Highly Enantioselective Addition of Phenylethynylzinc to Aldehydes Catalyzed by Chiral Cyclopropane-Based Amino Alcohols
Abstract
:1. Introduction
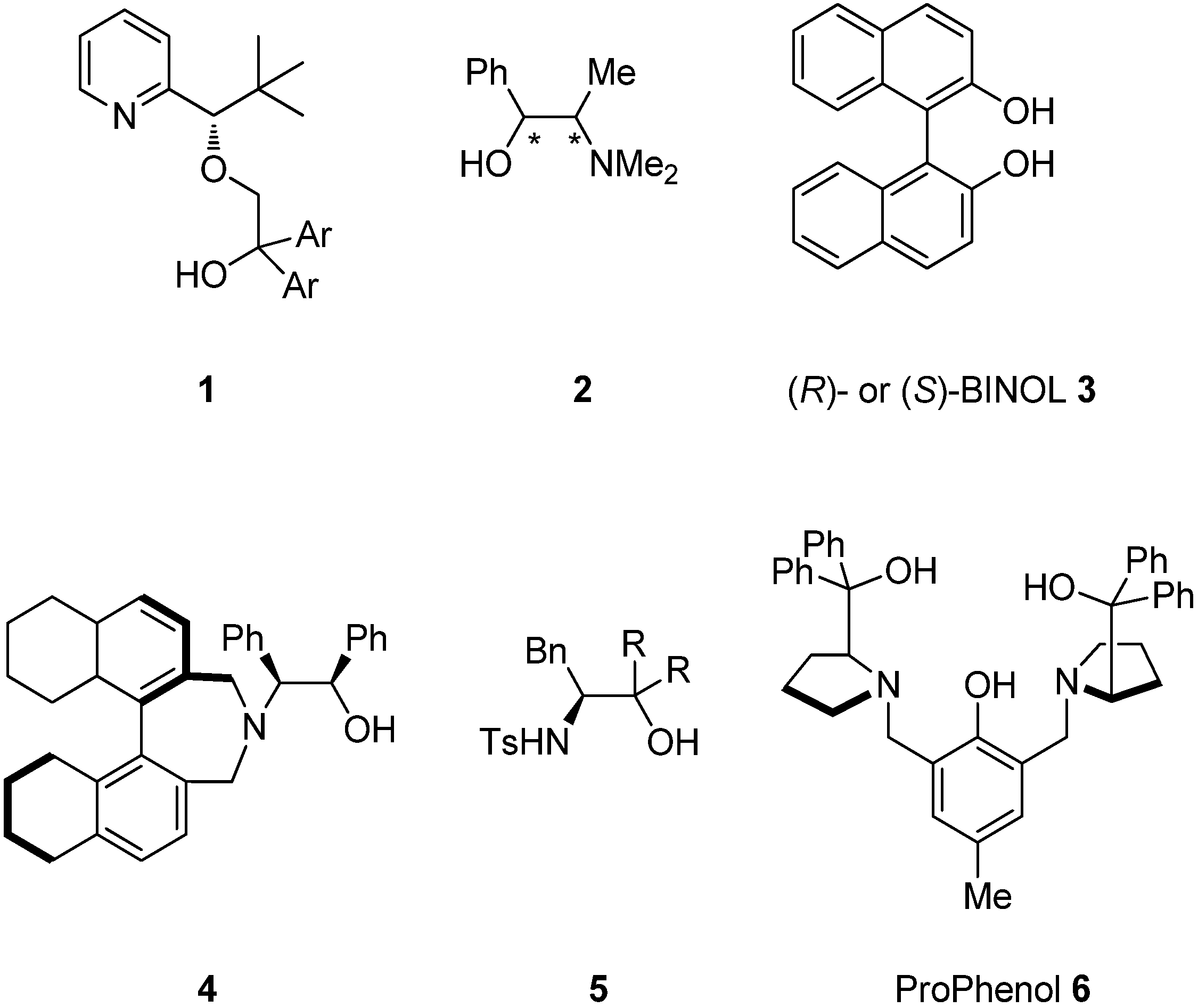

2. Results and Discussion

| Entry | Ligand | Time (h) | Yield (%) b | Ee (%) c | Config. d |
|---|---|---|---|---|---|
| 1 | 7a | 20 | 89 | 10 | S |
| 2 | 7b | 20 | 90 | 16 | S |
| 3 | 7c | 20 | 94 | 80 | S |
| 4 | 7d | 20 | 90 | 22 | S |
| 5 | 7e | 20 | 91 | 79 | S |
| Entry | Ligand (mol %) | Solvent | Time (h) | Temp (°C) | Yield (%) b | Ee (%) c |
|---|---|---|---|---|---|---|
| 1 | 10 | Toluene | 20 | 25 | 94 | 80 |
| 2 | 10 | Toluene | 48 | 0 | 91 | 93 |
| 3 | 10 | Toluene | 48 | −10 | 83 | 86 |
| 4 | 10 | Toluene | 48 | −20 | 40 | 79 |
| 5d | 10 | Toluene | 48 | 0 | 95 | 90 |
| 6 | 20 | Toluene | 48 | 0 | 97 | 94 |
| 7 | 5 | Toluene | 48 | 0 | 75 | 85 |
| 8 | 10 | Heptane | 48 | 0 | 80 | 83 |
| Entry | R | Product | Yield (%) b | Ee (%) c |
|---|---|---|---|---|
| 1 | Ph | 9a | 91 | 93 |
| 2 | p-FC6H4 | 9b | 90 | 94 |
| 3 | o-BrC6H4 | 9c | 96 | 94 |
| 4 | p-NO2C6H4 | 9d | 92 | 93 |
| 5 | o-CH3C6H4 | 9e | 91 | 98 |
| 6 | m-CH3C6H4 | 9f | 89 | 95 |
| 7 | p-CH3C6H4 | 9g | 90 | 95 |
| 8 | o-CH3OC6H4 | 9h | 81 | 96 |
| 9 | m-CH3OC6H4 | 9i | 80 | 94 |
| 10 | p-CH3OC6H4 | 9j | 85 | 97 |
| 11 | o-ClC6H4 | 9k | 95 | 90 |
| 12 | m-ClC6H4 | 9l | 93 | 93 |
| 13 | p-ClC6H4 | 9m | 92 | 93 |
| 14 | 1-Naphthyl | 9n | 91 | 98 |
| 15 | 2-Naphthyl | 9o | 80 | 92 |
| 16 | Cyclohexyl | 9p | 92 | 84 |
| 17 | Isopropyl | 9q | 91 | 88 |
3. Experimental
3.1. General Methods and Materials
3.2. X-Ray Crystallographic Data of the Ligand 7e
3.3. General Procedure for the Asymmetric Alkynylation of Aldehydes
4. Conclusions
Supplementary Materials
Acknowledgments
Conflicts of Interest
References
- Trost, B.M.; Quintard, A. Asymmetric catalytic alkynylation of acetaldehyde: Application to the synthesis of (+)-tetrahydropyrenophorol. Angew. Chem. Int. Ed. Engl. 2012, 51, 6704–6708. [Google Scholar] [CrossRef]
- Chinkov, N.; Warm, A.; Carreira, E.M. Asymmetric autocatalysis enables an improved synthesis of efavirenz. Angew. Chem. Int. Ed. 2011, 50, 2957–2961. [Google Scholar] [CrossRef]
- Tan, L.; Chen, C.; Tillyer, R.D.; Grabowski, E.J.J.; Reider, P.J. A novel highly enantioselective ketone alkynylation reaction mediated by chiral zinc aminoalkoxides. Angew. Chem. Int. Ed. 1999, 38, 711–713. [Google Scholar] [CrossRef]
- Reber, S.; Knöpfel, T.F.; Carreira, E.M. Enantioselective total synthesis of (R)-strongylodiols A and B. Tetrahedron 2003, 59, 6813–6817. [Google Scholar] [CrossRef]
