Antibacterial Activity Composition of the Fermentation Broth of Streptomyces djakartensis NW35
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
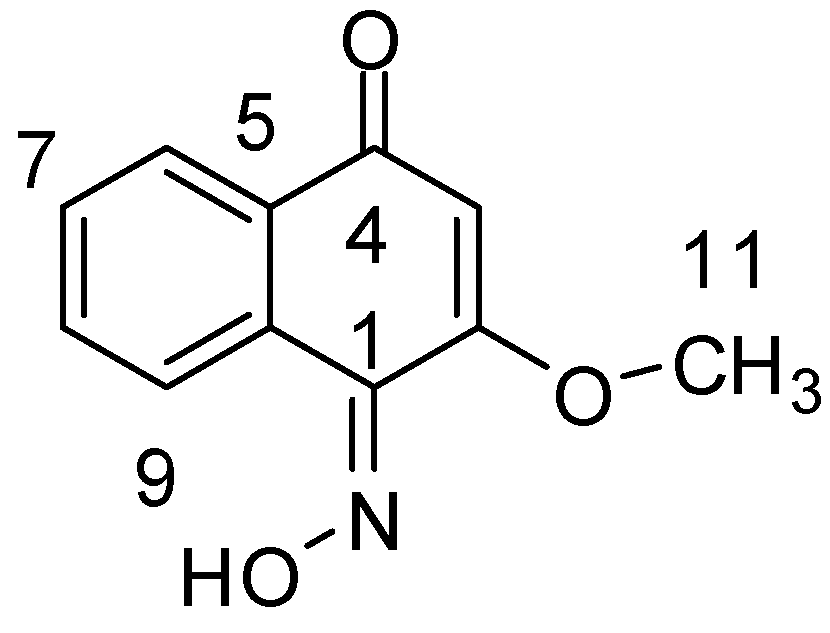
| Position | 1H-NMR | 13C-NMR | HMBC |
|---|---|---|---|
| 1 | 164.8 | ||
| 2 | 160.8 | ||
| 3 | 6.16 (1H, s) | 105.2 | C-1 |
| 4 | 182.6 | ||
| 5 | 124.9 | ||
| 6 | 8.10 (1H, d, J = 8.5 Hz) | 131.4 | C-4 |
| 7 | 7.24 (1H, t, J = 8.5 Hz) | 124.2 | |
| 8 | 7.59 (1H, t, J = 8.5 Hz) | 134.9 | |
| 9 | 7.20 (1H, d, J = 8.5 Hz,) | 119.9 | |
| 10 | 136.9 | ||
| 11 | 3.89 (3H, s) | 56.6 | C-2 |

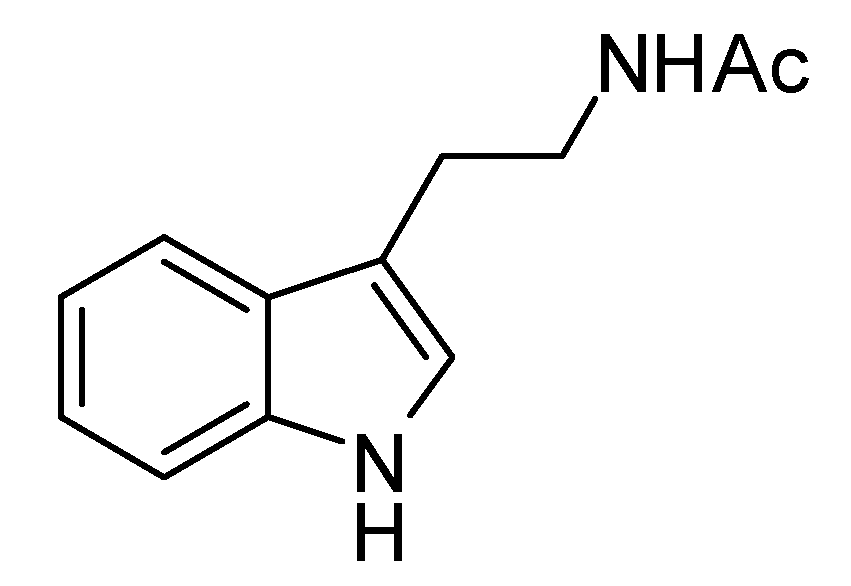
2.2. Bioactivity
| Test bacteria | Diameter of inhibition zone (mm) in 10 μL/disk (Mean ± S.D.) | ||
|---|---|---|---|
| Z-4-2 | Z-9-2 | Ampicillin | |
| Bacillus cereus | 11 ± 0.3 (+++) | 12 ± 0.2 (+++) | 14 ± 0.4 (+++) |
| Bacillus subtilis | 13 ± 0.2 (+++) | 15 ± 0.4 (+++) | 16 ± 0.3 (+++) |
| Staphylococcus aureus | 8 ± 0.3 (+++) | 9 ± 0.5 (+++) | 15 ± 0.1 (+++) |
| Escherichia coil | 9 ± 0.8 (+) | 10 ± 0.3 (+) | 13 ± 0.2 (+) |
| Pseudomonas aeruginosa | - | - | 7 ± 0.1 (+) |
| Pseuomonas syringae pv. Actinidiae | 15 ± 0.1 (++) | 17 ± 0.3 (++) | 11 ± 0.6 (+++) |
| Erwinia carotovora | - | - | - |
| MRSA | 13 ± 0.2 (++) | 12 ± 0.2 (++) | 17 ± 0.4 (+++) |
3. Experimental
3.1. General
3.2. Microorganism and Fermentation
3.3. Extraction and Isolation
3.4. Antibacterial Activity
4. Conclusions
Acknowledgments
References
- Lange, L.; Lopez, C.S. Microorganisms as a Source of Biologically Active Secondary Metabolites. In Corp Protection Agents from Nature: Natural Products, Analogues; Copping, L.G., Ed.; The Royal Society of Chemistry: Cambridge, UK, 1996. [Google Scholar]
- Watve, M.G.; Tickoo, R.; Jog, M.M.; Bhole, B.D. How many antibiotics are produced by the genus Streptomyces? Arch. Microbiol. 2001, 176, 386–390. [Google Scholar] [CrossRef]
