 (s, 3H, CH3), 2.29 (s, 3H, CH3). 13C-NMR (CDCl3) δ 168.8, 149.8, 141.5, 138.3, 136.0, 135.2, 134.0, 133.0(2C), 132.0, 129.9, 129.6, 129.4(2C), 129.2, 128.0(2C), 127.5, 125.2, 120.7, 120.0, 113.4, 113.4, 20.7, 20.3. IR (KBr) 1616 cm−1. Anal. Calcd. For C25H19N3O (MW 377.44): C, 79.55%; H, 5.07%; N, 11.13%; Found: C, 79.54%; H, 5.49%; N, 11.62%.
1-(Naphthalen-1-ylmethyl)-2-(pyridin-2-yl)-1H-benzo[d]imidazole (33). Yield: 70%. Mp. 150.7–151.6 °C. 1H-NMR (CDCl3) δ 8.48 (d, J = 8.0 Hz, 1H), 8.44 (d, J = 4.2 Hz, 1H), 8.19 (d, J = 8.4 Hz, 1H), 7.95 (d, J = 8.1 Hz, 1H), 7.91 (d, J = 8.1 Hz, 1H), 7.80 (td, J1 = 7.8 Hz, J2 = 1.4 Hz, 1H), 7.72 (d, J = 8.2 Hz, 1H), 7.63 (t, J = 7.2 Hz, 1H), 7.57 (t, J = 7.3 Hz, 1H), 7.37–7.32 (m, 1H), 7.26–7.17 (m, 4H), 6.70 (s, 2H, CH2), 6.63 (d, J = 7.1 Hz, 1H). 13C-NMR (CDCl3) δ 150.5, 148.7, 142.8, 139.9, 137.1, 136.8, 133.6, 132.8, 130.5, 129.0, 127.6, 126.4, 125.9, 125.6, 124.5, 123.8, 123.7, 123.0, 122.9, 122.5, 120.3, 110.8, 46.8. IR (KBr) 1447 cm−1. Anal. Calcd. For C23H17N3 (MW 335.40): C, 82.36%; H, 5.11%; N, 12.53%; Found: C, 82.86%; H, 5.10%; N, 12.07%.
5-Fluoro-1-(naphthalen-1-ylmethyl)-2-(pyridin-2-yl)-1H-benzo[d]imidazole (34). Yield: 55%. Mp. 155.7–155.9 °C. 1H-NMR (CDCl3) δ 8.50–8.41 (m, 2H), 8.16 (d, J = 8.3 Hz, 1H), 7.91 (d, J = 7.9 Hz, 1H), 7.81 (td, J1 = 7.7 Hz, J2 = 1.8 Hz, 1H), 7.73 (d, J = 8.1 Hz, 1H), 7.64–7.54 (m, 3H), 7.27–7.19 (m, 2H), 7.10 (dd, J1 = 8.9 Hz, J2 = 4.6 Hz, 1H), 6.96 (td, J1 = 9.1 Hz, J2 = 2.4 Hz, 1H), 6.68 (s, 2H, CH2), 6.61 (d, J = 7.2 Hz, 1H). 13C-NMR (CDCl3) δ 159.8 (d, J = 238.2 Hz), 151.7, 150.2, 148.8, 143.1 (d, J = 12.5 Hz), 136.9, 133.6, 132.5, 130.5, 129.0, 127.8, 127.7, 126.5, 126.0, 125.6, 124.6, 124.0, 122.9, 122.4, 114.0, 112.2 (d, J = 26.3 Hz), 111.2 (d, J = 10.2 Hz), 105.7 (d, J = 24.0 Hz), 47.0. IR (KBr) 1456 cm−1. Anal. Calcd. For C23H16FN3 (MW 353.39): C, 78.17%; H, 4.56%; N, 11.89% Found: C, 78.07%; H, 5.01%; N, 12.02%.
