Immunomodulatory Effects of Hedysarum polybotrys Extract in Mice Macrophages, Splenocytes and Leucopenia
Abstract
:1. Introduction
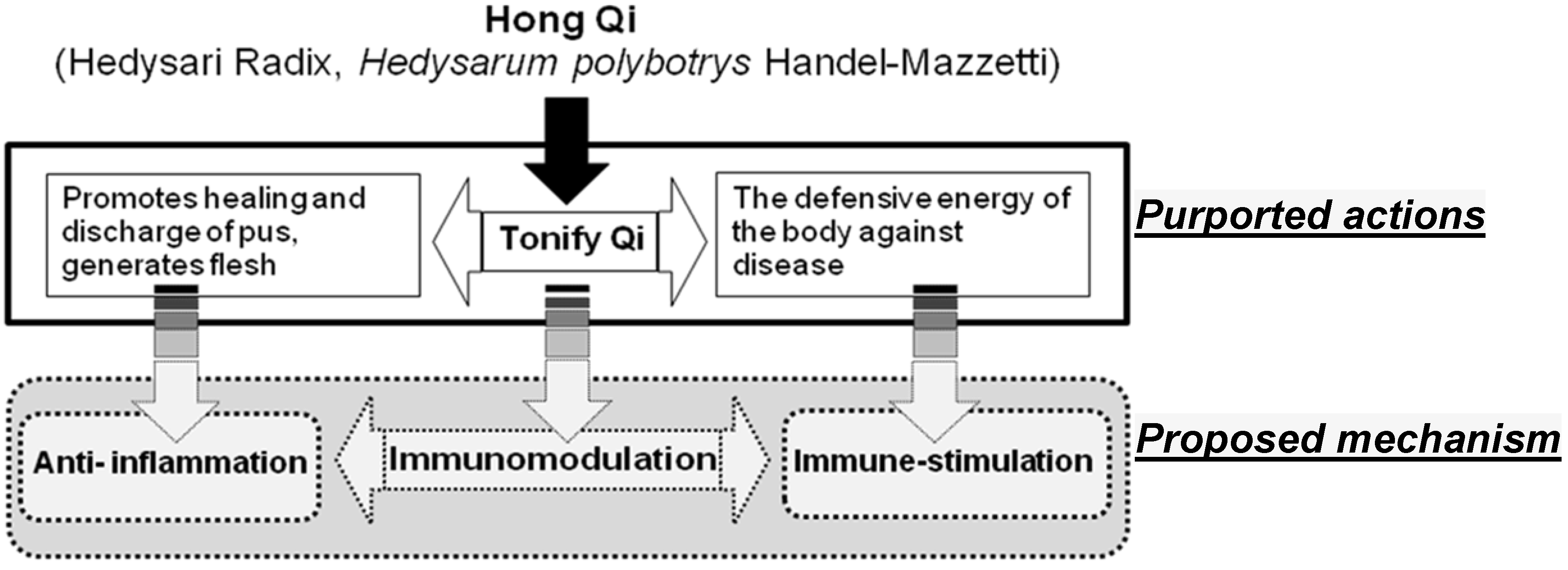
2. Results
2.1. Total Proanthocyanidin in H. polybotrys Extracts
| Samples | Quality control | NO inhibition effects | ||||
|---|---|---|---|---|---|---|
| Yield (%) | Proanthocyanidin a (mg/g) | Formononetin b (mg/g) | IC50 of NO− scavenging c | IC50 of NO inhibition in cells d | ||
| H | 33.70 | 2.60 | 0.013 ± 0.001 | 0.24 mg/mL | >200 μg/mL | |
| M | 15.16 | 7.68 | 0.204 ± 0.001 | 0.50 mg/mL | >200 μg/mL | |
| M-H | 14.41 | 3.75 | N.D. | 0.18 mg/mL | >200 μg/mL | |
| M-EA | 0.75 | 36.14 | 5.457 ± 0.174 | 0.15 mg/mL | 55.75 μg/mL | |
| Formononetin | - | - | - | 188.28 μM | 73.00 μM | |
2.2. Concentrations and Bioactivities of Formononetin in H. polybotrys Extracts




2.3. Attenuation of NO-Induced Cytotoxicity by M-EA Extract
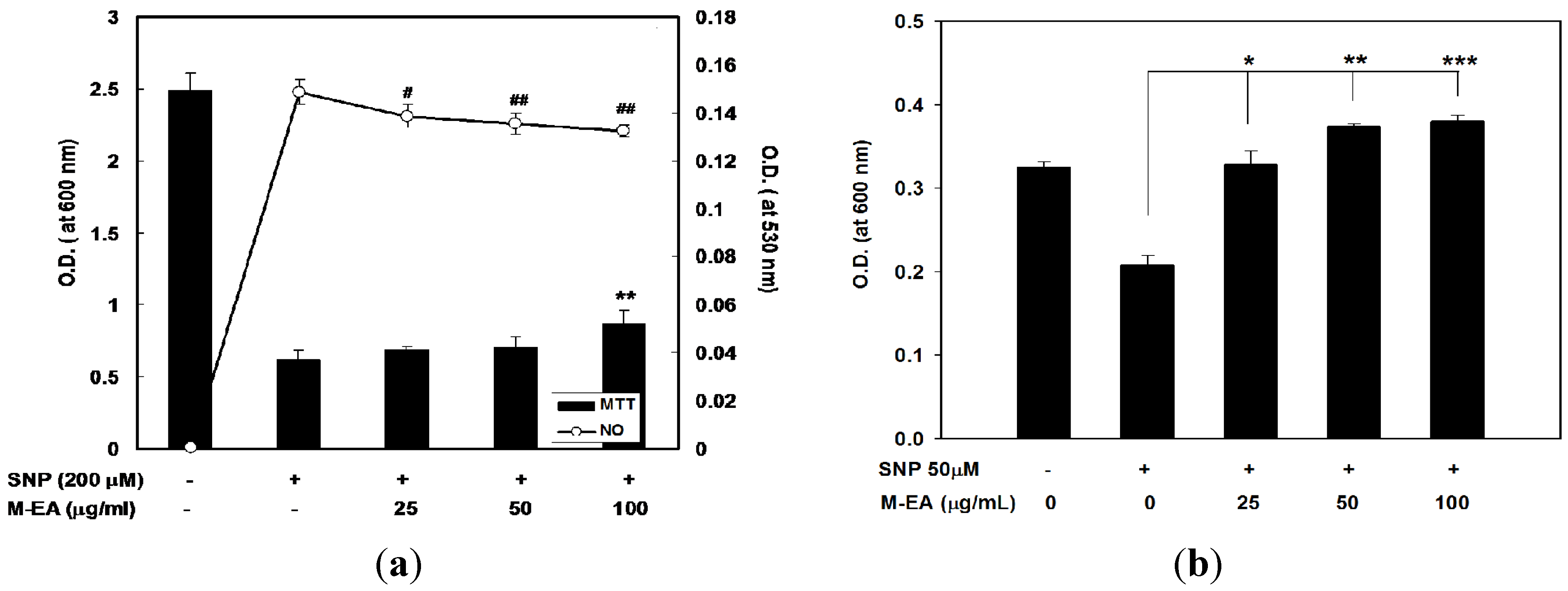
2.4. Alleviating Daunoblastina-Induced Leucopenia by M-EA Extract

3. Discussion
4. Experimental
4.1. Preparation of H. polybotrys Extracts
4.2. Total Proanthocyanidin Contents Analysis
4.3. Chromatographic Analysis
4.4. NO Radical-Scavenging Assay
4.5. RAW 264.7 Cell Cultures
4.6. Experimental Animals
4.7. Preparation of Splenocyte Suspensions
4.8. Induction of Inflammatory Response
4.9. T Induction of NO-Induced Cytotoxicity
4.10. Induction of Leucopenia in Mice
4.11. Cell Viability Assay
4.12. Measurement of Nitrite Formation
4.13. Measurement of Prostaglandin E2
4.14. Western Blot Assay
4.15. Statistical Analysis
5. Conclusions
Acknowledgments
Conflicts of Interest
References and Notes
- Li, S. Bencao Gangmu (Compendium of Materia Medica); 1578AD. [Google Scholar]
- Yen, K.Y. The Illustrated Chinese Materia Medica Crude Drugs, 1st ed.; SMC Publishing Institute: Taipei, Taiwan, 1992. [Google Scholar]
- Yao, W.; Yang, H.; Ding, G. Mechanisms of Qi-blood circulation and Qi deficiency syndrome in view of blood and interstitial fluid circulation. J. Tradit. Chin. Med. 2013, 33, 538–544. [Google Scholar] [CrossRef]
