Synthesis, Antimycobacterial Activity and In Vitro Cytotoxicity of 5-Chloro-N-phenylpyrazine-2-carboxamides
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
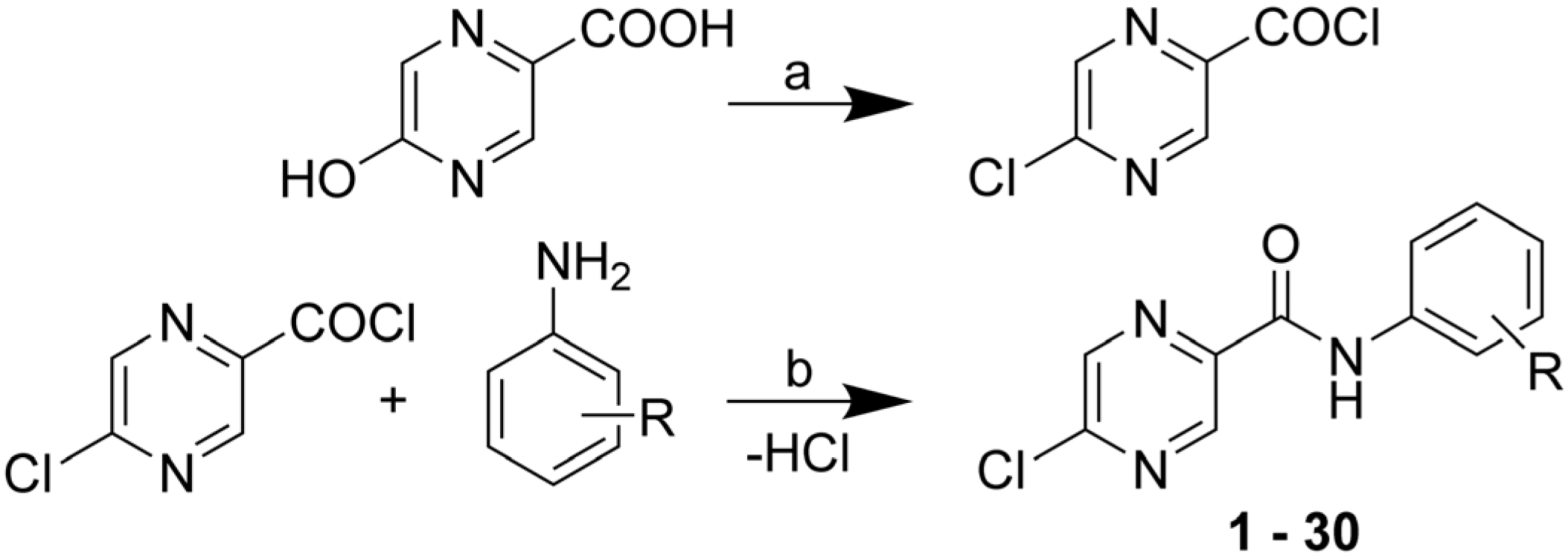
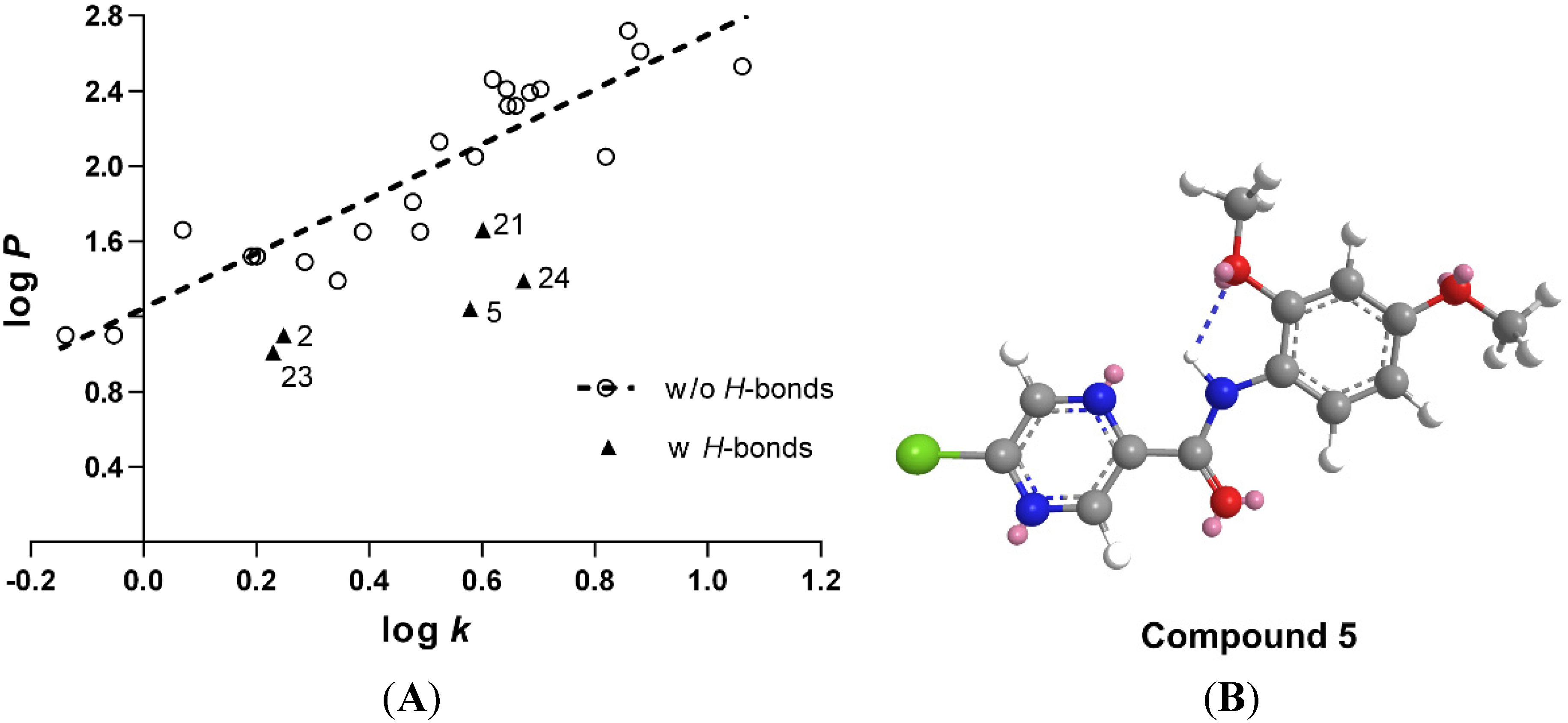
2.2. Lipophilicity
2.3. Biological Activity
2.3.1. In Vitro Antimycobacterial Activity
| Compound | Antimycobacterial activity MIC (μg/mL) | Cytotoxicity | Lipophilicity | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | R | M. tbc b | M.kansasii b | M.avium b | M. avium b | IC50 (μM) | SI c | logP | logk |
| 1 | H | 3.13 (1.56) | 25 | >100 | 50 | 2.55 | 0.19 | 1.49 | 0.286 |
| 2 | 2-OH | 3.13 (0.78) | n.d. | >100 | >100 | 30.00 | 2.39 | 1.10 | 0.248 |
| 3 | 3-OH | 6.25 | 50 | >100 | 100 | 32.10 | 1.28 | 1.10 | −0.053 |
| 4 | 4-OH | 3.13 (12.5) | 100 | >100 | 50 | 68.60 | 5.47 | 1.10 | −0.138 |
| 5 | 2,4-(OCH3)2 | >50 | >50 | >50 | >50 | n.a. | n.a. | 1.24 | 0.579 |
| 6 | 2,5-(CH3)2 | 1.56 (1.56) | >100 | >100 | >100 | 11.66 | 1.96 | 2.46 | 0.618 |
| 7 | 4-C2H5 | 1.56 (0.78) | n.d. | >100 | >100 | 7.17 | 2.41 | 2.39 | 0.685 |
| 8 | 4-i-Pr | 1.56 | n.d. | >100 | >100 | 14.44 | 2.55 | 2.72 | 0.859 |
| 9 | 2-F | 6.25 | 12.5 | >100 | >100 | n.a. | n.a. | 1.65 | 0.490 |
| 10 | 3-F | 6.25 | 12.5 | >100 | >100 | n.a. | n.a. | 1.65 | 0.389 |
| 11 | 2,4-F2 | 3.13 | 6.25 | >50 | >50 | n.a. | n.a. | 1.81 | 0.477 |
| 12 | 2-Cl | 3.13 (0.78) | n.d. | >100 | >100 | 6.72 | 0.58 | 2.05 | 0.819 |
| 13 | 3-Cl | 6.25 (3.13) | 25 | 25 | 25 | n.a. | n.a. | 2.05 | 0.587 |
| 14 | 3,4-Cl2 | 3.13 | >100 | >100 | >100 | 9.10 | 0.88 | 2.61 | 0.881 |
| 15 | 2,4,5-Cl3 | >100 | >100 | >100 | >100 | n.a. | n.a. | 3.16 | n.a. |
| 16 | 3-Br | 25 | 25 | >100 | >100 | n.a. | n.a. | 2.32 | 0.646 |
| 17 | 4-Br | 3.13 | 6.25 | >100 | >100 | n.a. | n.a. | 2.32 | 0.660 |
| 18 | 2-Cl-4-I | 12.5 | 1.56 | >50 | >50 | n.a. | n.a. | 3.41 | n.a. |
| 19 | 2-CH3-5-F | 3.13 (6.25) | 25 | >100 | >100 | n.a. | n.a. | 2.13 | 0.525 |
| 20 | 2-Cl-5-CH3 | 1.56 | 25 | >100 | >100 | 15.84 | 2.86 | 2.53 | 1.061 |