- Escorihuela, J.; Altava, B. C2 symmetrical nickel complexes derived from α-amino amides as efficient catalysts for the enantioselective addition of dialkylzinc reagents to aldehydes. Tetrahedron 2013, 69, 551–558. [Google Scholar] [CrossRef]
- Rachwalski, M.; Jarzynski, S. Aziridine ring-containing chiral ligands as highly efficient catalysts in asymmetric synthesis. Tetrahedron 2013, 24, 421–425. [Google Scholar]
- Turlington, M.; Du, Y.; Ostrum, S.G.; Santosh, V.; Wren, K.; Lin, T.; Sabat, M.; Pu, L. From highly enantioselective catalytic reaction of 1,3-diynes with aldehydes to facile asymmetric synthesis of polycyclic compounds. J. Am. Chem. Soc. 2011, 133, 11780–11794. [Google Scholar]
- Ishizaki, M.; Hoshino, O. Efficient ligands, chiral 2-[2,2-dimethyl-1-(2-pyridyl)propoxy]-1, 1-diarylethanols for highly enantioselective addition of alkynylzinc reagents to various aldehydes. Tetrahedron 1994, 5, 1901–1904. [Google Scholar]
- Frantz, D.E.; Fässler, R.; Carreira, E.M. Facile enantioselective synthesis of propargylic alcohols by direct addition of terminal alkynes to aldehydes. J. Am. Chem. Soc. 2000, 122, 1806–1807. [Google Scholar] [CrossRef]
- Anand, N.K.; Carreira, E.M. A simple, mild, catalytic, enantioselective addition of terminal acetylenes to aldehydes. J. Am. Chem. Soc. 2001, 123, 9687–9688. [Google Scholar] [CrossRef]
- Sasaki, H.; Boyall, D.; Carreira, E.M. Facile, asymmetric addition of acetylene to aldehydes: In Situ generation of reactive zinc acetylide. Helv. Chim. Acta 2001, 84, 964–971. [Google Scholar] [CrossRef]
- Boyall, D.; Frantz, D.E.; Carreira, E.M. Efficient enantioselective additions of terminal alkynes and aldehydes under operationally convenient conditions. Org. Lett. 2002, 4, 2605–2606. [Google Scholar] [CrossRef]
- El-Sayed, E.; Anand, N.K.; Carreira, E.M. Asymmetric synthesis of γ-hydroxy α,β-unsaturated aldehydes via enantioselective direct addition of propargyl acetate to aldehydes. Org. Lett. 2001, 3, 3017–3020. [Google Scholar] [CrossRef]
- Boyall, D.; López, F.; Sasaki, H.; Frantz, D.; Carreira, E.M. Enantioselective addition of 2-methyl-3-butyn-2-ol to aldehydes: Preparation of 3-hydroxy-1-butynes. Org. Lett. 2000, 2, 4233–4236. [Google Scholar] [CrossRef]
- Gao, G.; Moore, D.; Xie, R.-G.; Pu, L. Highly enantioselective phenylacetylene additions to both aliphatic and aromatic aldehydes. Org. Lett. 2002, 4, 4143–4146. [Google Scholar] [CrossRef]
- Moore, D.; Pu, L. BINOL-catalyzed highly enantioselective terminal alkyne additions to aromatic aldehydes. Org. Lett. 2002, 4, 1855–1857. [Google Scholar] [CrossRef]
- Xu, M.-H.; Pu, L. A new 1,1'-binaphthyl-based catalyst for the enantioselective phenylacetylene addition to aromatic aldehydes without using a titanium complex. Org. Lett. 2002, 4, 4555–4557. [Google Scholar] [CrossRef]
