Crystal Structure, Antibacterial and Cytotoxic Activities of a New Complex of Bismuth(III) with Sulfapyridine
Abstract
:1. Introduction
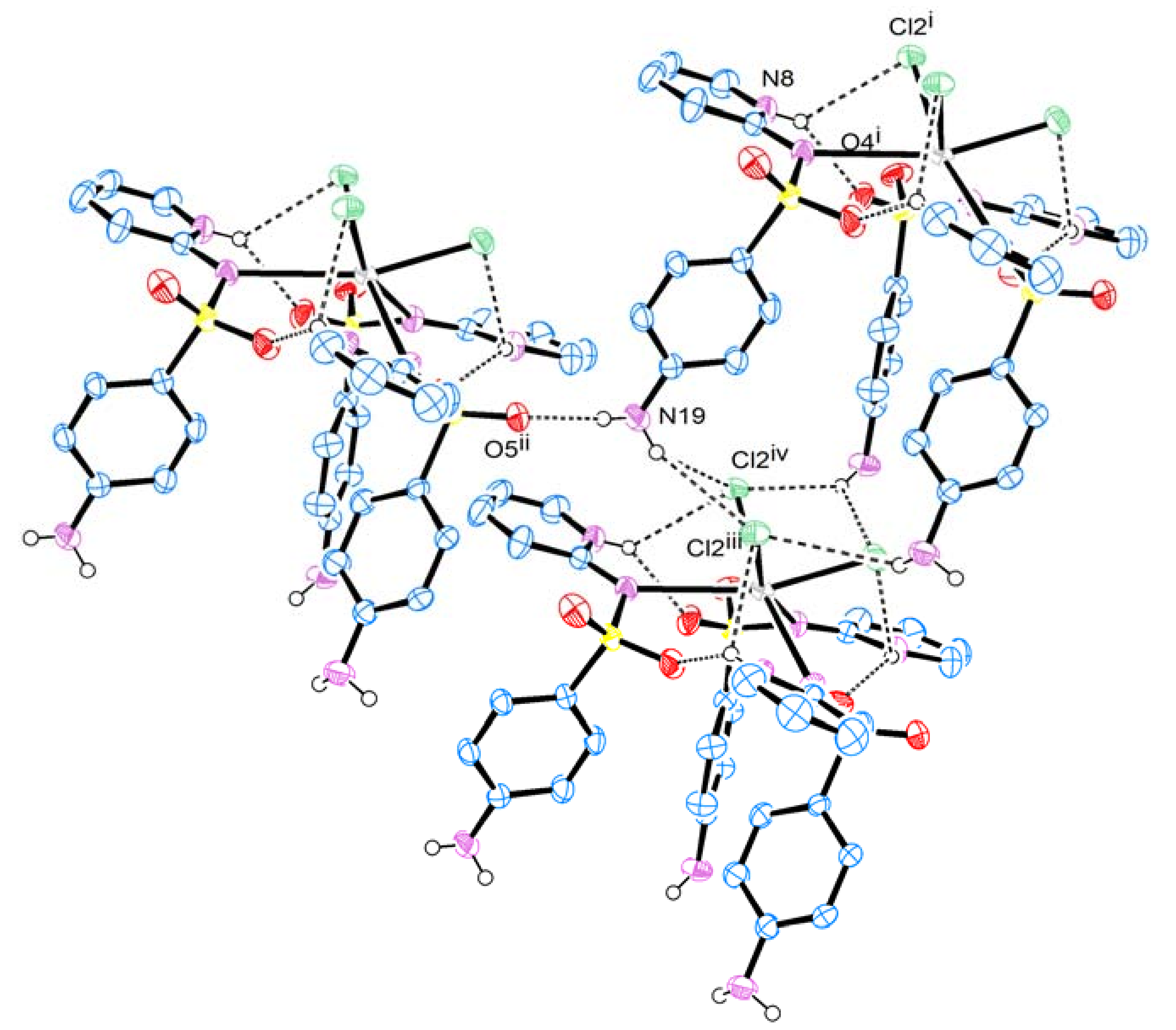
2. Results and Discussion
3. Experimental
3.1. General and Instruments
3.2. Synthesis
3.3. Microbial Strains and Growth Conditions
3.4. Determination of Minimal Inhibitory Concentration
3.5. Cell Line and Culture
3.6. Cytotoxicity Assays
4. Conclusions
Supplementary Materials
Acknowledgments
References
- Payne, D.J. Desperately Seeking New Antibiotics. Science 2008, 321, 1644–1645. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.; Davies, D. Origins and evolution of antibiotic resistance. Microbiol. Mol. Biol. Rev. 2010, 74, 417–433. [Google Scholar] [CrossRef] [PubMed]
- Silver, L.L. Challenges of antibacterial discovery. Clin. Microbiol. Rev. 2011, 24, 71–109. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.M. The biosynthesis of folic acid. II. Inhibition by sulfonamides. J. Biol. Chem. 1962, 237, 536–540. [Google Scholar] [PubMed]
- Huovinen, P.; Sundström, L.; Swedberg, G.; Sköld, O. Trimethoprim and sulfonamide resistance. Antimicrob. Agents Chemother. 1995, 39, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Sun, H. Biocoordination chemistry of bismuth: Recent advances. Coord. Chem. Rev. 2007, 251, 2354–2366. [Google Scholar] [CrossRef]
- Ford, A.C.; Malfertheiner, P.; Giguere, M.; Santana, J.; Khan, M.; Moayyedi, P. Adverse events with bismuth salts for Helicobacter pylori eradication: Systematic review and meta-analysis. World J. Gastroenterol. 2008, 14, 7361–7370. [Google Scholar] [CrossRef] [PubMed]
- Dore, M.P.; Farina, V.; Cuccu, M.; Mameli, L.; Massarelli, G.; Graham, D.Y. Twice-a-Day Bismuth-Containing Quadruple Therapy for Helicobacter Pylori Eradication: A Randomized Trial of 10 and 14 Days. Helicobacter 2011, 16, 295–300. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, A.; Gisbert, J.P.; McNamara, D.; O’Morain, C. Treatment of Helicobacter pylori Infection 2010. Helicobacter 2010, 15, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Rimbara, E.; Fischbach, L.A.; Graham, D.Y. Optimal therapy for Helicobacter pylori infections. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Liang, X.; Zheng, Q.; Liu, W.; Xiao, S.; Gu, W.; Lu, H. High Efficacy of 14-Day Triple Therapy-Based, Bismuth-Containing Quadruple Therapy for Initial Helicobacter pylori Eradication. Helicobacter 2010, 15, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Thurston, J.H.; Marlier, E.M.; Whitmire, K.H. Towards a molecular model for bismuth(III) subsalicylate. Synthesis and solid-state structure of [Bi(Hsal)3(bipy)(C7H8]2 and [Bi(Hsal)(sal)(1,10-phenanthroline)(C7H8]2. Chem. Commun. 2002, 2834–2835. [Google Scholar] [CrossRef]
