Phenolic Esters of O-Desmethylvenlafaxine with Improved Oral Bioavailability and Brain Uptake
Abstract
:1. Introduction
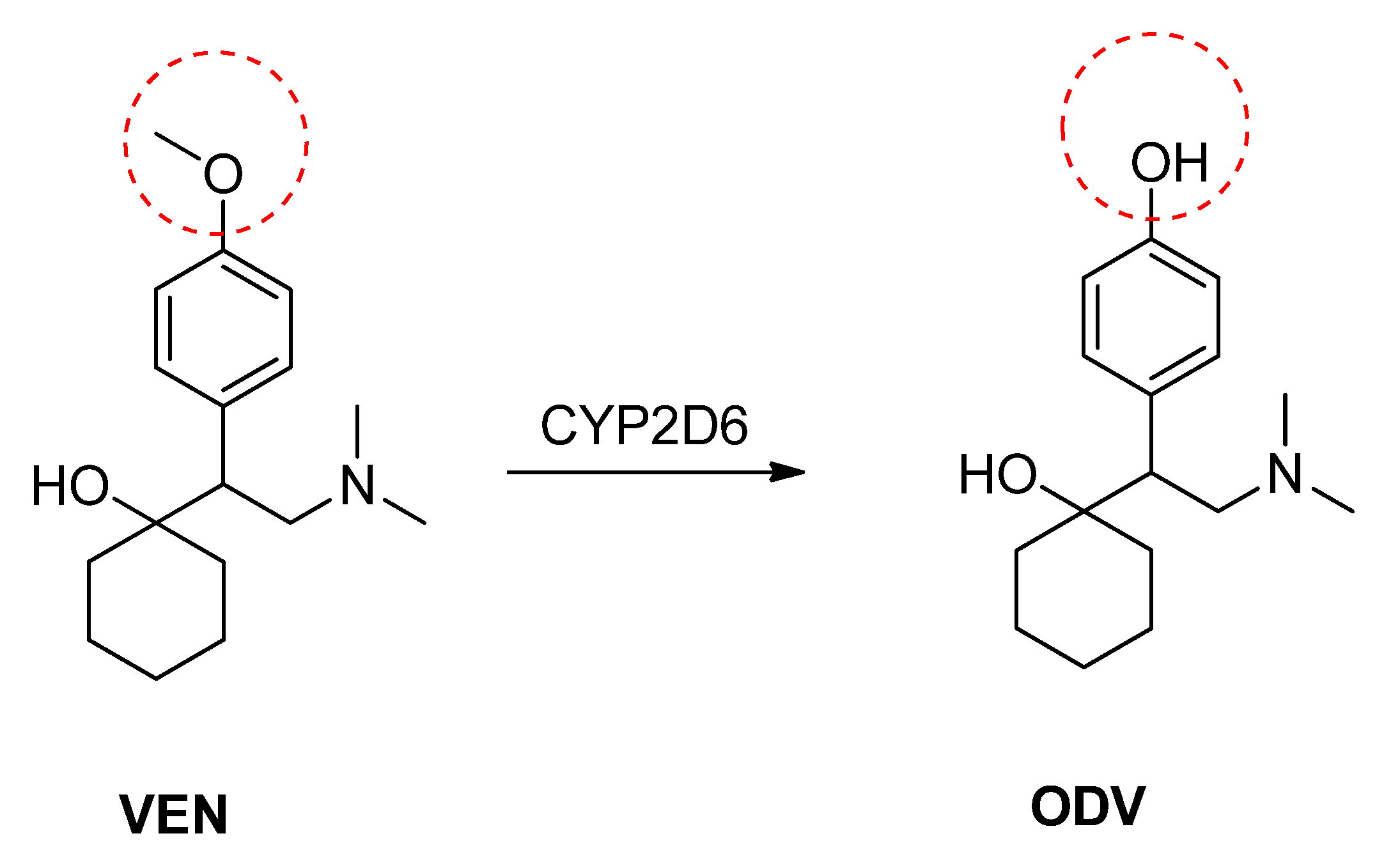
2. Results and Discussion
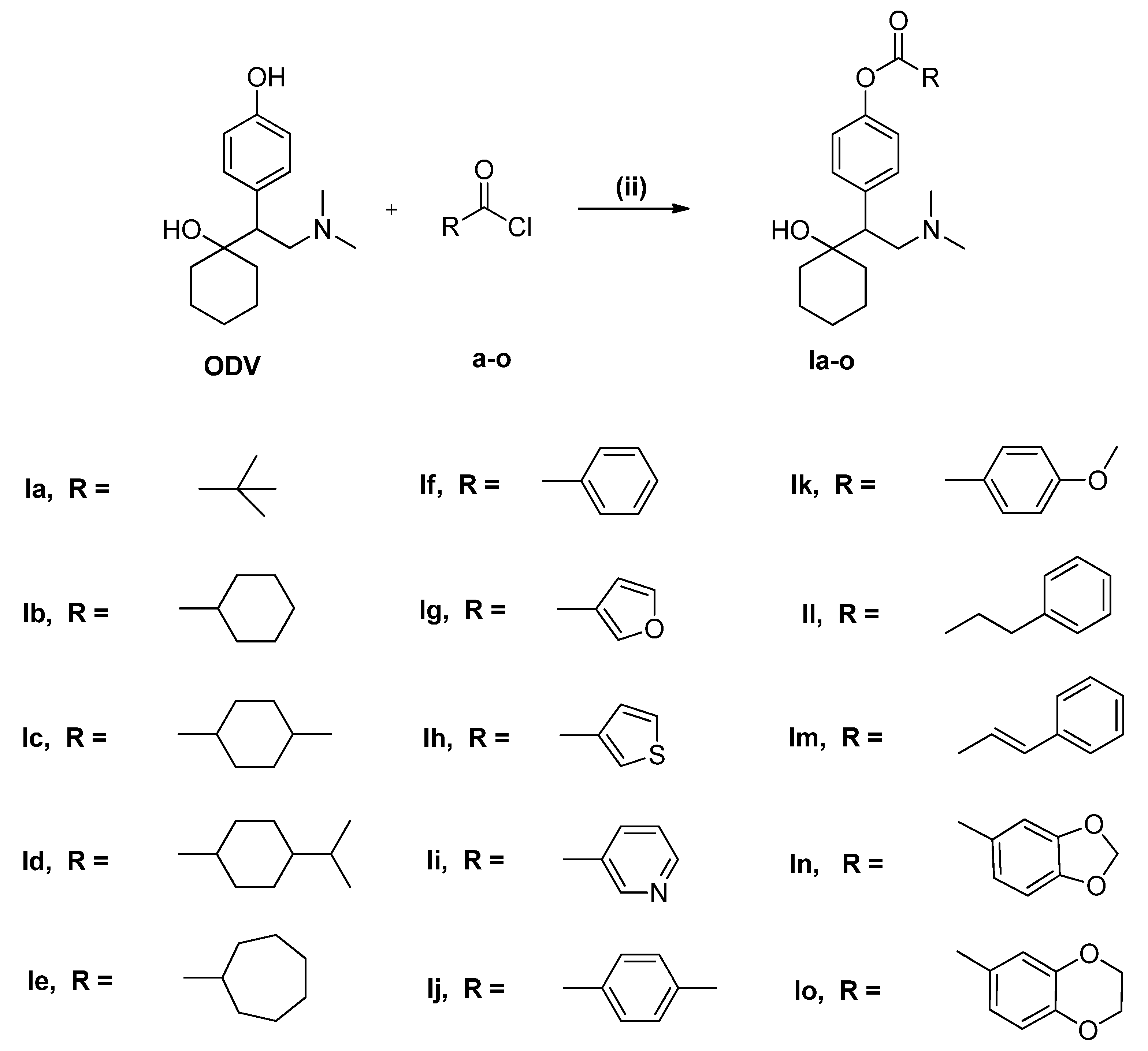
2.1. Chemistry
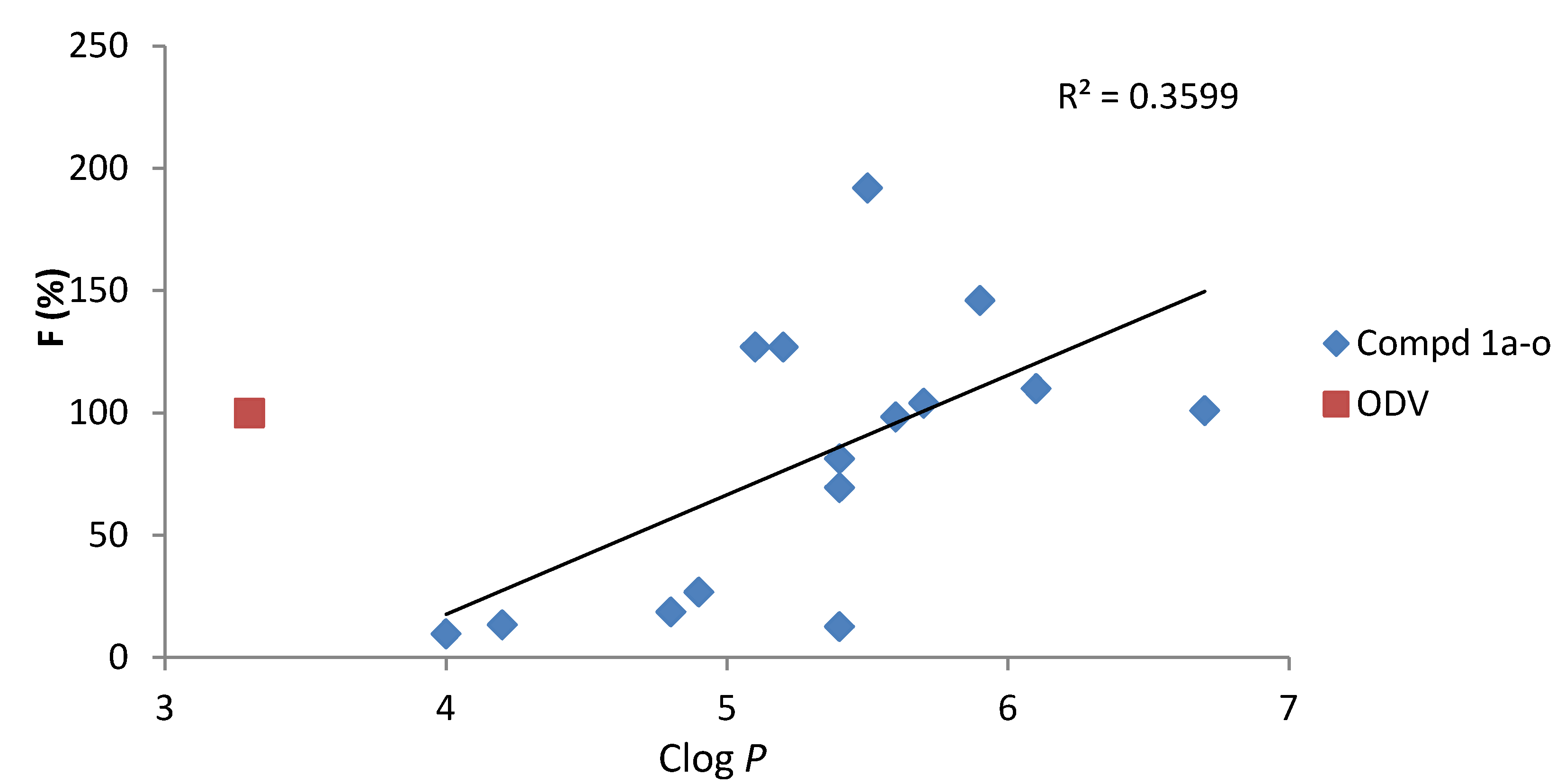
2.2. Pharmacokinetic (PK) Studies in Rat
| Compd. | Clog P | Cmax | Tmax | t1/2 | AUC0-t | AUC0-∞ | MRT | F |
|---|---|---|---|---|---|---|---|---|
| μg/L | h | h | μg/L × h | μg/L × h | h | % | ||
| ODV | 3.3 | 66.6 ± 14.6 | 1.25 ± 1.06 | 4.14 ± 1.21 | 219 ± 56 | 222 ± 59 | 6.03 ± 2.82 | 100 |
