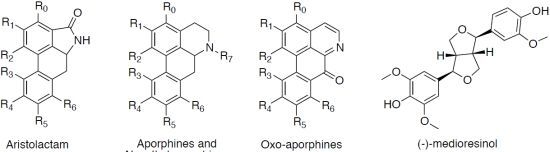
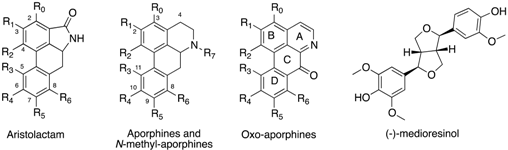
Natural Aristolactams and Aporphine Alkaloids as Inhibitors of CDK1/Cyclin B and DYRK1A
Abstract
:1. Introduction
2. Results and Discussion
| Compounds | R0 | R1 | R2 | R3 | R4 | R5 | R6 | R7 | DYRK1A (IC50 in μM) a | CDK1/Cyclin B (IC50 in μM) a |
| Aristolactams | ||||||||||
| aristolactam AII (1) | H | OH | OCH3 | H | H | H | H | H | >30 | >30 |
| aristolactam BII (2) | H | OCH3 | OCH3 | H | H | H | H | H | >30 | >30 |
| velutinam (3) | H | OCH3 | OCH3 | H | H | H | OH | H | 0.6 | 1.5 |
| aristolactam AIIIA (4) | H | OH | OCH3 | H | OH | H | H | H | 0.08 | 0.2 |
| N-Methylaporphines | ||||||||||
| (+)-corydine (6) | H | OCH3 | OH | OCH3 | OCH3 | H | H | CH3 | >30 | >30 |
| (−)-roemerine (7) | H | O-CH2-O | H | H | H | H | CH3 | 15.0 | >30 | |
| (+)-bulbocapnine (9) | H | O-CH2-O | OH | OCH3 | H | H | CH3 | >30 | >30 | |
| (+)-N-methyllindcarpine (10) | H | OH | OCH3 | OH | OCH3 | H | H | CH3 | >30 | >30 |
| (+)-boldine (16) | H | OH | OCH3 | H | OCH3 | OH | H | CH3 | >30 | >30 |
| Aporphines | ||||||||||
| (+)-actinodaphnine (11) | H | O-CH2-O | H | OCH3 | OH | H | H | >30 | >30 | |
| (+)-11-methoxynorneolistine (12) | H | O-CH2-O | OCH3 | O-CH2-O | H | H | 2.5 | >30 | ||
| (−)-O-methylisopiline (14) | OCH3 | OCH3 | OCH3 | H | H | H | H | H | >30 | >30 |
| (+)-N-nornuciferine (15) | H | OCH3 | OCH3 | H | H | H | H | H | 4.2 | >30 |
| Oxo-aporphines | ||||||||||
| liriodenine (8) | H | O-CH2-O | H | H | H | H | - | 3.1 | >30 | |
| lysicamine (13) | H | OCH3 | OCH3 | H | H | H | H | - | 2.4 | >30 |
| Other | ||||||||||
| (−)-medioresinol (5) | 0.1 | 1.3 | ||||||||
| 6-bromoindirubin-3'-monoxime b | 0.52 | 0.32 | ||||||||
3. Experimental
3.1. General
3.2. Plant Material
3.3. Extraction and Isolation Procedures
3.4. Preparation and Assay of Protein Kinases
4. Conclusions
Supplementary Materials
Acknowledgments
References and Notes
- Hunter, T. Protein kinases and phosphatases: The yin and yang of protein phosphorylation and signaling. Cell 1995, 80, 225–236. [Google Scholar] [CrossRef]
- Ferrer, I.; Barrachina, M.; Puig, B.; Martinez de Lagran, M.; Marti, E.; Avila, J.; Dierssen, M. Constitutive Dyrk1A is abnormally expressed in Alzheimer disease, Down syndrome, Pick disease, and related transgenic models. Neurobiol. Dis. 2005, 20, 392–400. [Google Scholar] [CrossRef]
- Becker, W.; Sippl, W. Activation, Regulation and Inhibition of DYRK1A. FEBS J. 2011, 278, 246–256. [Google Scholar] [CrossRef]
- Wegiel, J.; Gong, C.-X.; Hwang, Y.-W. The role of DYRK1A in neurodegenerative diseases. FEBS J. 2011, 278, 236–245. [Google Scholar] [CrossRef]
- Harper, J.W.; Adams, P.D. Cyclin-dependent kinases. Chem. Rev. 2001, 101, 2511–2526. [Google Scholar] [CrossRef]