- Andrews, P.C.; Ferrero, R.L.; Junk, P.C.; Kumar, I.; Luu, Q.; Nguyen, K.; Taylor, J.W. Bismuth(III) complexes derived from non-steroidal anti-inflammatory drugs and their activity against Helicobacter pylori. Dalton Trans. 2010, 39, 2861–2868. [Google Scholar] [CrossRef] [PubMed]
- Turel, I.; Goli, L.; Bukovec, P.; Gubina, M. Antibacterial tests of bismuth(III)-quinolone (ciprofloxacin, cf) compounds against Helicobacter pylori and some other bacteria. Crystal structure of (cfH2)2[Bi2Cl10]4H2O. J. Inorg. Biochem. 1998, 71, 53–60. [Google Scholar] [CrossRef]
- Turel, I.; Leban, I.; Bukovec, N. Crystal structure and characterization of the bismuth(III) compound with quinolone family member (Ciprofloxacin). Antibacterial study. J. Inorg. Biochem. 1997, 66, 241–245. [Google Scholar] [CrossRef]
- Diemer, R.; Dittes, U.; Nuber, B.; Seifried, V.; Opferkuch, W.; Keppler, B.K. Synthesis, characterization and molecular structures of some bismuth(III) complexes with thiosemicarbazones and dithiocarbazonic acid methylester derivatives with activity against Helicobacter Pylori. Met. Based Drugs 1995, 2, 271–292. [Google Scholar] [CrossRef] [PubMed]
- Nomiya, K.; Sekino, K.; Ishikawa, M.; Honda, A.; Yokoyama, M.; Kasuga, N.C.; Yokoyama, H.; Nakano, S.; Onodera, K. Syntheses, crystal structures and antimicrobial activities of monomeric 8-coordinate, and dimeric and monomeric 7-coordinate bismuth(III) complexes with tridentate and pentadentate thiosemicarbazones and pentadentate semicarbazone ligands. J. Inorg. Biochem. 2004, 98, 601–615. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Lu, Y.; Yang, M.; Li, Y.; Zhang, L.; Xie, S. One dodecahedral bismuth(III) complex derived from 2-acetylpyridine N(4)-pyridylthiosemicarbazone: Synthesis, crystal structure and biological evaluation. Dalton Trans. 2012, 41, 12882–12887. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yang, M.; Niu, J.; Zhang, L.; Xie, S. A nine-coordinated bismuth(III) complex derived from pentadentate 2,6-diacetylpyridine bis(4N-methylthiosemicarbazone): Crystal structure and both in vitro and in vivo biological evaluation. Inorg. Chem. 2012, 51, 12521–12526. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, L.; Yang, M.; Niu, J.; Zhou, J. Synthesis, crystal structures, in vitro biological evaluation of zinc(II) and bismuth(III) complexes of 2-acetylpyrazine N(4)-phenylthiosemicarbazone. Bioorg. Med. Chem. Lett. 2012, 22, 2418–2423. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; An, G.; Yang, M.; Li, M.; Zhu, X. Synthesis, characterization, crystal structure and biological activities of the unusual main group 8-coordinate bismuth (III) complex derived from 2-acetylpyrazine N4- pyridylthiosemicarbazone. Inorg. Chem. Commun. 2012, 20, 37–40. [Google Scholar] [CrossRef]