- Spellberg, B.; Guidos, R.; Gilbert, D.; Bradley, J.; Boucher, H.W.; Scheld, W.M.; Bartlett, J.G.; Edwards, J. Infectious Diseases Society of America: The epidemic of antibiotic-resistant infections: A call to action for the medical community from the Infectious Diseases Society of America. Clin. Infect. Dis. 2008, 46, 155–164. [Google Scholar] [CrossRef]
- Donadio, S.; Maffioli, S.; Monciardini, P.; Sosio, M.; Jabes, D. Antibiotic discovery in the 21st century: current trends and future perspectives. J. Antibiot. 2010, 63, 423–430. [Google Scholar] [CrossRef]
- Elliot Juhnke, M.; Mathre, D.E.; Sands, D.C. Identification and characterization of rhizosphere-competent bacteria of wheat. Appl. Environ. Microbol. 1987, 53, 2793–2799. [Google Scholar]
- Nie, Y.L.; Wang, C.X.; Zheng, Y.B.; Jang, H. Novel Secondary Metabolites with Cytotoxic Activities Produced by Verrucosispora sp. FIM06031 from Marine Sponge. Nat. Prod. Res. Dev. 2011, 23, 797–800. [Google Scholar]
- National Committee for Clinical Laboratory Standards, Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Document M7-A6; NCCLS: Wayne, PA, USA, 2003.
- Sample Availability: Samples of Streptomyces djakartensis NW35, N-acetyltryptamine and (E)-2-methoxy-1,4 naphthoquinone-1-oxime are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhang, W.; Wei, S.; Zhang, J.; Wu, W. Antibacterial Activity Composition of the Fermentation Broth of Streptomyces djakartensis NW35. Molecules 2013, 18, 2763-2768. https://doi.org/10.3390/molecules18032763
Zhang W, Wei S, Zhang J, Wu W. Antibacterial Activity Composition of the Fermentation Broth of Streptomyces djakartensis NW35. Molecules. 2013; 18(3):2763-2768. https://doi.org/10.3390/molecules18032763
Chicago/Turabian StyleZhang, Wenjuan, Shaopeng Wei, Jiwen Zhang, and Wenjun Wu. 2013. "Antibacterial Activity Composition of the Fermentation Broth of Streptomyces djakartensis NW35" Molecules 18, no. 3: 2763-2768. https://doi.org/10.3390/molecules18032763