- Zhao, J.; Yu, Q.T.; Li, P.; Zhou, P.; Zhang, Y.J.; Wang, W. Determination of nine active components in Radix Hedysari and Radix Astragali using capillary HPLC with diode array detection and MS detection. J. Sep. Sci. 2008, 31, 255–261. [Google Scholar] [CrossRef]
- Burns, J.J.; Zhao, L.; Taylor, E.W.; Spelman, K. The influence of traditional herbal formulas on cytokine activity. Toxicology 2010, 278, 140–159. [Google Scholar] [CrossRef]
- Spelman, K.; Burns, J.; Nichols, D.; Winters, N.; Ottersberg, S.; Tenborg, M. Modulation of cytokine expression by traditional medicines: A review of herbal immunomodulators. Altern. Med. Rev. 2006, 11, 128–150. [Google Scholar]
- Haddad, P.S.; Azar, G.A.; Groom, S.; Boivin, M. Natural health products, modulation of immune function and prevention of chronic diseases. Evid. Based Complement. Alternat. Med. 2005, 2, 513–520. [Google Scholar] [CrossRef]
- Lee, Y.S.; Han, O.K.; Park, C.W.; Suh, S.I.; Shin, S.W.; Yang, C.H.; Jeon, T.W.; Lee, E.S.; Kim, K.J.; Kim, S.H.; et al. Immunomodulatory effects of aqueous-extracted Astragali radix in methotrexate-treated mouse spleen cells. J. Ethnopharmacol. 2003, 84, 193–198. [Google Scholar] [CrossRef]
- Kang, H.; Ahn, K.S.; Cho, C.; Bae, H.S. Immunomodulatory effect of Astragali Radix extract on murine TH1/TH2 cell lineage development. Biol. Pharm. Bull. 2004, 27, 1946–1950. [Google Scholar] [CrossRef]
- Shon, Y.H.; Nam, K.S. Protective effect of Astragali radix extract on interleukin-1 beta-induced inflammation in human amnion. Phytother. Res. 2003, 17, 1016–1020. [Google Scholar] [CrossRef]
- Lee, Y.S.; Han, O.K.; Park, C.W.; Yang, C.H.; Jeon, T.W.; Yoo, W.K.; Kim, S.H.; Kim, H.J. Pro-inflammatory cytokine gene expression and nitric oxide regulation of aqueous extracted Astragali radix in RAW 264.7 macrophage cells. J. Ethnopharmacol. 2005, 100, 289–294. [Google Scholar] [CrossRef]
- Ryu, M.; Kim, E.H.; Chun, M.; Kang, S.; Shim, B.; Yu, Y.B.; Jeong, G.; Lee, J.S. Astragali Radix elicits anti-inflammation via activation of MKP-1, concomitant with attenuation of p38 and Erk. J. Ethnopharmacol. 2008, 115, 184–193. [Google Scholar] [CrossRef]