- Domenico, P.; Salo, R.J.; Novick, S.G.; Schoch, P.E.; van Horn, K.; Cunha, B.A. Enhancement of bismuth antibacterial activity with lipophilic thiol chelators. Antimicrob. Agents Chemother. 1997, 41, 1697–1703. [Google Scholar] [PubMed]
- Andrews, P.C.; Busse, M.; Deacon, G.B.; Ferrero, R.L.; Junk, P.C.; MacLellan, J.G.; Vom, A. Remarkable in vitro bactericidal activity of bismuth(III) sulfonates against Helicobacter pylori. Dalton Trans. 2012, 41, 11798–11806. [Google Scholar] [CrossRef] [PubMed]
- Ge, R.; Sun, X.; Gu, Q.; Watt, R.M.; Tanner, J.A.; Wong, B.C.; Xia, H.H.; Huang, J.D.; He, Q.Y.; Sun, H. A proteomic approach for the identification of bismuth-binding proteins in Helicobacter pylori. J. Biol. Inorg. Chem. 2007, 12, 831–842. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhou, Q.; Ge, R. Inhibition of fumarase by bismuth(III): Implications for the tricarboxylic acid cycle as a potential target of bismuth drugs in Helicobacter pylori. Biometals 2012, 25, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Guerra, W.; Silva, I.R.; Azevedo, E.A.; Monteiro, A.R.S.; Bucciarelli-Rodriguez, M.; Chartone-Souza, E.; Silveira, J.N.; Fontes, A.P.S.; Pereira-Maia, E.C. Three New Complexes of Platinum(II) with Doxycycline, Oxytetracycline and Chlortetracycline and their Antimicrobial Activity. J. Braz. Chem. Soc. 2006, 17, 1627–1633. [Google Scholar] [CrossRef]
- Guerra, W.; Azevedo, E.A.; Monteiro, A.R.S.; Bucciarelli-Rodriguez, M.; Chartone-Souza, E.; Nascimento, A.M.A.; Fontes, A.P.S.; le Moyec, L.; Pereira-Maia, E.C. Synthesis, characterization, and antibacterial activity of three palladium(II) complexes of tetracyclines. J. Inorg. Biochem. 2005, 99, 2348–2354. [Google Scholar] [CrossRef] [PubMed]
- Chartone-Souza, E.; Loyola, T.L.; Bucciarelli-Rodriguez, M.; Menezes, M.A.; Rey, N.A.; Pereira-Maia, E.C. Synthesis and characterization of a tetracycline-platinum (II) complex active against resistant bacteria. J. Inorg. Biochem. 2005, 95, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Breunig, H.J; Nema, M.G.; Silvestru, C.; Richard, A.S.; Varga, A. [2-{E(CH2CH2)2NCH2}C6H4]nBiX3–n (E = O, NMe; X = Cl, Br, I; n = 1–3) and [2-(Me2NCH2)C6H4]BiBr2—New Hypervalent Organobismuth(III) Compounds. Z. Anorg. Allg. Chem. 2010, 636, 2378–2386. [Google Scholar] [CrossRef]