| 1a | 4.8 | 7.67 ± 1.21 | 1.50 ± 0.71 | 2.13 ± 0.83 | 41.1 ± 3.8 | 41.1 ± 3.7 | 4.70 ± 0.47 | 18.7 |
| 1b | 5.4 | 18.2 ± 3.4 | 1.50 ± 0.24 | 3.95 ± 1.84 | 152 ± 15 | 156 ± 20 | 6.99 ± 1.40 | 69.5 |
| 1c | 5.2 | 98.8 ± 14.5 | 0.50 ± 0.12 | 5.47 ± 1.50 | 278 ± 86 | 293 ± 103 | 4.81 ± 2.06 | 127 |
| 1d | 6.7 | 33.3 ± 18.0 | 0.92 ± 0.83 | 6.40 ± 5.30 | 222 ± 114 | 246 ± 148 | 6.31 ± 0.44 | 101 |
| 1e | 5.9 | 118 ± 16 | 0.33 ± 0.04 | 2.39 ± 0.15 | 321 ± 83 | 321 ± 93 | 4.37 ± 1.39 | 146 |
| 1f | 5.6 | 47.0 ± 13.4 | 2.00 ± 1.41 | 4.61 ± 2.28 | 216 ± 89 | 220 ± 87 | 4.53 ± 1.96 | 98.4 |
| 1g | 4.2 | 9.36 ± 3.60 | 0.58 ± 0.59 | 1.65 ± 0.11 | 29.4 ± 0.2 | 29.4 ± 0.2 | 4.14 ± 0.10 | 13.4 |
| 1h | 4.9 | 15.7 ± 4.6 | 0.50 ± 0.07 | 2.53 ± 1.65 | 58.7 ± 23.9 | 58.9 ± 23.6 | 4.92 ± 1.14 | 26.8 |
| 1i | 4.0 | 10.5 ± 2.7 | 0.50 ± 0.12 | 1.19 ± 0.80 | 21.3 ± 2.8 | 21.3 ± 2.8 | 2.35 ± 0.40 | 9.73 |
| 1j | 6.1 | 101 ± 31 | 0.42 ± 0.09 | 2.49 ± 0.44 | 241 ± 61 | 251 ± 80 | 2.77 ± 0.80 | 110 |