- Hortelano, S.; Castrillo, A.; Alvarez, A.M.; Bosca, L. Contribution of cyclopentenone prostaglandins to the resolution of inflammation through the potentiation of apoptosis in activated macrophages. J. Immunol. 2000, 165, 6525–6531. [Google Scholar]
- Heigold, S.; Bauer, G. RAW 264.7 macrophages induce apoptosis selectively in transformed fibroblasts: Intercellular signaling based on reactive oxygen and nitrogen species. J. Leukoc. Biol. 2002, 72, 554–563. [Google Scholar]
- Ma, X.Q.; Shi, Q.; Duan, J.A.; Dong, T.T.; Tsim, K.W. Chemical analysis of Radix Astragali (Huangqi) in China: A comparison with its adulterants and seasonal variations. J. Agric. Food Chem. 2002, 50, 4861–4866. [Google Scholar] [CrossRef]
- Halabalaki, M.; Alexi, X.; Aligiannis, N.; Lambrinidis, G.; Pratsinis, H.; Florentin, I.; Mitakou, S.; Mikros, E.; Skaltsounis, A.L.; Alexis, M.N. Estrogenic activity of isoflavonoids from Onobrychis ebenoides. Planta Med. 2006, 72, 488–493. [Google Scholar] [CrossRef]
- Shrestha, S.P.; Amano, Y.; Narukawa, Y.; Takeda, T. Nitric oxide production inhibitory activity of flavonoids contained in trunk exudates of Dalbergia sissoo. J. Nat. Prod. 2008, 71, 98–101. [Google Scholar] [CrossRef]
- Chin, Y.W.; Jung, H.A.; Liu, Y.; Su, B.N.; Castoro, J.A.; Keller, W.J.; Pereira, M.A.; Kinghorn, A.D. Anti-oxidant constituents of the roots and stolons of licorice (Glycyrrhiza glabra). J. Agric. Food Chem. 2007, 55, 4691–4697. [Google Scholar] [CrossRef]
- Legler, D.F.; Bruckner, M.; Uetz-von Allmen, E.; Krause, P. Prostaglandin E2 at new glance: Novel insights in functional diversity offer therapeutic chances. Int. J. Biochem. Cell Biol. 2010, 42, 198–201. [Google Scholar] [CrossRef]
- Badn, W.; Hegardt, P.; Fellert, M.A.; Darabi, A.; Esbjornsson, M.; Smith, K.E.; Janelidze, S.; Salford, L.G.; Visse, E.; Siesjo, P. Inhibition of inducible nitric oxide synthase enhances anti-tumour immune responses in rats immunized with IFN-gamma-secreting glioma cells. Scand. J. Immunol. 2007, 65, 289–297. [Google Scholar] [CrossRef]