6-Fluoro-1-(naphthalen-1-ylmethyl)-2-(pyridin-2-yl)-1H-benzo[d]imidazole (35). Yield = 50%. Mp. 160.7–161.1 °C. 1H-NMR (CDCl3) δ 8.49–8.40 (m, 2H), 8.16 (d, J = 8.3 Hz, 1H), 7.92 (d, J = 8.0 Hz, 1H),7.84 (dd, J1 = 9.0 Hz, J2 = 4.9 Hz, 1H), 7.79 (dt, J1 = 8.0 Hz, J2 = 1.6 Hz, 1H), 7.74 (d, J = 8.2 Hz, 1H), 7.63 (t, J2 = 7.7 Hz, J2 = 1.5 Hz, 1H), 7.57 (t, J = 7.2 Hz, 1H), 7.28–7.19 (m, 2H), 7.07 (td, J1 = 9.2 Hz, J2 = 2.4 Hz, 1H), 6.88 (dd, J1 = 8.8 Hz, J2 = 2.5 Hz, 1H), 6.65 (s, 2H, CH2), 6.63 (d, J = 6.9 Hz, 1H). 13C-NMR (CDCl3) δ 160.3 (d, J = 241.5 Hz), 151.2, 150.3, 148.8, 139.2, 137.4 (d, J = 13.4 Hz), 136.9, 133.7, 132.3, 130.5, 129.0, 127.8, 126.5, 126.0, 125.6, 124.3, 123.9, 122.9, 122.42, 121.1 (d, J = 10.3 Hz), 111.6 (d, J = 25.1 Hz), 97.4 (d, J = 27.3 Hz), 47.1. IR (KBr) cm−1: 1446. Anal. Calcd. For C23H16FN3 (MW 353.39): C, 78.17%; H, 4.56%; N, 11.89%. Found: C, 78.56%; H, 4.58%; N, 11.42%.
5,6-Dimethyl-1-(naphthalen-1-ylmethyl)-2-(pyridin-2-yl)-1H-benzo[d]imidazole (36). Yield = 85%. Mp. 155.5-156.1 °C. 1H-NMR (CDCl3) δ 8.44 (d, J = 7.8 Hz, 1H), 8.40 (d, J = 3.8 Hz, 1H), 8.19 (d, J = 8.4 Hz, 1H), 7.92 (d, J = 7.5 Hz, 1H), 7.77 (t, J = 7.8 Hz, 1H), 7.73-7.67 (m, 2H), 7.67-7.61 (m, 1H), 7.60-7.55 (m, 1H), 7.23-7.16 (m, 2H), 6.99 (s, 1H), 6.65 (s, 2H, CH2), 6.58 (d, J = 7.1 Hz, 1H), 2.40 (s, 3H, CH3), 2.28 (s, 3H, CH3). 13C-NMR (CDCl3) δ 150.7, 148.6, 141.5, 136.7, 135.7, 133.6, 133.2, 133.0, 132.0, 130.5, 129.0, 127.4, 126.3, 125.8, 125.7, 124.3, 123.5, 122.7, 122.5, 120.9, 120.2, 110.7, 46.7, 20.6, 20.4. IR (KBr) 1446 cm−1. Anal. Calcd. For C25H21N3 (MW 363.45): C, 82.61%; H, 5.82%; N, 11.56%. Found: C, 82.21%; H, 6.31%; N, 11.55%.
1-(Naphthalen-1-ylmethyl)-2-(pyridin-3-yl)-1H-benzo[d]imidazole (37). Yield = 80%. Mp. 160.2–161.1 °C. 1H-NMR (CDCl3) δ 8.88–8.86 (s, 1H), 8.58 (d, J = 4.8 Hz, 1H), 7.94 (dt, J1 = 8.0 Hz, J2 = 1.6 Hz, 1H), 7.91–7.84 (m, 3H), 7.77 (d, J = 8.3 Hz, 1H), 7.56–7.50 (m, 2H), 7.33–7.16 (m, 4H), 7.12 (d, J = 7.9 Hz, 1H), 6.79 (d, J = 7.1 Hz, 1H), 5.84 (s, 2H, CH2). 13C-NMR (CDCl3) δ 151.3, 150.9, 149.6, 143.3, 136.4, 136.4, 133.8, 130.9, 130, 129.3, 128.6, 127.0, 126.4, 126.3, 125.7, 123.8, 123.6, 123.2, 122.9, 121.9, 120.3, 110.6, 46.6. IR (KBr) 1450 cm−1. Anal. Calcd. For C23H17N3 (MW 335.40): C, 82.36%; H, 5.11%; N, 12.53%. Found: C, 82.16%; H, 5.60%; N, 12.67%.