| 1k | 5.7 | 96.3 ± 24.7 | 1.75 ± 0.35 | 4.32 ± 0.82 | 228 ± 38 | 231 ± 47 | 4.95 ± 1.85 | 104 |
| 1l | 5.4 | 12.4 ± 13.5 | 1.50 ± 0.71 | 8.61 ± 3.09 | 27.8 ± 23.6 | 33.9 ± 23.7 | 9.29 ± 3.43 | 12.7 |
| 1m | 5.4 | 30.4 ± 5.9 | 1.25 ± 0.35 | 6.35 ± 4.09 | 178 ± 60 | 197 ± 84 | 6.75 ± 1.60 | 81.3 |
| 1n | 5.5 | 141 ± 48 | 1.00 ± 0.58 | 8.29 ± 5.12 | 421 ± 146 | 456 ± 153 | 4.42 ± 0.74 | 192 |
| 1o | 5.1 | 87.7 ± 13.3 | 0.44 ± 0.10 | 9.02 ± 1.74 | 278 ± 85 | 318 ± 84 | 6.83 ± 0.73 | 127 |
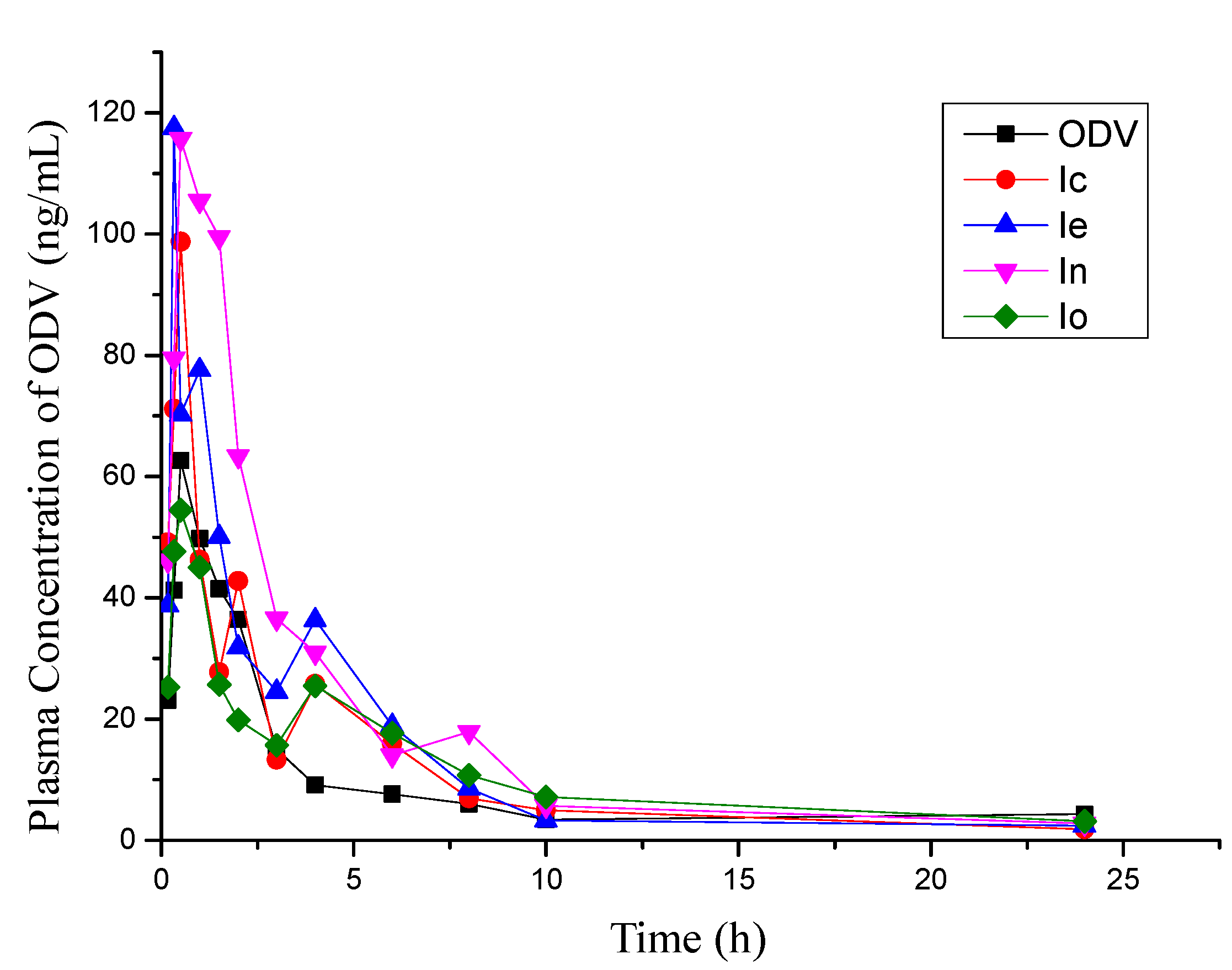
2.3. Brain Uptake Studies in Rat