| 21 | 5-Cl-2-OH | 1.56 (6.25) | 12.5 | 12.5 | 12.5 | 40.59 | 7.39 | 1.66 | 0.602 |
| 22 | 3-Cl-4-OH | 3.13 (0.39) | >100 | 50 | 25 | 12.9 | 1.17 | 1.66 | 0.070 |
| 23 | 2-OH-5-NO2 | 1.56 (1.56) | n.d. | 50 | 50 | 1.52 | 0.29 | 1.01 | 0.230 |
| 24 | 2-NO2 | 12.5 | n.d. | >100 | >100 | n.a. | n.a. | 1.39 | 0.674 |
| 25 | 3-NO2 | 3.13 | n.d. | >100 | >100 | 32.70 | 2.91 | 1.39 | 0.344 |
| 26 | 3-CN | 25 | 3.13 | >50 | >50 | n.a. | n.a. | 1.52 | 0.192 |
| 27 | 4-CN | >100 | >100 | >100 | >100 | n.a. | n.a. | 1.52 | 0.201 |
| 28 | 3-CF3 | 3.13 (6.25) | 12.5 | >100 | >100 | 41.39 | 3.99 | 2.41 | 0.643 |
| 29 | 4-CF3 | 1.56 (3.13) | 12.5 | >100 | >100 | 8.50 | 1.64 | 2.41 | 0.704 |
| 30 | 4-COOH-3-OH | 3.13 | n.d. | >100 | >100 | n.a. | n.a. | 0.66 | n.a. |
| 5-Cl-PZA | − | 25 | 12.5 | >100 | >100 | 1594 | 10.0 | −0.41 | n.a. |
| PZA | − | 6.25–12.5 | >100 | >100 | >100 | >104 | >196 | −1.31 | −0.687 |
| INH | − | 0.39–0.78 | 12.5–25 | 12.5–25 | 3.13–6.25 | 79 × 103 d | n.a. | −0.64 | −0.743 |
2.3.2. In Vitro Cytotoxicity
| Compound | M. tbc H37Rv | HepG2 | CHO-K1 | ACHN | |||
|---|---|---|---|---|---|---|---|
| MIC (µM) | IC50 (µM) | SI | IC50 (µM) | SI | IC50 (µM) | SI | |
| 4 | 12.5 | 69 | 5.5 | 48 ± 4 | 3.8 | 100 ± 39 | 8.0 |
| 21 | 5.5 | 41 | 7.4 | 39 ± 2 | 7.1 | 51 ± 14 | 9.3 |
| 30 | 10.7 | n.a. | n.a. | 502 ± 122 | 47.1 | 371 ± 96 | 34.8 |
| 5-Cl PZA | 158.7 | 1594 | 10.0 | 290 ± 43 | 0.9 | 540 ± 120 | 1.7 |
2.3.3. In Vitro Antibacterial and Antifungal Activity
3. Experimental
3.1. General
3.2. Synthesis and Purification of Final Compounds
3.3. Data of the Prepared Target Compounds
3.4. Determination of Lipophilicity by HPLC (Logk)
3.5. Biological Methods
3.5.1. Evaluation of In Vitro Antimycobacterial Activity
3.5.2. HepG2 Cytotoxicity Determination
3.5.3. CHO-K1 and ACHN Cytotoxicity Determination
3.5.4. Evaluation of In Vitro Antibacterial Activity
3.5.5. Evaluation of In Vitro Antifungal Activity
4. Conclusions
Supplementary Materials
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Tuberculosis Report 2013; WHO/HTM/TB/2013.11; WHO: Geneva, Switzerland, 2013; pp. 8–21. [Google Scholar]