5-Fluoro-1-(naphthalen-1-ylmethyl)-2-(pyridin-3-yl)-1H-benzo[d]imidazole (38). Yield = 58%. Mp. 163.4–163.9 °C. 1H-NMR (CDCl3) δ 8.94 (d, J = 0.01Hz, 1H), 8.66 (dd, J1 = 4.9 Hz, J2 = 1.7 Hz, 1H), 8.04–7.96 (m, 2H), 7.95–7.91 (m, 1H), 7.86 (d, J = 8.3 Hz, 1H), 7.64–7.58 (m, 3H), 7.40–7.30 (m, 2H), 7.10 (dd, J1 = 8.9 Hz, J2 = 4.6 Hz, 1H), 7.01 (td, J1 = 9.1, J2 = 2.4 Hz, 1H), 6.85 (dd, J1 = 7.2 Hz, J2 = 1.3 Hz, 1H), 5.91 (s, 2H, CH2). 13C-NMR (CDCl3) δ 159.9 (d, J = 238.7 Hz), 152.7, 151.0, 149.5, 143.7 (d, J = 13.0 Hz), 136.3, 133.9, 132.9, 130.6, 129.3, 128.8, 127.8, 127.0, 126.5, 125.7, 123.7, 122.8, 121.8, 114.1, 112.2 (d, J = 26.1 Hz), 111.1 (d, J = 10.4 Hz), 106.0 (d, J = 24.0 Hz), 46.8. IR (KBr) 1476 cm−1. Anal. Calcd. For C23H16FN3 (MW 353.39): C, 78.17%; H, 4.56%; N, 11.89%. Found: C, 78.27%; H, 5.05%; N, 12.32%.
6-Fluoro-1-(naphthalen-1-ylmethyl)-2-(pyridin-3-yl)-1H-benzo[d]imidazole (39). Yield = 56%. Mp. 156.1–157.0 °C. 1H-NMR (CDCl3) δ 8.93 (s, 1H), 8.64 (dd, J1 = 4.9 Hz, J2 = 1.7 Hz, 1H), 8.01–7.94 (m, 2H), 7.93–7.89 (m, 1H), 7.89–7.82 (m, 2H), 7.64–7.57 (m, 2H), 7.35 (t, J = 7.7 Hz, 1H), 7.30 (dd, J1 = 8.0 Hz, J2 = 4.9 Hz, 1H), 7.09 (dt, J1 = 9.2 Hz, J2 = 2.4 Hz, 1H), 6.89–6.84 (m, 2H), 5.86 (s, 2H, CH2). 13C-NMR (CDCl3) δ 160.2 (d, J = 241.8 Hz), 156.4, 152.0 (d, J = 3.5 Hz), 150.9, 149.5, 139.7, 136.7 (d, J = 13.0 Hz), 136.2, 133.9, 130.4, 129.3, 128.8, 127.0, 126.5, 125.6, 123.6, 122.8, 121.8, 121.2 (d, J = 10.0 Hz), 114.1, 111.7 (d, J = 25.1 Hz), 97.3 (d, J = 27.8 Hz), 46.8. IR (KBr) 1480 cm−1. Anal. Calcd. For For C23H16FN3 (MW 353.39): C, 78.17%; H, 4.56%; N, 11.89%. Found: C, 78.61%; H, 4.44%; N, 11.79%.
5,6-Dimethyl-1-(naphthalen-1-ylmethyl)-2-(pyridin-3-yl)-1H-benzo[d]imidazole (40). Yield = 90%. Mp. 141.4–142.1 °C. 1H-NMR (CDCl3) δ 8.88 (s, 1H), 8.59 (d, J = 4.4 Hz, 1H), 7.99–7.90 (m, 3H), 7.83 (d, J = 8.2 Hz, 1H), 7.69 (s, 1H), 7.64–7.57 (m, 2H), 7.32 (t, J = 7.7 Hz, 1H), 7.27–7.21 (m, 1H), 6.94 (s, 1H), 6.85 (d, J = 7.1 Hz, 1H), 5.81 (s, 2H, CH2), 2.40 (s, 3H, CH3), 2.27 (s, 3H, CH3). 13C-NMR (CDCl3) δ 150.5, 150.4, 149.4, 141.9, 136.2, 135.1, 133.8, 133.2, 132.2, 131.2, 130.0, 129.2, 128.5, 126.9, 126.5, 126.4, 125.8, 123.5, 122.9, 122.0, 120.2, 110.6, 46.5, 20.6, 20.4. IR (KBr) 1440 cm−1. Anal. Calcd. For C25H21N3 (MW 363.45): C, 82.61%; H, 5.82%; N, 11.56%. Found: C, 83.11%; H, 5.92%; N, 11.57%.