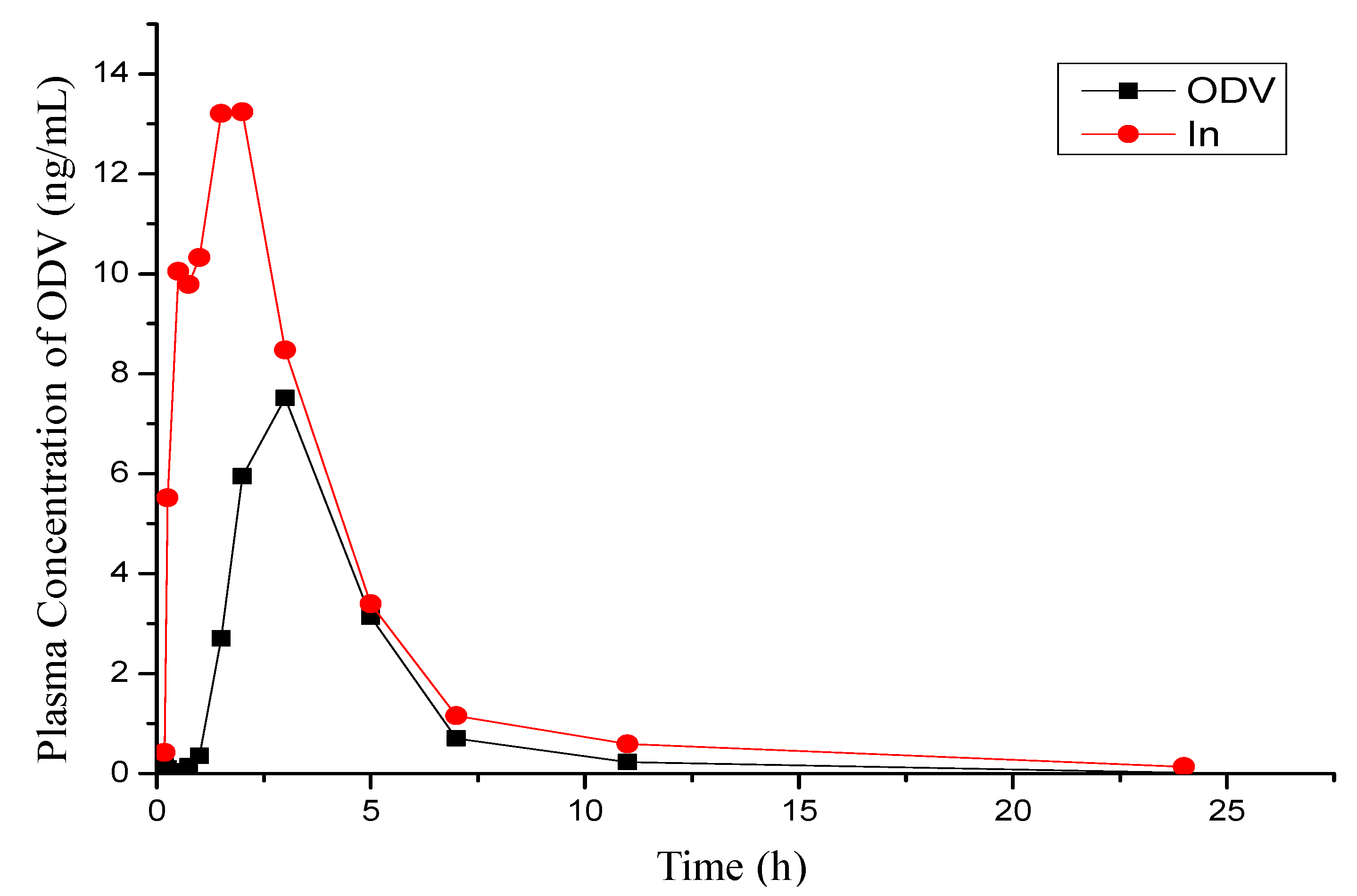
2.4. PK Studies of 1n and ODV in Beagle Dog
| Compd. | Cmax | Tmax | t1/2 | AUC0–t | AUC0–∞ | MRT | F |
|---|---|---|---|---|---|---|---|
| μg/L | h | h | μg/L × h | μg/L × h | h | % | |
| ODV | 7.55 ± 4.34 | 2.67 ± 0.58 | 1.55 ± 0.29 | 27.7 ± 12.1 | 27.8 ± 12.6 | 4.10 ± 0.70 | 100 |
| 1n | 20.8 ± 6.6 | 1.21 ± 0.61 | 3.41 ± 1.54 | 55.1 ± 17.2 | 55.5 ± 16.9 | 3.91 ± 0.76 | 199 |
3. Experimental
3.1. General
3.2. General Procedure for Synthesis of Compounds a–o
3.3. General Procedure for Synthesis of Compounds 1a–o
3.4. PK Studies in Rat
3.5. Brain Uptake Studies in Rat
3.6. PK Studies in Beagle Dog
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Richards, D. Prevalence and clinical course of depression: A review. Clin. Psychol. Rev. 2011, 31, 1117–1125. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Depression: A global public health concern; WHO: Geneva, Switzerland, 2012. Available online: http://www.who.int/mental_health/management/depression/who_paper_depression_wfmh_2012.pdf (accessed on 5 October 2012).