- Knockaert, M.; Greengard, P.; Meijer, L. Pharmacological inhibitors of cyclin-dependent kinases. Trends Pharmacol. Sci. 2002, 23, 417–425. [Google Scholar] [CrossRef]
- Shapiro, G.I. Cyclin-Dependent Kinase Pathways as Targets for Cancer Treatment. J. Clin. Oncol. 2006, 24, 1770–1783. [Google Scholar] [CrossRef]
- Malumbres, M.; Barbacid, M. Mammalian cyclin-dependent kinases. Trends Biochem. Sci. 2005, 30, 630–641. [Google Scholar] [CrossRef]
- Kamb, A. Cyclin-dependent kinase inhibitors and human cancer. Curr. Top. Microbiol. Immunol. 1998, 227, 139–148. [Google Scholar] [CrossRef]
- Pines, J. Cyclins and cyclin-dependent kinases: Take your partners. Trends Biochem. Sci. 1993, 18, 195–197. [Google Scholar] [CrossRef]
- Savage, M.J.; Gingrich, D.E. Advances in the development of kinase inhibitor therapeutics for Alzheimer’s disease. Drug Dev. Res. 2009, 70, 125–144. [Google Scholar] [CrossRef]
- Apel, C.; Dumontet, V.; Lozach, O.; Meijer, L.; Guéritte, F.; Litaudon, M. Phenanthrene derivatives from Appendicula reflexa as new CDK1/cyclin B inhibitors Phytochem. Lett. 2012, 5, 814–818. [Google Scholar]
- The species G. dumontetii, which was the first representative of the genus Goniothalamus in New Caledonia was discovered in October 1997 by one of us (M.L.) in the “Special Reserve of Nodela Flora” (South Province), and was named in 2007 by R.M.K. Sauders and Munzinger (Bot. J. Linn. Soc. 2007, 155, 497–503), following the chemical and biological studies carried out.
- Crohare, R.; Priestap, H.A.; Farina, M.; Cedola, M.; Ruveda, E.A. Aristololactams of Aristolochia argentina. Phytochemistry 1974, 13, 1957–1962. [Google Scholar] [CrossRef]
- Wang, E.-C.; Shih, M.-H.; Liu, M.-C.; Chen, M.-T.; Lee, G.-H. Studies on constituents of Saururus chinensis. Heterocycle 1996, 43, 969–976. [Google Scholar] [CrossRef]
- Omar, S.; Chee, C.L.; Ahmad, F.; Ni, J.X.; Jaber, H.; Huang, J.; Nakatsu, T. Phenanthrene lactams from Goniothalamus velutinus. Phytochemistry 1992, 31, 4395–4397. [Google Scholar] [CrossRef]
- Hedge, V.R.; Borges, S.; Patel, M.; Das, P.R.; Wu, B.; Gullo, V.P.; Chan, T.-Z. New potential antitumor compounds from the plant Aristolochia manshuriensis as inhibitors of the CDK2 enzyme. Bioorg. Med. Chem. 2010, 20, 1344–1346. [Google Scholar] [CrossRef]
- Li, N.; Wu, J.-L.; Hasegawa, T.; Sakai, J.-I.; Bai, L.-M.; Wang, L.-Y.; Kakuta, S.; Furuya, Y.; Ogura, H.; Kataoka, T.; et al. Bioactive lignans from Peperomia duclouxii. J. Nat. Prod. 2007, 70, 544–548. [Google Scholar] [CrossRef]
- Xiong, L.; Zhu, C.; Li, Y.; Tian, Y.; Lin, S.; Yuan, S.; Hu, J.; Hou, Q.; Chen, N.; Yang, Y.; Shi, J. Lignans and Neolignans from Sinocalamus affinis and Their Absolute Configurations. J. Nat. Prod. 2011, 74, 1188–1200. [Google Scholar] [CrossRef]