1-(Naphthalen-1-ylmethyl)-2-(pyridin-4-yl)-1H-benzo[d]imidazole (41). Yield = 93%. Mp. 163.3–163.8 °C. 1H-NMR (CDCl3) δ 8.56 (d, J = 6.1 Hz, 2H), 7.97–7.88 (m, 3H), 7.81 (d, J = 8.3 Hz, 1H), 7.62–7.54 (m, 4H), 7.33 (t, J = 7.6 Hz, 1H), 7.28–7.24 (m, 1H), 7.22–7.16 (m, 1H), 7.11 (d, J = 8.1 Hz, 1H), 6.81 (d, J = 7.1 Hz, 1H), 5.84 (s, 2H, CH2). 13C-NMR (CDCl3) δ 151.3, 150.5(2C), 143.2, 137.5, 136.6, 133.9, 130.9, 129.9, 129.4, 129.3, 128.7, 126.5, 125.7, 124.1, 123.6, 123.4, 122.9(2C), 121.9, 120.6, 110.7, 46.6. IR (KBr) 1449 cm−1. Anal. Calcd. For C23H17N3 (MW 335.40): C, 82.36%; H, 5.11%; N, 12.53%. Found: C, 82.86%; H, 5.20%; N, 12.77%.
5-Fluoro-1-(naphthalen-1-ylmethyl)-2-(pyridin-4-yl)-1H-benzo[d]imidazole (42). Yield = 53%. Mp. 151.5-152.5 °C. 1H-NMR (DMSO-d6) δ 8.65 (d, J = 5.2 Hz, 2H), 8.05–7.93 (m, 2H), 7.89 (d, J = 8.4 Hz, 1H), 7.70–7.55 (m, 5H), 7.38 (t, J = 7.7 Hz, 1H), 7.12 (dd, J1 = 8.8 Hz, J2 = 4.6 Hz, 1H), 7.03 (td, J1 = 8.9 Hz, J2 = 2.4 Hz, 1H), 6.86 (d, J = 7.2 Hz, 1H), 5.93 (s, 2H, CH2). 13C-NMR (CDCl3) δ 160.0 (d, J = 238.6 Hz), 152.7, 150.6(2C), 143.6 (d, J = 12.8 Hz), 137.2, 133.9, 133.1, 130.5, 129.9, 129.4, 128.9, 127.8 (d, J = 5.1 Hz), 127.1, 126.6, 125.7, 122.8(2C), 121.8, 112.7 (d, J = 26.3 Hz), 111.2 (d, J = 10.0 Hz), 106.2 (d, J = 24.2 Hz), 46.8. IR (KBr) 1471 cm−1. Anal. Calcd. For C23H16FN3 (MW 353.39): C, 78.17%; H, 4.56%; N, 11.89%. Found: C, 78.67%; H, 4.55%; N, 12.30%.
6-Fluoro-1-(naphthalen-1-ylmethyl)-2-(pyridin-4-yl)-1H-benzo[d]imidazole (43). Yield = 60%. Mp. 167.4–168.1°C. 1H-NMR (CDCl3) δ 8.63 (d, J = 5.6 Hz, 2H), 8.04–7.96 (m, 1H), 7.98–7.91 (m, 1H), 7.91–7.84 (m, 2H), 7.74–7.45 (m, 4H), 7.38 (t, J = 7.7 Hz, 1H), 7.12 (td, J1 = 8.9 Hz, J2 = 2.4 Hz, 1H), 6.91–6.84 (m, 2H), 5.89 (s, 2H, CH2). 13C-NMR (CDCl3) δ 160.4 (d, J = 241.6 Hz), 152.0, 150.5(2C), 139.6, 137.2, 133.9, 130.3, 129.9, 129.4, 128.9, 127.8 (d, J = 6.7 Hz), 127.1, 126.6, 125.7, 123.1, 122.8(2C), 121.8, 121.5 (d, J = 9.8 Hz), 112.0 (d, J = 25.3 Hz), 97.3 (d, J = 27.5 Hz), 46.8. IR (KBr) 1431 cm−1. Anal. Calcd. For C23H16FN3 (MW 353.39): C, 78.17%; H, 4.56%; N, 11.89%. Found: C, 78.38%; H, 5.05%; N, 12.21%.
5,6-Dimethyl-1-(naphthalen-1-ylmethyl)-2-(pyridin-4-yl)-1H-benzo[d]imidazole (44). Yield = 92%. Mp. 156.8–157.6 °C. 1H-NMR (CDCl3) δ 8.59 (s broad, 2H), 7.98 (s broad, 2H), 7.86 (d, J = 7.0 Hz, 1H), 7.74–7.53 (m, 5H), 7.39–7.31 (m, 1H), 6.96 (s, 1H), 6.86 (d, J = 6.8 Hz, 1H), 5.87 (s, 2H, CH2), 2.41 (s, 3H, CH3 ), 2.29 (s, 3H, CH3 ). 13C-NMR (CDCl3) δ 150.4(2C), 141.9, 137.7, 135.3, 133.9, 133.7, 132.5, 131.1, 129.9, 128.6, 127.9, 127.0, 126.5, 125.8, 123.1, 122.9, 122.8(2C), 122.0, 120.5, 110.6, 46.6, 20.6, 20.4. IR (KBr) 1455 cm−1. Anal. Calcd. For C25H21N3 (MW 363.45): C, 82.61%; H, 5.82%; N, 11.56%. Found: C, 83.07%; H, 6.30%; N, 12.01%.