- Moore, D.; Huang, W.-S.; Xu, M.-H.; Pu, L. Greatly enhanced enantioselectivity by an apparently remote steric effect in the 1,1'-binaphthyl-catalyzed alkynylzinc addition to aldehydes. Tetrahedron Lett. 2002, 43, 8831–8834. [Google Scholar] [CrossRef]
- Liu, L.; Pu, L. 3,3'-Functionalized octahydro-BINOL: A facile synthesis and its highenantioselectivity in the alkyne addition to aldehydes. Tetrahedron 2004, 60, 7427–7430. [Google Scholar] [CrossRef]
- Li, Z.-B.; Pu, L. BINOL-salen-catalyzed highly enantioselective alkyne additions to aromatic aldehydes. Org. Lett. 2004, 6, 1065–1068. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, S.-Y.; Yu, X.-Q.; Pu, L. 1,1-Binaphthyl ligands with bulky 3,3-thtertiaryalkyl substituents for the asymmetric alkyne addition to aromatic aldehydes. Tetrahedron 2007, 63, 4422–4428. [Google Scholar] [CrossRef]
- Yue, Y.; Turlington, M.; Yu, X.-Q.; Pu, L. 3,3'-Anisyl-substituted BINOL, H4BINOL, and H8BINOL ligands: Asymmetric synthesis of diverse propargylic alcohols and their ring-closing metathesis to chiral cycloalkenes. J. Org. Chem. 2009, 74, 8681–8689. [Google Scholar] [CrossRef]
- Chen, X.; Chen, W.; Wang, L.; Yu, X.-Q.; Huang, D.-S.; Pu, L. Synthesis of a C1 symmetric BINOL-terpyridine ligand and highly enantioselective methyl propiolate addition to aromatic aldehydes. Tetrahedron 2010, 66, 1990–1993. [Google Scholar] [CrossRef]
- Gao, G.; Wang, Q.; Yu, X.-Q.; Xie, R.-G.; Pu, L. Highly enantioselective synthesis of γ-hydroxy-α, β-acetylenic esters by asymmetric alkyne addition to aldehydes. Angew. Chem. Int. Ed. Engl. 2006, 45, 122–125. [Google Scholar] [CrossRef]
- Lu, G.; Li, X.; Chan, W.L.; Chan, A.S.C. Titanium-catalyzed enantioselective alkynylation of aldehydes. Chem. Commun. 2002, 21, 172–173. [Google Scholar]
- Li, X.; Lu, G.; Kwok, W.H.; Chan, A.S.C. Highly enantioselective alkynylzinc addition to aromatic aldehydes catalyzed by self-assembled titanium catalysts. J. Am. Chem. Soc. 2002, 124, 12636–12637. [Google Scholar]
- Lu, G.; Li, X.; Zhou, Z.; Chan, W.L.; Chan, A.S.C. Enantioselective alkynylation of aromatic aldehydes catalyzed by new chiral amino alcohol-based ligands. Tetrahedron 2001, 12, 2147–2152. [Google Scholar]
- Ruan, J.; Lu, G.; Xu, L.; Li, Y.-M.; Chan, A.S.C. Catalytic asymmetric alkynylation and arylation of aldehydes by an H8-binaphthyl-based amino alcohol ligand. Adv. Synth. Catal. 2008, 350, 76–84. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, R.; Xu, J.; Da, C.-S.; Yan, W.-J.; Chen, C. Highly enantioselective addition of phenylacetylene to aldehydes catalyzed by a β-sulfonamide alcohol–titanium complex. Angew. Chem. Int. Ed. 2003, 42, 5747–5749. [Google Scholar]