- Ferreira, M.L.R.; de Pascoli, I.C.; Nascimento, I.R.; Lopes, L.M.X.; Zukerman-Schpector, J. Aporphine and bisaporphine alkaloids from Aristolochia lagesiana var. intermedia. Phytochemistry 2010, 71, 469–478. [Google Scholar] [CrossRef]
- Guinaudeau, H.; Leboeuf, M.; Debray, M.; Cave, A.; Paris, R.R. Alkaloids of Colubrina faralaotra ssp. faralaotra. Planta Med. 1975, 27, 304–316. [Google Scholar] [CrossRef]
- Bhakuni, D.S.; Tewari, S.; Dhar, M.M. Aporphine alkaloids of annona squamosa. Phytochemistry 1972, 11, 1819–1822. [Google Scholar] [CrossRef]
- Simas, N.K.; Ferrari, S.F.; Pereira, S.N.; Leitao, G.G. Chemicalecological characteristics of herbivory of Siparuna guianensis seeds by buffy-headed marmosets (Callithrix flaviceps) in the Atlantic forest of southeastern Brazil. J. Chem. Ecol. 2001, 27, 93–108. [Google Scholar] [CrossRef]
- Chen, K.-S.; Wu, Y.-C.; Teng, C.-M.; Ko, F.-N.; Wu, T.-S. Bioactive alkaloids from Illigera luzonensis. J. Nat. Prod. 1997, 60, 645–647. [Google Scholar] [CrossRef]
- Denisenko, O.N.; Israilov, I.A.; Chelombitko, V.A.; Yunusov, M.S. Alkaloids of Corydalis marschalliana. Chem. Nat. Compd. 1993, 29, 690–691. [Google Scholar] [CrossRef]
- Kamenati, T.; Sugahara, T.; Fukumoto, K. Studies on total photolytic synthesis of alkaloids—III: The products of photo-pschorr reaction—Total synthesis of isocorydine. Tetrahedron 1971, 27, 5367–5374. [Google Scholar] [CrossRef]
- Johns, S.R.; Lamberton, J.A. Alkaloids of Phoebe clemensii Allen (family Lauraceae). Aust. J. Chem. 1967, 20, 1277–1281. [Google Scholar] [CrossRef]
- Stévigny, C.; Block, S.; Pauw-Gillet, M.C.; de Hoffmann, E.; de Llabres, G.; Adjakidje, V.; Quetin-Leclercq, J. Cytotoxic aporphine alkaloids from Cassytha filiformis. Planta Med. 2002, 68, 1042–1044. [Google Scholar] [CrossRef]
- Buchanan, M.S.; Carroll, A.R.; Pass, D.; Quinn, R.J. Aporphine Alkaloids from the Chinese Tree Neolitsea aurata var. paraciculata. Nat. Prod. Com. 2007, 2, 255–259. [Google Scholar]
- Orito, K.; Uchiito, S.; Satoh, Y.; Tatsuzawa, T.; Harada, R.; Tokuda, M. Aryl Radical Cyclizations of 1-(2'-Bromobenzyl)isoquinolines with AIBN-Bu3SnH: Formation of Aporphines and Indolo[2,1-a]isoquinolines. Org. Lett. 2000, 2, 307–310. [Google Scholar] [CrossRef]
- Hocquemiller, R.; Rasamizafy, S.; Cavé, A.; Moretti, C. Alcaloïdes des Annonacees XXXVII: Alcaloïdes du Guatteria scandens. J. Nat. Prod. 1983, 46, 335–341. [Google Scholar] [CrossRef]
- Montenegro, H.; Gutierrrez, M.; Romero, L.I.; Ortega-Barria, E.; Capson, T.L.; Cubilla Rios, L. Aporphine alkaloids from Guatteria spp. with leishmanicidal activity. Planta Med. 2003, 69, 677–679. [Google Scholar] [CrossRef]