1-(Naphthalen-2-ylmethyl)-2-(pyridin-2-yl)-1H-benzo[d]imidazole (45). Yield = 87%. Mp. 150.7–151.8 °C. 1H-NMR (CDCl3) δ 8.74 (d, J = 4.2 Hz, 1H), 8.59 (d, J = 8.0 Hz, 1H), 8.01 (d, J = 7.9 Hz, 1H), 7.95 (td, J = 7.9, 1.7 Hz, 1H), 7.89 (dd, J1 = 6.0 Hz, J2 = 3.4 Hz, 1H), 7.86 (d, J = 8.6 Hz, 1H), 7.80 (dd, J1 = 6.0 Hz, J2 = 3.4 Hz, 1H), 7.67 (s, 1H), 7.56–7.51 (m, 2H), 7.51-7.45 (m, 2H), 7.44–7.40 (m, 2H), 7.37 (d, J = 6.6 Hz, 1H), 6.48 (s, 2H, CH2).13C-NMR (CDCl3) δ 150.6, 150.1, 148.7, 142.8, 136.9, 136.9, 135.0, 133.3, 132.7, 128.5, 127.8, 127.7, 126.2, 125.9, 125.4, 124.9, 124.7, 123.9, 123.7, 122.9, 120.2, 110.8, 49.2. IR (KBr) 1466 cm−1. Anal. Calcd. For C23H17N3 (MW 335.40): C, 82.36%; H, 5.11%; N, 12.53%. Found: C, 82.06%; H, 5.61%; N, 12.05%.
5-Fluoro-1-(naphthalen-2-ylmethyl)-2-(pyridin-2-yl)-1H-benzo[d]imidazole (46). Yield = 62%. Mp. 165.7–166.4 °C. 1H-NMR (CDCl3) δ 8.65 (d, J = 3.9 Hz, 1H), 8.48 (d, J = 8.0 Hz, 1H), 7.87 (td, J1 = 7.9 Hz, J2 = 1.9 Hz, 1H), 7.83–7.76 (m, 2H), 7.72 (dd, J1 = 6.2 Hz, J2 = 3.3 Hz, 1H), 7.61–7.54 (m, 2H), 7.46 (d, J = 6.3 Hz, 1H), 7.45 (d, J = 6.2 Hz, 1H), 7.39–7.32 (m, 2H), 7.29 (dd, J1 = 9.0 Hz, J2 = 4.4 Hz, 1H), 7.02 (td, J1 = 9.1 Hz, J2 = 2.4 Hz, 1H), 6.37 (s, 2H, CH2). 13C-NMR (CDCl3) δ 159.8 (d, J = 238.1 Hz), 151.3, 150.3, 148.8, 143.2, 143.1, 137.0, 134.7, 133.4, 133.3, 132.8, 128.6, 127.8 (d, J = 9.7 Hz), 126.3, 126.0, 125.8, 125.4, 124.8 (d, J = 2.8 Hz), 124.1, 112.1 (d, J = 26.4 Hz), 111.3 (d, J = 10.2 Hz), 105.7 (d, J = 23.9 Hz), 49.4. IR (KBr) 1484 cm−1. Anal. Calcd. For C23H16FN3 (MW 353.39): C, 78.17%; H, 4.56%; N, 11.89%. Found: C, 77.95%; H, 5.02%; N, 12.35%.