- Andrews, J.M.; Ninan, P.T.; Nemeroff, C.B. Venlafaxine: A novel antidepressant that has a dual mechanism of action. Depression 1996, 4, 48–56. [Google Scholar] [CrossRef]
- Makhija, S.N.; Vavia, P.R. Stability indicating LC method for the estimation of venlafaxine in pharmaceutical formulations. J. Pharm. Biomed. Anal. 2002, 28, 1055–1059. [Google Scholar] [CrossRef]
- Preskorn, S. Pharmacotherapeutic profile of venlafaxine. Eur. Psychiatr. 1997, 12, 285s–294s. [Google Scholar] [CrossRef]
- Gutierrez, M.A.; Stimmel, G.L.; Aiso, J.Y. Venlafaxine: A 2003 update. Clin. Ther. 2003, 25, 2138–2154. [Google Scholar] [CrossRef]
- Ellingrod, V.L.; Perry, P.J. Venlafaxine: A heterocyclic antidepressant. Am. J. Hosp. Pharm. 1994, 51, 3033–3046. [Google Scholar]
- Klamerus, K.J.; Maloney, K.; Rudolph, R.L.; Sisenwine, S.F.; Jusko, W.J.; Chiang, S.T. Introduction of a composite parameter to the pharmacokinetics of venlafaxine and its active O-desmethyl metabolite. J. Clin. Pharmacol. 1992, 32, 716–724. [Google Scholar] [CrossRef]
- Schweizer, E.; Thielen, R.J.; Frazer, A. Venlafaxine: A novel antidepressant compound. Expert Opin. Investig. Drugs 1997, 6, 65–78. [Google Scholar] [CrossRef]
- Perry, R.; Cassagnol, M. Desvenlafaxine: A new serotonin-norepinephrine reuptake inhibitor for the treatment of adults with major depressive disorder. Clin. Ther. 2009, 31, 1374–1404. [Google Scholar] [CrossRef]
- Muth, E.A.; Haskins, J.T.; Moyer, J.A.; Husbands, G.E.; Nielsen, S.T.; Sigg, E.B. Antidepressant biochemical profile of the novel bicyclic compound Wy-45030, an ethyl cyclohexanol derivative. Biochem. Pharmacol. 1986, 35, 4493–4497. [Google Scholar] [CrossRef]
- Rudorfer, M.V.; Potter, W.Z. The role of metabolites of antidepressants in the treatment of depression. CNS Drugs 1997, 7, 273–312. [Google Scholar] [CrossRef]
- Ereshefsky, L.; Dugan, D. Review of the pharmacokinetics, pharmacogenetics, and drug interaction potential of antidepressants: Focus on venlafaxine. Depress. Anxiety 2000, 12, 30–44. [Google Scholar] [CrossRef]
- De Ruiter, J.; Holston, P.L. New Drug Review 2008. US Pharm. 2008, 33, 30–44. [Google Scholar]
- Desvenlafaxine: Application withdrawal. Desvenlafaxine: Withdrawal of marketing application for depression also. Prescrire Int. 2009, 18, 197.
- Liebowitz, M.R.; Tourian, K.A.; Hwang, E.; Mele, L. A double-blind, randomized, placebo-controlled study assessing the efficacy and tolerability of desvenlafaxine 10 and 50 mg/day in adult outpatients with major depressive disorder. BMC Psychiatry 2013, 13, 94–102. [Google Scholar] [CrossRef]
- Iwata, N.T.K.; Hwang, E.; Mele, L.; Vialet, C. Efficacy and safety of desvenlafaxine 25 and 50 mg/day in a randomized, placebo-controlled study of depressed outpatients. J. Psychiatr. Pract. 2013, 19, 5–14. [Google Scholar] [CrossRef]
- Kubo, S.H.; Cody, R.J. Clinical pharmacokinetics of the angiotensin converting enzyme inhibitors. A review. Clin. Pharmacokinet. 1985, 10, 377–391. [Google Scholar] [CrossRef]
- Mizuno, N.; Niwa, T.; Yotsumoto, Y.; Sugiyama, Y. Impact of drug transporter studies on drug discovery and development. Pharmacol. Rev. 2003, 55, 425–461. [Google Scholar]
- Baudy, R.B.; Butera, J.A.; Abou-Gharbia, M.A.; Chen, H.; Harrison, B.; Jain, U.; Magolda, R.; Sze, J.Y.; Brandt, M.R.; Cummons, T.A.; et al. Prodrugs of perzinfotel with improved oral bioavailability. J. Med. Chem. 2009, 52, 771–778. [Google Scholar] [CrossRef]