- Gunawardana, Y.A.; Geewanda, P.; Huck-Meng, L.; Bick, I.; Ralph, C. Alkaloids of Hedycarya angustifolia. Heterocycles 1987, 26, 447–456. [Google Scholar] [CrossRef]
- Sobarzo-Sanchez, E.; Cassels, B.K.; Saitz-Barria, C.; Jullian, C. Oxazine- and oxazole-fused derivatives of the alkaloid boldine and their complete structural and spectral assignments by HMQC and HMBC experiments. Magn. Reson. Chem. 2001, 39, 361–368. [Google Scholar] [CrossRef]
- Braz-F, R.; Gabriel, S.J.; Gomes, C.M.R.; Gottlieb, O.R.; Bichara, M.D.G.A.; Maia, J.G.S. Oxoaporphine alkaloids from Fusea longifolia and Siparuna guianensis. Phytochemistry 1976, 15, 1187–1188. [Google Scholar] [CrossRef]
- Guinaudeau, H.; Leboeuf, M.; Cavé, A. Aporphine Alkaloids. II. J. Nat. Prod. 1979, 42, 325–360. [Google Scholar] [CrossRef]
- Guinaudeau, H.; Leboeuf, M.; Cavé, A. Aporphinoid Alkaloids, III. J. Nat. Prod. 1983, 46, 761–835. [Google Scholar]
- Guinaudeau, H.; Leboeuf, M.; Cavé, A. Aporphinoid alkaloids. J. Nat. Prod. 1994, 57, 1033–1135. [Google Scholar] [CrossRef]
- Castedo, L.; Tojo, G. Phenantrene Alkaloids. In The Alkaloid; Brossi, A., Ed.; Academic: New York, NY, USA, 1990; Volume 39, pp. 99–138. [Google Scholar]
- Chen, Z.-L.; Zhu, D.-Y. Aristolochia Alkaloids. In The Alkaloids: Chemistry and Pharmacology; Brossi, A., Ed.; New York, NY, USA, 1987; Volume 31, pp. 29–65. [Google Scholar]
- Kumar, V.; Poonam, P.A.K.; Parmar, V.S. Naturally occurring aristolactams, aristolochic acids and dioxoaporphines and their biological activities. Nat. Prod. Rep. 2003, 20, 565–583. [Google Scholar] [CrossRef]
- Bentley, K.W. β-Phenylethylamines and the isoquinoline alkaloids. Nat. Prod. Rep. 2006, 23, 444–463. [Google Scholar] [CrossRef]
- Zhang, Y.-N.; Zhong, X.-G.; Zheng, Z.-P.; Hu, X.-D.; Zuo, J.-P.; Hu, L.-H. Discovery and synthesis of new immunosuppressive alkaloids from the stem of Fissistigma oldhamii (Hemsl.) Merr. Bioorg. Med. Chem. 2007, 15, 988–996. [Google Scholar] [CrossRef]
- Choi, Y.L.; Kim, J.K.; Choi, S.-U.; Min, Y.-K.; Bae, M.-A.; Kim, B.T.; Heo, J.-N. Synthesis of aristolactam analogues and evaluation of their antitumor activity. Bioorg. Med. Chem. Lett. 2009, 19, 3036–3040. [Google Scholar] [CrossRef]
- Hedge, V.R.; Borges, S.; Pu, H.; Patel, M.; Gullo, V.P.; Wu, B.; Kirchmeier, P.; William, M.J.; Madison, V.; Fischmann, T.; Chan, T.-Z. Semi-synthetic aristolactams—inhibitors of CDK2 enzyme. Bioorg. Med. Chem. Lett. 2010, 20, 1384–1387. [Google Scholar] [CrossRef]
- Li, L.; Wang, X.; Chen, J.; Ding, H.; Zhang, Y.; Hu, T.-C.; Hu, L.-H.; Jiang, H.-L.; Shen, X. The natural product Aristolactam AIIIa as a new ligand targeting the polo-box domain of polo-like kinase 1 potently inhibits cancer cell proliferation. Acta Pharm. Sin. 2009, 30, 1443–1453. [Google Scholar] [CrossRef]