- Cynamon, M.H.; Speirs, R.J.; Welch, J.T. In vitro antimycobacterial activity of 5-chloropyrazinamide. Antimicrob. Agents Chemother. 1998, 42, 462–463. [Google Scholar]
- Zimhony, O.; Cox, J.S.; Welch, J.T.; Vilcheze, C.; Jacobs, W.R. Pyrazinamide inhibits the eukaryotic-like fatty acid synthetase I (FASI) of Mycobacterium tuberculosis. Nat. Med. 2000, 6, 1043–1047. [Google Scholar] [CrossRef]
- Boshoff, H.I.; Mizrahi, V.; Barry, C.E. Effects of pyrazinamide on fatty acid synthesis by whole mycobacterial cells and purified fatty acid synthase I. J. Bacteriol. 2002, 184, 2167–2172. [Google Scholar] [CrossRef]
- Ngo, S.C.; Zimhony, O.; Chung, W.J.; Sayahi, H.; Jacobs, W.R.; Welch, J.T. Inhibition of isolated mycobacterium tuberculosis fatty acid synthase I by pyrazinamide analogs. Antimicrob. Agents Chemother. 2007, 51, 2430–2435. [Google Scholar] [CrossRef]
- Sayahi, H.; Pugliese, K.M.; Zimhony, O.; Jacobs, W.R.; Shekhtman, A.; Welch, J.T. Analogs of the antituberculous agent pyrazinamide are competitive inhibitors of NADPH binding to M. tuberculosis fatty acid synthase I. Chem. Biodivers. 2012, 9, 2582–2596. [Google Scholar] [CrossRef]
- Ahmad, Z.; Tyagi, S.; Minkowski, A.; Almeida, D.; Nuermberger, E.L.; Peck, K.M.; Welch, J.T.; Baughn, A.S.; Jacobs, W.R.; Grosset, J.H. Activity of 5-chloropyrazinamide in mice infected with Mycobacterium tuberculosis or Mycobacterium bovis. Indian J. Med. Res. 2012, 135, 808–814. [Google Scholar]
- Zhang, Y.; Mitchison, D. The curious characteristics of pyrazinamide: A review. Int. J. Tuberc. Lung Dis. 2003, 7, 6–21. [Google Scholar]
- Dolezal, M.; Kesetovic, D.; Zitko, J. Antimycobacterial evaluation of pyrazinoic acid reversible derivatives. Curr. Pharm. Des. 2011, 17, 3506–3514. [Google Scholar] [CrossRef]