- Xie, Q.; Wang, X.; Jiang, Z.; Qiu, Z. Design, synthesis, and bioavailability evaluation of coumarin-based prodrug of meptazinol. Bioorg. Med. Chem. Lett. 2005, 15, 4953–4956. [Google Scholar] [CrossRef]
- Kahns, A.H.; Møss, J.; Bundgaard, H. Improved oral bioavailability of salicylamide in rabbits by a 1,3-benzoxazine-2,4-dione prodrug. Int. J. Pharm. 1992, 78, 199–202. [Google Scholar] [CrossRef]
- Horn, A.S.; Kelly, P.; Westerink, B.H.; Dijkstra, D. A prodrug of ADTN: Selectivity of dopaminergic action and brain levels of ADTN. Eur. J. Pharmacol. 1979, 60, 95–99. [Google Scholar] [CrossRef]
- Bandgar, B.P.; Sarangdhar, R.J.; Viswakarma, S.; Ahamed, F.A. Synthesis and biological evaluation of orally active prodrugs of indomethacin. J. Med. Chem. 2011, 54, 1191–1201. [Google Scholar] [CrossRef]
- De Graaf, M.; Nevalainen, T.J.; Scheeren, H.W.; Pinedo, H.M.; Haisma, H.J.; Boven, E. A methylester of the glucuronide prodrug DOX-GA3 for improvement of tumor-selective chemotherapy. Biochem. Pharmacol. 2004, 68, 2273–2281. [Google Scholar] [CrossRef]
- Tian, J.W.; Jiang, W.L.; Zhong, Y.; Meng, Q.; Gai, Y.; Zhu, H.B.; Hou, J.; Xing, Y.; Li, Y.X. Preclinical pharmacology of TP1, a novel potent triple reuptake inhibitor with antidepressant properties. Neuroscience 2011, 196, 124–130. [Google Scholar] [CrossRef]
- Ibáñez, E.; Plano, D.; Font, M.; Calvo, A.; Prior, C.; Palop, J.A.; Sanmartín, C. Synthesis and antiproliferative activity of novel symmetrical alkylthio- and alkylseleno-imidocarbamates. Eur. J.Med. Chem. 2011, 46, 265–274. [Google Scholar] [CrossRef]
- Zhang, L.P. Compounds, preparation process, and uses thereof used for interrupting reuptake of 5-hydroxytryptamine and norepinephrine or treating diseases such as depression et al. WO Patent 2006133652, 21 December 2006. [Google Scholar]
- Bhatt, J.; Jangid, A.; Venkatesh, G.; Subbaiah, G.; Singh, S. Liquid chromatography-tandem mass spectrometry (LC-MS-MS) method for simultaneous determination of venlafaxine and its active metabolite O-desmethyl venlafaxine in human plasma. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2005, 829, 75–81. [Google Scholar] [CrossRef]
- Liu, W.; Cai, H.L.; Li, H.D. High performance liquid chromatography-electrospray ionization mass spectrometry (HPLC-MS/ESI) method for simultaneous determination of venlafaxine and its three metabolites in human plasma. J. Chromatogr. BAnalyt. Technol. Biomed. Life Sci. 2007, 850, 405–411. [Google Scholar] [CrossRef]
- Rosendorff, C.; Cranston, W.I. Effects of intrahypothalamic and intraventricular norepinephrine and 5-hydroxytryptamine on hypothalamic blood flow in the conscious rabbit. Circ. Res. 1971, 28, 492–502. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 1a–o are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhang, Y.; Yang, Y.; Zhao, S.; Yang, Z.; Yang, H.; Fawcett, J.P.; Li, Y.; Gu, J.; Sun, T. Phenolic Esters of O-Desmethylvenlafaxine with Improved Oral Bioavailability and Brain Uptake. Molecules 2013, 18, 14920-14934. https://doi.org/10.3390/molecules181214920
Zhang Y, Yang Y, Zhao S, Yang Z, Yang H, Fawcett JP, Li Y, Gu J, Sun T. Phenolic Esters of O-Desmethylvenlafaxine with Improved Oral Bioavailability and Brain Uptake. Molecules. 2013; 18(12):14920-14934. https://doi.org/10.3390/molecules181214920
Chicago/Turabian StyleZhang, Yang, Yan Yang, Sen Zhao, Zhichao Yang, Hong Yang, J. Paul Fawcett, Youxin Li, Jingkai Gu, and Tiemin Sun. 2013. "Phenolic Esters of O-Desmethylvenlafaxine with Improved Oral Bioavailability and Brain Uptake" Molecules 18, no. 12: 14920-14934. https://doi.org/10.3390/molecules181214920