- Swaffar, D.S.; Holley, C.J.; Fitch, R.W.; Elkin, K.R.; Zhang, C.; Sturgill, J.P.; Menachery, M.D. Phytochemical investigation and in vitro cytotoxic evaluation of alkaloids from Abuta rufescens. Planta Med. 2012, 78, 230–232. [Google Scholar] [CrossRef]
- Costa, E.V.; Pinheiro, M.L.B.; Barison, A.; Maia, B.H.L.N.S.; Campos, F.R.; Salvador, M.J.; Cabral, E.C.; Eberlin, M.N. Alkaloids from the bark of Guatteria hispida and their evaluation as antioxidant and antimicrobial agents. J. Nat. Prod. 2010, 73, 1180–1183. [Google Scholar] [CrossRef]
- Chang, H.-C.; Chang, F.-R.; Wu, Y.-C.; Lai, Y.-H. Anti-cancer effect of liriodenine on human lung cancer cells. Kaohsiung J. Med. Sci. 2004, 20, 365–371. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Chen, S.-Y.; Chen, C.-H. Liriodenine induces G1/S cell cycle arrest in human colon cancer cells via nitric oxide- and p53-mediated pathway. Process Biochem. 2012, 47, 1460–1468. [Google Scholar] [CrossRef]
- Kim, K.H.; Moon, E.; Choi, S.U.; Kim, S.Y.; Lee, K.R. Biological evaluation of phenolic constituents from the trunk of Berberis koreana. Bioorg. Med. Chem. Lett. 2011, 21, 2270–2273. [Google Scholar] [CrossRef]
- Li, F.; Awale, S.; Tezuka, Y.; Kadota, S. Cytotoxic constituents from Brazilian red propolis and their structure-activity relationship. Bioorg. Med. Chem. 2008, 16, 5434–5440. [Google Scholar] [CrossRef]
- Beauchard, A.; Ferandin, Y.; Frère, S.; Lozach, O.; Blairvacq, M.; Meijer, L.; Thiéry, V.; Besson, T. Synthesis of novel 5-substituted indirubins as potential inhibitor of protein kinases. Bioorg. Med. Chem. 2006, 14, 6434–6443. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 1–15 are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Marti, G.; Eparvier, V.; Morleo, B.; Ven, J.L.; Apel, C.; Bodo, B.; Amand, S.; Dumontet, V.; Lozach, O.; Meijer, L.; et al. Natural Aristolactams and Aporphine Alkaloids as Inhibitors of CDK1/Cyclin B and DYRK1A. Molecules 2013, 18, 3018-3027. https://doi.org/10.3390/molecules18033018
Marti G, Eparvier V, Morleo B, Ven JL, Apel C, Bodo B, Amand S, Dumontet V, Lozach O, Meijer L, et al. Natural Aristolactams and Aporphine Alkaloids as Inhibitors of CDK1/Cyclin B and DYRK1A. Molecules. 2013; 18(3):3018-3027. https://doi.org/10.3390/molecules18033018
Chicago/Turabian StyleMarti, Guillaume, Véronique Eparvier, Barbara Morleo, Jessica Le Ven, Cécile Apel, Bernard Bodo, Séverine Amand, Vincent Dumontet, Olivier Lozach, Laurent Meijer, and et al. 2013. "Natural Aristolactams and Aporphine Alkaloids as Inhibitors of CDK1/Cyclin B and DYRK1A" Molecules 18, no. 3: 3018-3027. https://doi.org/10.3390/molecules18033018