- Dolezal, M.; Zitko, J.; Jampilek, J. Pyrazinecarboxylic Acid Derivatives with Antimycobacterial Activity. In Understanding Tuberculosis—New Approaches to Fighting Against Drug Resistance; Cardona, P.-J., Ed.; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Servusova, B.; Vobickova, J.; Paterova, P.; Kubicek, V.; Kunes, J.; Dolezal, M.; Zitko, J. Synthesis and antimycobacterial evaluation of N-substituted 5-chloropyrazine-2-carboxamides. Bioorg. Med. Chem. Lett. 2013, 23, 3589–3591. [Google Scholar] [CrossRef]
- Wardell, S.; de Souza, M.V.N.; Vasconcelos, T.R.A.; Ferreira, M.D.L.; Wardell, J.L.; Low, J.N.; Glidewell, C. Patterns of hydrogen bonding in mono- and disubstituted N-arylpyrazinecarboxamides. Acta Crystallogr. Sect. B Struct. Sci. 2008, 64, 84–100. [Google Scholar] [CrossRef]
- Zitko, J.; Paterova, P.; Kubicek, V.; Mandikova, J.; Trejtnar, F.; Kunes, J.; Dolezal, M. Synthesis and antimycobacterial evaluation of pyrazinamide derivatives with benzylamino substitution. Bioorg. Med. Chem. Lett. 2013, 23, 476–479. [Google Scholar] [CrossRef]
- Tostmann, A.; Boeree, M.J.; Peters, W.H.M.; Roelofs, H.M.J.; Aarnoutse, R.E.; van der Ven, A.; Dekhuijzen, P.N.R. Isoniazid and its toxic metabolite hydrazine induce in vitro pyrazinamide toxicity. Int. J. Antimicrob. Agents 2008, 31, 577–580. [Google Scholar] [CrossRef]
- Bispo, M.D.F.; Goncalves, R.S.B.; Lima, C.H.D.; Cardoso, L.N.D.; Lourenco, M.C.S.; de Souza, M.V.N. Synthesis and Antitubercular Evaluation of N-Arylpyrazine and N,N’-Alkyl-diylpyrazine-2-carboxamide Derivatives. J. Heterocycl. Chem. 2012, 49, 1317–1322. [Google Scholar] [CrossRef]
- Servusova, B.; Eibinova, D.; Dolezal, M.; Kubicek, V.; Paterova, P.; Pesko, M.; Kral’ova, K. Substituted N-Benzylpyrazine-2-carboxamides: Synthesis and Biological Evaluation. Molecules 2012, 17, 13183–13198. [Google Scholar] [CrossRef]
- Tostmann, A.; Boeree, M.J.; Aarnoutse, R.E.; de Lange, W.C.M.; van der Ven, A.; Dekhuijzen, R. Antituberculosis drug-induced hepatotoxicity: Concise up-to-date review. J. Gastroenterol. Hepatol. 2008, 23, 192–202. [Google Scholar] [CrossRef]
- Singh, M.; Sasi, P.; Rai, G.; Gupta, V.H.; Amarapurkar, D.; Wangikar, P.P. Studies on toxicity of antitubercular drugs namely isoniazid, rifampicin, and pyrazinamide in an in vitro model of HepG2 cell line. Med. Chem. Res. 2011, 20, 1611–1615. [Google Scholar] [CrossRef]