- Ferrari, M.B.; Cappacchi, L.C.; Calvaca, L.; Gasparri, G.F. The Crystal and Molecular Structure of Trichlorotris-(3-sulphanilamido-6-methoxypyridazine)bismuth(III). Acta Crystallogr. B 1972, 28, 1169–1173. [Google Scholar] [CrossRef]
- Soran, A.; Breunig, H.J.; Lippolis, V.; Arca, M.; Silvestru, C. Syntheses, solid-state structures, solution behavior of hypervalent organobismuth(III) compounds [2-(Et2NCH2)C6H4]nBiX3-n and DFT characterization of [2-(Me2NCH2)C6H4]nBiX3-n [X = Cl, Br, I; n = 1–3]. J. Organomet. Chem. 2010, 695, 850–862. [Google Scholar] [CrossRef]
- Geary, W.J. The use of conductivity measurements in organic solvents for the characterisation of coordination compounds. Coord. Chem. Rev. 1971, 7, 81–122. [Google Scholar] [CrossRef]
- Cook, D. Vibrational spectra of pyridinium salts. Can. J. Chem. 1961, 39, 2009–2024. [Google Scholar] [CrossRef]
- Kremer, E.; Facchin, G.; Estévez, E.; Alborés, P.; Baran, E.J.; Ellena, J.; Torre, M.H. Copper complexes with heterocyclic sulfonamides: Synthesis, spectroscopic characterization, microbiological and SOD-like activities: Crystal structure of [Cu(sulfisoxazole)2(H2O)4]·2H2O. J. Inorg. Biochem. 2006, 100, 1167–1175. [Google Scholar] [CrossRef] [PubMed]
- Mondelli, M.; Bruné, V.; Borthagaray, G.; Ellena, J.; Nascimento, O.R.; Leite, C.Q.; Batista, A.A.; Torre, M.H. New Ni(II)-sulfonamide complexes: Synthesis, structural characterization and antibacterial properties. X-ray diffraction of [Ni(sulfisoxazole)2(H2O)4]·2H2O and [Ni(sulfapyridine)2]. J. Inorg. Biochem. 2008, 102, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Macías, B.; García, I.; Villa, M.V.; Borrás, J.; Castiñeiras, A.; Sanz, F. Synthesis and characterization of sulfonamides containing 8-aminoquinoline and their Ni(II) complexes. Crystalline structures of the Ni complexes. Polyhedron 2002, 21, 1229–1234. [Google Scholar] [CrossRef]
- Tiekink, E.R.T. Antimony and bismuth compounds in oncology. Crit. Rev. Oncol. Hematol. 2002, 42, 217–224. [Google Scholar] [CrossRef]
- Li, H.; Lai, C.S.; Wu, J.; Ho, P.C.; Vos, D.; Tiekink, E.R.T. Cytotoxicity, qualitative structure-activity relationship (QSAR), and anti-tumor activity of bismuth dithiocarbamate complexes. J. Inorg. Biochem. 2007, 101, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Wallqvist, A.; Covell, D.G. Anticancer metal compounds in NCI’s tumor-screening database: Putative mode of action. Biochem. Pharmacol. 2005, 69, 1009–1039. [Google Scholar] [CrossRef] [PubMed]
- CRYSALISPRO, Version 1.171.33.55 (release 05-01-2010 CrysAlis 171.NET). Oxford Diffraction Ltd.: Oxfordshire, UK, 2010.