- Hai, L.Q.; Zhang, Q.Y.; Liang, H.; Zhao, Y.Y.; Du, N.S. Studies on chemical constituents of Hedysarum polybotrys. Yao Xue Xue Bao 2003, 38, 592–595. [Google Scholar]
- Li, X.; Zhu, Z.Y.; Wang, B.; Lou, Z.Y.; Chai, Y.F. Determination of three constituents in Radix Astragali by HPLC-MS. Yao Xue Xue Bao 2006, 41, 793–796. [Google Scholar]
- Wang, Y.; Zhu, Y.; Gao, L.; Yin, H.; Xie, Z.; Wang, D.; Zhu, Z.; Han, X. Formononetin attenuates IL-1β-induced apoptosis and NF-κB activation in INS-1 cells. Molecules 2012, 17, 10052–10064. [Google Scholar] [CrossRef]
- Tenore, G.C.; Manfra, M.; Stiuso, P.; Coppola, L.; Russo, M.; Monterrey, I.M.G.; Campiglia, P. Antioxidant profile and in vitro cardiac radical-scavenging versus pro-oxidant effects of commercial red grape juices (Vitis vinifera L. cv. Aglianico N.). J. Agric. Food Chem. 2012, 60, 9680–9687. [Google Scholar] [CrossRef]
- Li, J.Z.; Yu, S.Y.; Wu, J.H.; Shao, Q.R.; Dong, X.M. Paeoniflorin protects myocardial cell from doxorubicin-induced apoptosis through inhibition of NADPH oxidase. Can. J. Physiol. Pharmacol. 2012, 90, 1569–1575. [Google Scholar] [CrossRef]
- Tulipani, S.; Mezzetti, B.; Capocasa, F.; Bompadre, S.; Beekwilder, J.; de Vos, C.H.; Capanoglu, E.; Bovy, A.; Battino, M. Antioxidants, phenolic compounds, and nutritional quality of different strawberry genotypes. J. Agric. Food Chem. 2008, 56, 696–704. [Google Scholar] [CrossRef]
- Huang, G.C.; Wu, L.S.; Chen, L.G.; Yang, L.L.; Wang, C.C. Immuno-enhancement effects of Huang Qi Liu Yi Tang in a murine model of cyclophosphamide-induced leucopenia. J. Ethnopharmacol. 2007, 109, 229–235. [Google Scholar]
- Pavlovic, R.; Santaniello, E. Peroxynitrite and nitrosoperoxycarbonate, a tightly connected oxidizing-nitrating couple in the reactive nitrogen-oxygen species family: New perspectives for protection from radical-promoted injury by flavonoids. J. Pharm. Pharmacol. 2007, 59, 1687–1695. [Google Scholar] [CrossRef]