- Singh, M.; Sasi, P.; Gupta, V.H.; Rai, G.; Amarapurkar, D.N.; Wangikar, P.P. Protective effect of curcumin, silymarin and N-acetylcysteine on antitubercular drug-induced hepatotoxicity assessed in an in vitro model. Hum. Exp. Toxicol. 2012, 31, 788–797. [Google Scholar] [CrossRef]
- Owen, T.C. Tetrazolium Compounds for Cell Viability Assays. U.S. Patent 5,185,450, 9 February 1993. [Google Scholar]
- Jones, R.N.; Barry, A.L. Optimal dilution susceptibility testing conditions, recommendations for MIC interpretation, and quality control guidelines for the ampicillin-sulbactam combination. J. Clin. Microbiol. 1987, 25, 1920–1925. [Google Scholar]
- National Committee for Clinical Laboratory Standards. Method for Antifungal Disc Diffusion Susceptibility Testing of Yeasts: Approved Guideline M44-A; NCCLS: Wayne, PA, USA, 2004. [Google Scholar]
- Sample Availability: Samples of the compounds 1–30 are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zitko, J.; Servusová, B.; Paterová, P.; Mandíková, J.; Kubíček, V.; Kučera, R.; Hrabcová, V.; Kuneš, J.; Soukup, O.; Doležal, M. Synthesis, Antimycobacterial Activity and In Vitro Cytotoxicity of 5-Chloro-N-phenylpyrazine-2-carboxamides. Molecules 2013, 18, 14807-14825. https://doi.org/10.3390/molecules181214807
Zitko J, Servusová B, Paterová P, Mandíková J, Kubíček V, Kučera R, Hrabcová V, Kuneš J, Soukup O, Doležal M. Synthesis, Antimycobacterial Activity and In Vitro Cytotoxicity of 5-Chloro-N-phenylpyrazine-2-carboxamides. Molecules. 2013; 18(12):14807-14825. https://doi.org/10.3390/molecules181214807
Chicago/Turabian StyleZitko, Jan, Barbora Servusová, Pavla Paterová, Jana Mandíková, Vladimír Kubíček, Radim Kučera, Veronika Hrabcová, Jiří Kuneš, Ondřej Soukup, and Martin Doležal. 2013. "Synthesis, Antimycobacterial Activity and In Vitro Cytotoxicity of 5-Chloro-N-phenylpyrazine-2-carboxamides" Molecules 18, no. 12: 14807-14825. https://doi.org/10.3390/molecules181214807