- Altomare, A.; Burla, M.C.; Camalli, M.; Cascarano, G.L.; Giacovazzo, C.; Guagliardi, A.; Moliterni, A.G.G.; Polidori, G.; Spagna, R. SIR97: A new tool for crystal structure determination and refinement. J. Appl. Crystallogr. 1999, 32, 115–119. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX suite for small-molecule single-crystal crystallography. J. Appl. Crystallogr. 1999, 32, 837–838. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXL-97—A Program for Crystal Structure Refinement; University of Goettingen: Göttingen, Germany, 1997. [Google Scholar]
- Flack, H.D. On enantiomorph-polarity estimation. Acta Crystallogr. 1983, A39, 876–881. [Google Scholar] [CrossRef]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; van de Streek, J. Mercury: Visualization and analysis of crystal structures. J. Appl. Crystallogr. 2006, 39, 453–457. [Google Scholar] [CrossRef]
- Weber, S. XRDIFF: Simulation of X-ray diffraction patterns. J. Appl. Crystallogr. 1997, 30, 565–566. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compound [BiCl3(C11H11N3O2S)3] are available from the authors. |
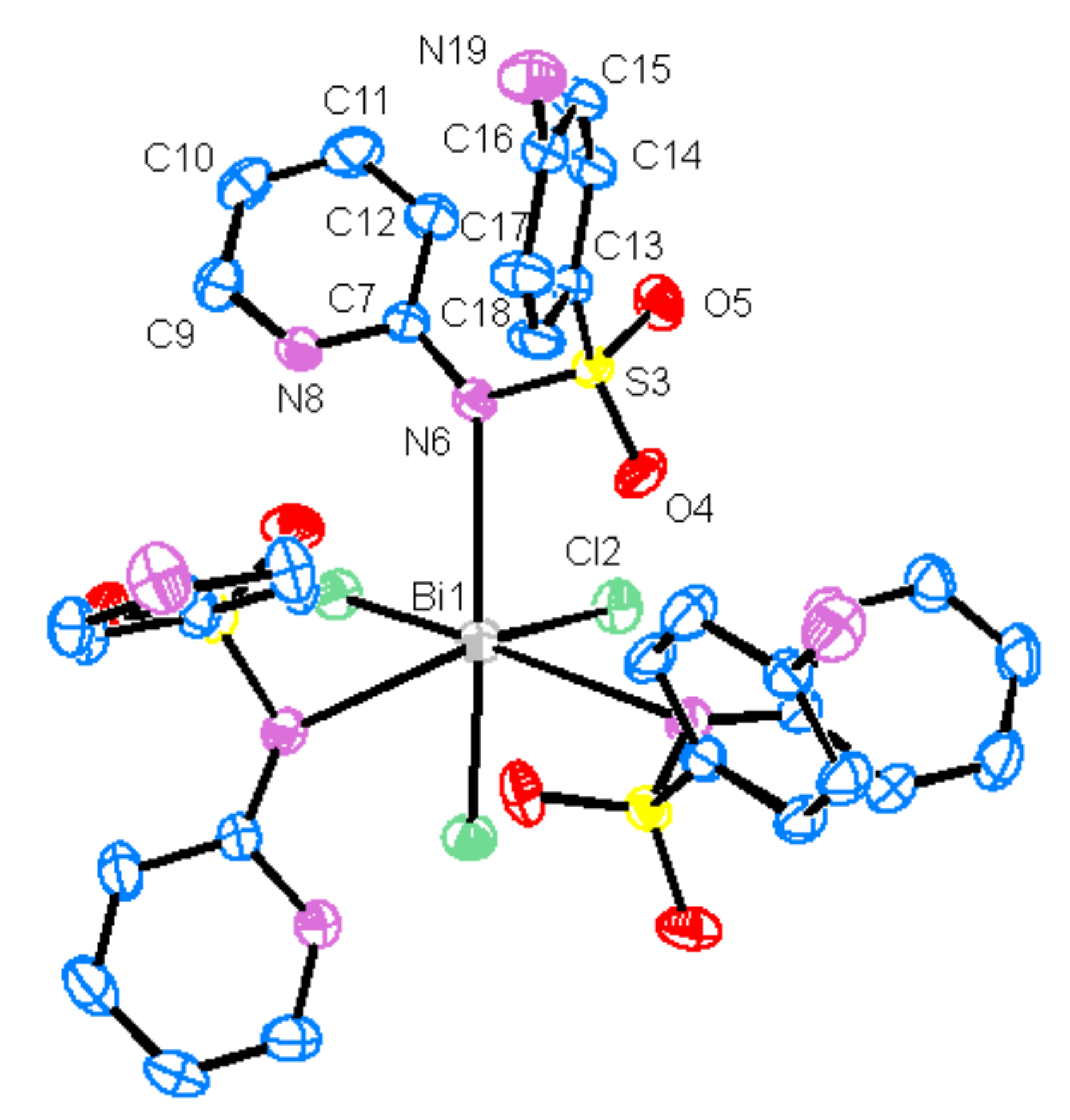

| Empirical formula | C33H33BiCl3N9O6S3 |
|---|---|
| Formula weight | 1063.22 |
| Temperature | 293(2) K |
| Wavelength | 0.71073 Å |
| Crystal system | Trigonal |
| Space group | R 3 |
| Unit cell dimensions | a = 17.9557(3), b = 17.9557(3), c = 10.19691 (12) Å, α = 90, β = 90, γ = 120° |
| Volume | 2847.12(7) Å3 |
| Z | 3 |
| Density (calculated) | 1.860 Mg/m3 |
| Absorption coefficient | 5.08 mm−1 |
| F(000) | 1572 |
| Crystal size | 0.23 × 0.25 × 0.29 mm3 |
| Theta range for data collection | 4.00 to 37.9° |
| Index ranges | −27 ≤ h ≤ 30, −29 ≤ k ≤ 15,−9 ≤ l ≤ 17 |
| Reflections collected | 6528 |
| Independent reflections | 4005(Rint = 0.024) |
| Absorption correction | Empirical |
| Refinement method | Full-matrix least-squares on F2 |
| Data/restraints/parameters | 4005/4/172 |
| Goodness-of-fitonF2 | 1.00 |
| Final R indices (for 3857 reflections with I > 2σ(I)) | R1 = 0.023, wR2 = 0.055 |
| R indices (all data) | R1 = 0.024, wR2 = 0.057 |