- Kang, Y.-F.; Liu, L.; Wang, R.; Yan, W.-J.; Zhou, Y.-F. The use of bifunctional catalyst systems in the asymmetric addition of alkynylzinc to aldehydes. Tetrahedron 2004, 15, 3155–3159. [Google Scholar]
- Xu, Z.; Chen, C.; Xu, J.; Miao, M.; Yan, W.; Wang, R. Highly enantioselective addition of phenylacetylene to aldehydes catalyzed by a camphor sulfonamide ligand. Org. Lett. 2004, 6, 1193–1195. [Google Scholar] [CrossRef]
- Han, Z.-J.; Da, C.-S.; Xu, Z.-Q.; Ni, M.; Wang, R. The asymmetric addition of phenylacetylene to aldehydes catalyzed by l-leucine derived chiral sulfonamide alcohol ligands. J. Mol. Cat. A 2005, 236, 32–37. [Google Scholar] [CrossRef]
- Ni, M.; Wang, R.; Han, Z.-J.; Mao, B.; Da, C.-S.; Liu, L.; Chen, C. Synthesis of new C2-symmetrical bissulfonamide ligands and application in the enantioselective addition of alkynylzinc to aldehydes and ketones. Adv. Synth. Catal. 2005, 347, 1659–1665. [Google Scholar] [CrossRef]
- Xu, Z.; Lin, L.; Xu, J.; Yan, W.; Wang, R. Asymmetric addition of phenylacetylene to aldehydes catalyzed by β-sulfonamide alcohol-titanium complex. Adv. Synth. Catal. 2006, 348, 506–514. [Google Scholar] [CrossRef]
- Lin, L.; Jiang, X.; Liu, W.; Qiu, L.; Xu, Z.; Xu, J.; Chan, A.S.C.; Wang, R. Highly enantioselective synthesis of γ-hydroxy-α, β-acetylenic esters catalyzed by a β-sulfonamide alcohol. Org. Lett. 2007, 9, 2329–2332. [Google Scholar] [CrossRef]
- Qiu, L.; Wang, Q.; Lin, L.; Liu, X.D.; Jiang, X.X.; Zhao, Q.Y.; Hu, G.W.; Wang, R. Highly enantioselective addition of terminal alkynes to aldehydes catalyzed by a new chiral β-sulfonamide alcohol/Ti(OiPr)4/Et2Zn/R3N catalyst system. Chirality 2009, 21, 316–323. [Google Scholar] [CrossRef]
- Trost, B.M.; Weiss, A.H.; von Wangelin, A.J. Dinuclear Zn-catalyzed asymmetric alkynylation of unsaturated aldehydes. J. Am. Chem. Soc. 2006, 128, 8–9. [Google Scholar]
- Trost, B.M.; Sieber, J.D.; Qian, W.; Dhawan, R.; Ball, Z.T. Asymmetric total synthesis of soraphen A: A flexible alkyne strategy. Angew. Chem. Int. Ed.Engl. 2009, 48, 5478–5481. [Google Scholar] [CrossRef]
- Trost, B.M.; Chan, V.S.; Yamamoto, D. Enantioselective prophenol-catalyzed addition of 1,3-diynes to aldehydes to generate synthetically versatile building blocks and diyne natural products. J. Am. Chem. Soc. 2010, 132, 5186–5192. [Google Scholar] [CrossRef]
- Trost, B.M.; O’Boyle, B.M. Exploiting orthogonally reactive functionality: Synthesis and stereochemical assignment of (−)-ushikulide A. J. Am. Chem. Soc. 2008, 130, 16190–16192. [Google Scholar] [CrossRef]