6-Fluoro-1-(naphthalen-2-ylmethyl)-2-(pyridin-2-yl)-1H-benzo[d]imidazole (47). Yield = 55%. Mp. 170.5–171.3 °C. 1H-NMR (CDCl3) δ 8.65 (d, J = 5.8 Hz, 1H), 8.46 (d, J = 8.0 Hz, 1H), 7.9–7.76 (m, 4H), 7.73 (dd, J1 = 6.2 Hz, J2 = 3.4 Hz, 1H), 7.58 (s, 1H), 7.47 (d, J = 6.3 Hz, 1H), 7.46 (d, J = 6.2 Hz, 1H), 7.40–7.31 (m, 2H), 7.13–7.02 (m, 2H), 6.34 (s, 2H, CH2). 13C-NMR (CDCl3) δ 160.3 (d, J = 211.9 Hz), 150.4, 148.7, 145.1, 139.2, 137.2, 137.0, 134.5, 133.3, 132.8, 128.6, 128.1, 127.8 (d, J = 12.1 Hz), 126.3, 126.0, 125.5, 124.8, 124.5, 124.0, 121.0 (d, J = 9.8 Hz), 111.5 (d, J = 25.1 Hz), 97.4 (d, J = 27.9 Hz), 49.4. IR (KBr) 1464 cm−1. Anal. Calcd. For C23H16FN3 (MW 353.39): C, 78.17%; H, 4.56%; N, 11.89%. Found: C, 78.15%; H, 4.52%; N, 12.33%.
5,6-Dimethyl-1-(naphthalen-2-ylmethyl)-2-(pyridin-2-yl)-1H-benzo[d]imidazole (48). Yield = 77%. Mp. 147.7–148.5 °C. 1H-NMR (CDCl3) δ 8.61 (d, J = 4.3 Hz, 1H), 8.45 (d, J = 7.9 Hz, 1H), 7.89–7.75 (m, 3H), 7.67 (s, 1H), 7.58–7.49 (m, 2H), 7.48–7.41 (m, 2H), 7.36 (d, J = 8.5 Hz, 1H), 7.31–7.26 (m, 1H), 7.15 (s, 1H), 6.33 (s, 2H, CH2), 2.41 (s, 3H, CH3 ), 2.35 (s, 3H, CH3). 13C-NMR (CDCl3) δ 150.8, 148.6, 141.5, 136.8, 135.6, 135.3, 133.3, 133.1, 132.7, 131.9, 128.4, 127.8, 127.7, 126.2, 125.8, 125.4, 125.2, 124.9, 124.5, 123.6, 120.1, 110.8, 49.1, 20.8, 20.4. IR (KBr) 1440 cm−1. Anal. Calcd. For C25H21N3 (MW 363.45): C, 82.61%; H, 5.82%; N, 11.56%. Found: C, 82.97%; H, 6.15%; N, 11.50%.
1-(Naphthalen-2-ylmethyl)-2-(pyridin-3-yl)-1H-benzo[d]imidazole (49). Yield = 86%. Mp. 146.3–147.0 °C. 1H-NMR (CDCl3) δ 9.02 (s broad, 1H), 8.74 (s broad, 1H), 8.06 (d, J = 7.3 Hz, 1H), 7.97 (d, J = 7.6 Hz, 1H), 7.92–7.82 (m, 2H), 7.72 (d, J = 7.6 Hz, 1H), 7.57–7.46 (m, 3H), 7.44–7.37 (m, 2H), 7.35–7.24 (m, 3H), 5.65 (s, 2H, CH2). 13C-NMR (CDCl3) δ 150.9, 136.8, 136.3, 135.0, 133.4, 133.3, 133.0, 131.7, 129.3, 127.9, 127.8, 126.8, 126.5, 124.6, 123.8, 123.6, 123.2, 120.3, 114.1, 112.8, 110.7, 110.7, 48.6. IR (KBr) 1459.5 cm−1. Anal. Calcd. For C23H17N3 (MW 335.40): C, 82.36%; H, 5.11%; N, 12.53%. Found: C, 81.99%; H, 5.54%; N, 12.55%.
5-Fluoro-1-(naphthalen-2-ylmethyl)-2-(pyridin-3-yl)-1H-benzo[d]imidazole (50). Yield = 66%. Mp. 175.7–176.8 °C 1H-NMR (CDCl3) δ 9.00 (s, 1H), 8.74 (s broad, 1H), 8.05 (d, J = 7.8 Hz, 1H), 7.93–7.80 (m, 3H), 7.73 (d, J = 7.9 Hz, 1H), 7.56–7.50 (m, 2H), 7.48–7.46 (m, 1H), 7.43–7.37 (m, 1H), 7.26 (d, J = 8.5 Hz, 1H), 7.13 (td, J1 = 9.2 Hz, J2 = 2.3 Hz, 1H), 6.99 (dd, J1 = 8.5 Hz, J2 = 2.4 Hz, 1H), 5.62 (d, J = 14.7 Hz, 2H, CH2). 13C-NMR (CDCl3) δ 160.1 (d, J = 234.2 Hz), 151.9, 151.1, 149.7, 139.7, 136.6 (d, J = 8.2 Hz), 133.4, 133.0, 132.8, 129.5, 127.9 (d, J = 4.8 Hz), 126.8, 126.6, 126.3, 124.6, 123.6, 123.5, 121.2 (d, J = 10.1 Hz), 112.3, 111.7 (d, J = 25.7 Hz), 105.9, 97.4 (d, J = 27.5 Hz), 48.8. IR (KBr) 1478 cm−1. Anal. Calcd. For C23H16FN3 (MW 353.39): C, 78.17%; H, 4.56%; N, 11.89%. Found: C, 77.88%; H, 4.96%; N, 11.39%.