| Largest diff. peak and hole | 1.37 and −0.55 eÅ−3 |
| Absolute structure [5] | Flack parameter = 0.003(3) |
| Bi1—Cl2 | 2.5442 (7) | Bi1—N6 | 2.924 (2) | ||
| C7—N6 | 1.349 (4) | C7—N8 | 1.359 (4) | ||
| C9—N8 | 1.344 (4) | C16—N19 | 1.383 (4) | ||
| D—H···A | D—H | H···A | D···A | D—H···A | |
| N8—H8···O4 i | 0.86 | 2.13 | 2.858 (3) | 142 | |
| N8—H8···Cl2 i | 0.86 | 2.81 | 3.340 (3) | 122 | |
| N19—H19A···O5 ii | 0.85 (1) | 2.14 (1) | 2.995 (4) | 177 (5) | |
| N19—H19B···Cl2 iii | 0.86 (1) | 2.83 (4) | 3.568 (3) | 146 (5) | |
| N19—H19B···Cl2 iv | 0.85(1) | 3.12 (4) | 3.702 (3) | 127 (4) | |
| Bacterial strains | MIC a/µM | ||
|---|---|---|---|
| sp | [Bi(sp)3Cl3] | ||
| E. coli | ATCC 25922 | >1027 | 241 |
| S. aureus | ATCC 25923 | 385 | 90 |
| P. aeruginosa | ATCC 24853 | 1027 | 120 |
| S. typhimur ium | ATCC 13311 | 96 | 30 |
| S. sonnei | ATCC 11060 | 1027 | 241 |
| S. dysenteriae | ATCC 13313 | 128 | 30 |
© 2013 by the authors. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marzano, I.M.; Franco, M.S.; Silva, P.P.; Augusti, R.; Santos, G.C.; Fernandes, N.G.; Bucciarelli-Rodriguez, M.; Chartone-Souza, E.; Pereira-Maia, E.C. Crystal Structure, Antibacterial and Cytotoxic Activities of a New Complex of Bismuth(III) with Sulfapyridine. Molecules 2013, 18, 1464-1476. https://doi.org/10.3390/molecules18021464
Marzano IM, Franco MS, Silva PP, Augusti R, Santos GC, Fernandes NG, Bucciarelli-Rodriguez M, Chartone-Souza E, Pereira-Maia EC. Crystal Structure, Antibacterial and Cytotoxic Activities of a New Complex of Bismuth(III) with Sulfapyridine. Molecules. 2013; 18(2):1464-1476. https://doi.org/10.3390/molecules18021464
Chicago/Turabian StyleMarzano, Ivana M., Marina S. Franco, Priscila P. Silva, Rodinei Augusti, Geandson C. Santos, Nelson G. Fernandes, Mônica Bucciarelli-Rodriguez, Edmar Chartone-Souza, and Elene C. Pereira-Maia. 2013. "Crystal Structure, Antibacterial and Cytotoxic Activities of a New Complex of Bismuth(III) with Sulfapyridine" Molecules 18, no. 2: 1464-1476. https://doi.org/10.3390/molecules18021464