- Zhong, J.C.; Wang, M.A.; Guo, H.C.; Bian, Q.H.; Wang, M. Design and synthesis of 1,4-amino alcohol ligands with a chiral cyclopropane backbone for asymmetric diethylzinc addition to aromatic aldehydes. Synlett 2006, 11, 1667–1670. [Google Scholar]
- Zhong, J.C.; Wang, M.A.; Guo, H.C.; Yin, M.M.; Wang, M. Asymmetric diethylzinc addition and phenyl transfer to aldehydes using chiral cis-cyclopropane-based amino alcohols. Tetrahedron Asymmetry 2007, 18, 734–741. [Google Scholar] [CrossRef]
- Zhong, J.C.; Hou, S.C.; Bian, Q.H.; Yin, M.M.; Na, R.S.; Zheng, B.; Li, Z.Y.; Liu, S.Z.; Wang, M. Highly enantioselective zinc/amino alcohol-catalyzed alkynylation of aldehydes. Chem. Eur. J. 2009, 15, 3069–3071. [Google Scholar] [CrossRef]
- Li, Z.Y.; Wang, M.; Bian, Q.H.; Zheng, B.; Mao, J.Y.; Li, S.N.; Liu, S.Z.; Wang, M.A.; Zhong, J.C.; Guo, H.C. Highly enantioselective addition of trimethylsilylacetylene to aldehydes catalyzed by a zinc-amino-alcohol complex. Chem. Eur. J. 2011, 17, 5782–5786. [Google Scholar] [CrossRef]
- Zheng, B.; Wang, M.; Li, Z.Y.; Bian, Q.H.; Mao, J.Y.; Li, S.N.; Liu, S.Z.; Zhong, J.C.; Guo, H.C. Asymmetric henry reaction catalyzed by a Zn-amino alcohol system. Tetrahedron Asymmetry 2011, 22, 1156–1160. [Google Scholar] [CrossRef]
- Mao, J.Y.; Nie, X.; Wang, M.; Wang, Q.; Zheng, B.; Bian, Q.H.; Zhong, J.C. Catalytic asymmetric nitroaldol (Henry) reactions with copper(II)/cyclopropane-based bisoxazoline complexes. Tetrahedron Asymmetry 2012, 23, 965–971. [Google Scholar] [CrossRef]
- Dahmen, S. Enantioselective alkynylation of aldehydes catalyzed by [2.2]paracyclophane-based ligands. Org. Lett. 2004, 6, 2113–2116. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 7a–d are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zheng, B.; Li, Z.; Liu, F.; Wu, Y.; Shen, J.; Bian, Q.; Hou, S.; Wang, M. Highly Enantioselective Addition of Phenylethynylzinc to Aldehydes Catalyzed by Chiral Cyclopropane-Based Amino Alcohols. Molecules 2013, 18, 15422-15433. https://doi.org/10.3390/molecules181215422
Zheng B, Li Z, Liu F, Wu Y, Shen J, Bian Q, Hou S, Wang M. Highly Enantioselective Addition of Phenylethynylzinc to Aldehydes Catalyzed by Chiral Cyclopropane-Based Amino Alcohols. Molecules. 2013; 18(12):15422-15433. https://doi.org/10.3390/molecules181215422
Chicago/Turabian StyleZheng, Bing, Zhiyuan Li, Feipeng Liu, Yanhua Wu, Junjian Shen, Qinghua Bian, Shicong Hou, and Ming Wang. 2013. "Highly Enantioselective Addition of Phenylethynylzinc to Aldehydes Catalyzed by Chiral Cyclopropane-Based Amino Alcohols" Molecules 18, no. 12: 15422-15433. https://doi.org/10.3390/molecules181215422