6-Fluoro-1-(naphthalen-2-ylmethyl)-2-(pyridin-3-yl)-1H-benzo[d]imidazole (51). Yield = 62%. Mp. 166.8–167.4 °C. 1H-NMR (CDCl3) δ 9.00 (s, 1H), 8.75 (d, J = 4.8 Hz, 1H), 8.07 (d, J = 8.1 Hz, 1H), 7.94–7.83 (m, 2H), 7.77–7.70 (m, 1H), 7.61 (dd, J1 = 9.1 Hz, J2 = 2.4 Hz, 1H),7.56–7.50 (m, 2H), 7.48 (s, 1H), 7.42 (dd, J1 = 8.0 Hz, J2 = 4.8 Hz, 1H), 7.31–7.24 (m, 1H), 7.22 (dd, J1 = 8.8 Hz, J2 = 4.5 Hz, 1H), 7.06 (td, J1 = 9.0 Hz, J2 = 2.3 Hz, 1H), 5.65 (s, 2H, CH2).13C-NMR (CDCl3) δ 159.9 (d, J = 238.9 Hz), 151.1, 149.7, 144.4, 143.7, 136.7, 133.4, 133.0, 130.0, 129.4, 129.4, 127.8 (d, J = 4.5 Hz), 126.9, 126.6, 125.9, 124.6, 123.5, 118.4, 116.1, 112.2 (d, J = 26.3 Hz), 111.1 (d, J = 10.2 Hz), 106.0 (d, J = 24.3 Hz), 48.8. IR (KBr) 1500 cm−1. Anal. Calcd. For C23H16FN3 (MW 353.39): C, 78.17%; H, 4.56%; N, 11.89%. Found: C, 78.30%; H, 4.59%; N, 11.92%.
5,6-Dimethyl-1-(naphthalen-2-ylmethyl)-2-(pyridin-3-yl)-1H-benzo[d]imidazole (52). Yield = 96%. Mp. 138.5–139.5 °C. 1H-NMR (CDCl3) δ 8.99 (s broad, 1H), 8.70 (s broad, 1H), 8.03 (d, J = 7.5 Hz, 1H), 7.94–7.82 (m, 2H), 7.77–7.66 (m, 2H), 7.56–7.44 (m, 3H), 7.36 (s broad, 1H), 7.29 (d, J = 6.5 Hz, 1H), 7.08 (s, 1H), 5.60 (s, 2H, CH2), 2.44 (s, 3H, CH3 ), 2.35 (s, 3H, CH3 ). 13C-NMR (CDCl3) δ 150.7, 142.0, 136.5, 135.0, 133.7, 133.5, 133.1, 132.9, 132.2, 129.3, 127.9, 127.8, 126.7, 126.4, 124.5, 123.6, 123.5, 120.3, 110.6, 107.1, 103.8, 103.0, 48.5, 20.6, 20.4. IR (KBr) 1447 cm−1. Anal. Calcd. For C25H21N3 (MW 363.45): C, 82.61%; H, 5.82%; N, 11.56%. Found: C, 82.66%; H, 5.92%; N, 11.66%.
1-(Naphthalen-2-ylmethyl)-2-(pyridin-4-yl)-1H-benzo[d]imidazole (53). Yield = 94%. Mp. 133.0-131.0 °C. 1H-NMR (CDCl3) δ 8.73 (d, J = 5.9 Hz, 2H), 7.97 (d, J = 8.1 Hz, 1H), 7.94–7.86 (m, 2H), 7.73 (d, J = 6.6 Hz, 1H), 7.67 (d, J = 5.9 Hz, 2H), 7.58–7.48 (m, 3H) 7.45–7.37 (m, 1H), 7.36–7.28 (m, 3H), 5.68 (s, 2H, CH2). 13C-NMR (CDCl3) δ 150.5(2C), 143.2, 137.7, 136.5, 133.3, 129.4, 127.9, 127.8, 126.8, 126.5, 124.6, 124.2, 123.5, 123.4, 123.2(2C), 120.6, 119.5, 119.3, 118.7, 110.7, 48.7. IR (KBr) 1437 cm−1. Anal. Calcd. For C23H17N3 (MW 335.40): C, 82.36%; H, 5.11%; N, 12.53%. Found: C, 81.22%; H, 5.60%; N, 12.68%.