- Sumanont, Y.; Murakami, Y.; Tohda, M.; Vajragupta, O.; Matsumoto, K.; Watanabe, H. Evaluation of the nitric oxide radical scavenging activity of manganese complexes of curcumin and its derivative. Biol. Pharm. Bull. 2004, 27, 170–173. [Google Scholar] [CrossRef]
- Chen, L.G.; Yang, L.L.; Wang, C.C. Anti-inflammatory activity of mangostins from Garcinia mangostana. Food Chem. Toxicol. 2008, 46, 688–693. [Google Scholar] [CrossRef]
- Huan, S.K.; Lee, H.H.; Liu, D.Z.; Wu, C.C.; Wang, C.C. Cantharidin-induced cytotoxicity and cyclooxygenase 2 expression in human bladder carcinoma cell line. Toxicology 2006, 223, 136–143. [Google Scholar] [CrossRef]
- Tseng, S.H.; Lee, H.H.; Chen, L.G.; Wu, C.H.; Wang, C.C. Effects of three purgative decoctions on inflammatory mediators. J. Ethnopharmacol. 2006, 105, 118–124. [Google Scholar]
- Sample Availability: Samples are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Huang, G.-C.; Lee, C.-J.; Wang, K.-T.; Weng, B.-C.; Chien, T.-Y.; Tseng, S.-H.; Wang, C.-C. Immunomodulatory Effects of Hedysarum polybotrys Extract in Mice Macrophages, Splenocytes and Leucopenia. Molecules 2013, 18, 14862-14875. https://doi.org/10.3390/molecules181214862
Huang G-C, Lee C-J, Wang K-T, Weng B-C, Chien T-Y, Tseng S-H, Wang C-C. Immunomodulatory Effects of Hedysarum polybotrys Extract in Mice Macrophages, Splenocytes and Leucopenia. Molecules. 2013; 18(12):14862-14875. https://doi.org/10.3390/molecules181214862
Chicago/Turabian StyleHuang, Guan-Cheng, Chia-Jung Lee, Kun-Teng Wang, Bor-Chun Weng, Ting-Yi Chien, Sung-Hui Tseng, and Ching-Chiung Wang. 2013. "Immunomodulatory Effects of Hedysarum polybotrys Extract in Mice Macrophages, Splenocytes and Leucopenia" Molecules 18, no. 12: 14862-14875. https://doi.org/10.3390/molecules181214862
APA StyleHuang, G.-C., Lee, C.-J., Wang, K.-T., Weng, B.-C., Chien, T.-Y., Tseng, S.-H., & Wang, C.-C. (2013). Immunomodulatory Effects of Hedysarum polybotrys Extract in Mice Macrophages, Splenocytes and Leucopenia. Molecules, 18(12), 14862-14875. https://doi.org/10.3390/molecules181214862