5-Fluoro-1-(naphthalen-2-ylmethyl)-2-(pyridin-4-yl)-1H-benzo[d]imidazole (54). Yield = 50%. Mp. 177.8–178.5 °C. 1H-NMR (CDCl3) δ 8.76 (s broad, 2H), 7.95–7.85 (m, 2H), 7.77–7.71 (m, 1H), 7.67 (s broad, 2H), 7.62 (dd, J1 = 9.2 Hz, J2 = 2.4 Hz, 1H), 7.58–7.51 (m, 2H), 7.48 (s, 1H), 7.32–7.26 (m, 1H), 7.22 (dd, J1 = 8.8 Hz, J2 = 4.4 Hz, 1H), 7.06 (td, J1 = 9.1 Hz, J2 = 2.4 Hz, 1H), 5.66 (s, 2H, CH2). 13C-NMR (CDCl3) δ 158.7, 151.5 (d, J = 214.6 Hz), 143.5, 138.7, 137.4, 133.3 (d, J = 12.0 Hz), 133.0, 132.9(2C), 131.7, 129.5, 127.8(2C), 127.7, 126.9, 126.6, 125.1, 124.6, 123.4, 119.1, 112.6 (d, J = 26.5 Hz), 111.2 (d, J = 11.0 Hz), 106.2 (d, J = 24.3 Hz), 48.8. IR (KBr) 1585 cm−1. Anal. Calcd. For C23H16FN3 (MW 353.39): C, 78.17%; H, 4.56%; N, 11.89%. Found: C, 78.66%; H, 4.57%; N, 11.79%.
6-Fluoro-1-(naphthalen-2-ylmethyl)-2-(pyridin-4-yl)-1H-benzo[d]imidazole (55). Yield = 53%. Mp. 163.5–164.1 °C. 1H-NMR (CDCl3) δ 8.79 (s broad, 2H), 8.00–7.82 (m, 3H), 7.80–7.60 (m, 2H), 7.59–7.44 (m, 3H), 7.37–7.25 (m, 2H), 7.15 (td, J1 = 9.2 Hz, J2 = 2.4 Hz, 1H), 6.99 (dd, J1 = 8.5 Hz, J2 = 2.4 Hz, 1H), 5.64 (s, 2H, CH2). 13C-NMR (CDCl3) δ 153.3 (d, J = 227.6 Hz), 150.4, 147.3, 144.0, 136.0, 134.2, 133.4, 132.7, 132.7, 129.6, 127.9(2C), 126.9, 126.6, 124.6, 124.1, 123.4(2C), 121.5 (d, J = 8.1 Hz), 120.5, 112.0 (d, J = 23.3 Hz), 97.4 (d, J = 26.9 Hz), 48.9. IR (KBr) 1497 cm−1. Anal. Calcd. For C23H16FN3 (MW 353.39): C, 78.17%; H, 4.56%; N, 11.89%. Found: C, 78.18%; H, 4.99%; N, 11.55%.
5,6-Dimethyl-1-(naphthalen-2-ylmethyl)-2-(pyridin-4-yl)-1H-benzo[d]imidazole (56). Yield = 90%. Mp. 133.7-134.5 °C. 1H-NMR (CDCl3) δ 8.69 (d, J = 6.0 Hz, 2H), 7.96–7.85 (m, 2H), 7.77–7.69 (m, 2H), 7.64 (d, J = 6.0 Hz, 2H), 7.58–7.51 (m, 2H), 7.49 (s, 1H), 7.33 (d, J = 8.5 Hz, 1H), 7.08 (s, 1H), 5.64 (s, 2H, CH2), 2.44 (s, 3H, CH3 ), 2.35 (s, 3H, CH3 ). 13C-NMR (CDCl3) δ 162.2, 161.3, 150.5, 150.4(2C), 142.2, 133.7, 129.4, 127.9, 127.8, 126.8, 126.5, 126.5, 124.8, 124.4, 123.6, 123.1(2C), 120.4, 115.5, 112.8, 110.7, 109.1, 96.5, 50.2. IR (KBr) 1450 cm−1. Anal. Calcd. For C25H21N3 (MW 363.45): C, 82.61%; H, 5.82%; N, 11.56%. Found: C, 83.06%; H, 5.88%; N, 11.78%.