Modulation of the Microenvironment Surrounding the Active Site of Penicillin G Acylase Immobilized on Acrylic Carriers Improves the Enzymatic Synthesis of Cephalosporins
Abstract
:1. Introduction

2. Results and Discussion
2.1. Immobilization of PGA through a Spacer
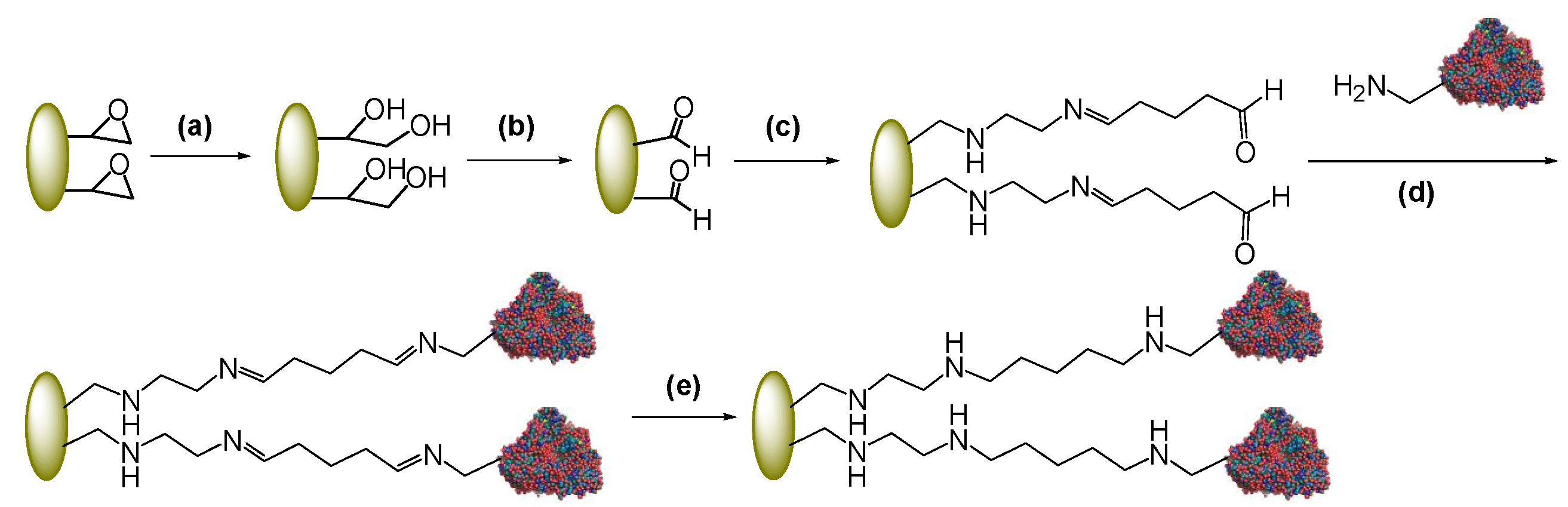
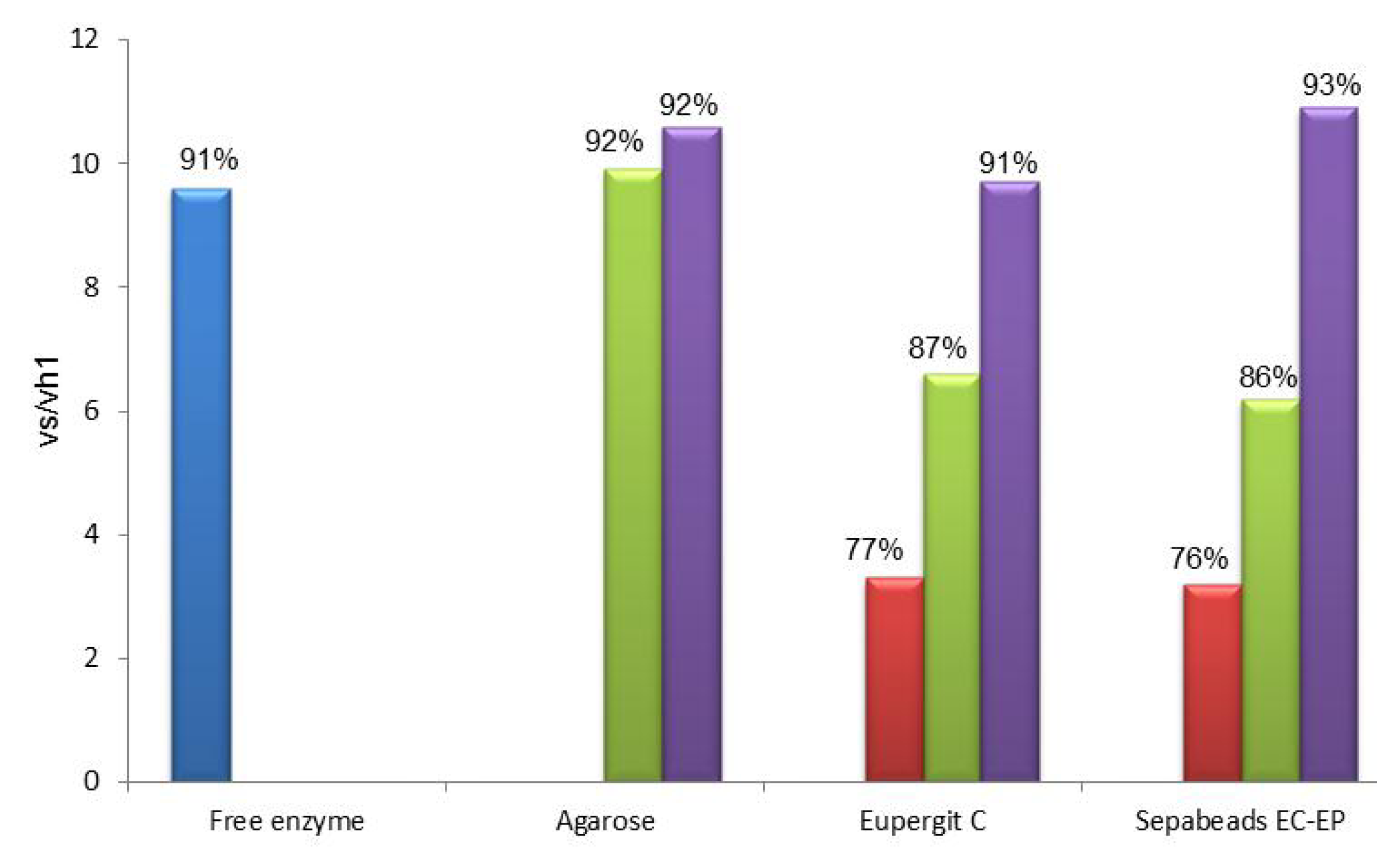
2.2. Post-Immobilization Modification of Epoxy Acrylic Carriers (Eupergit® C)
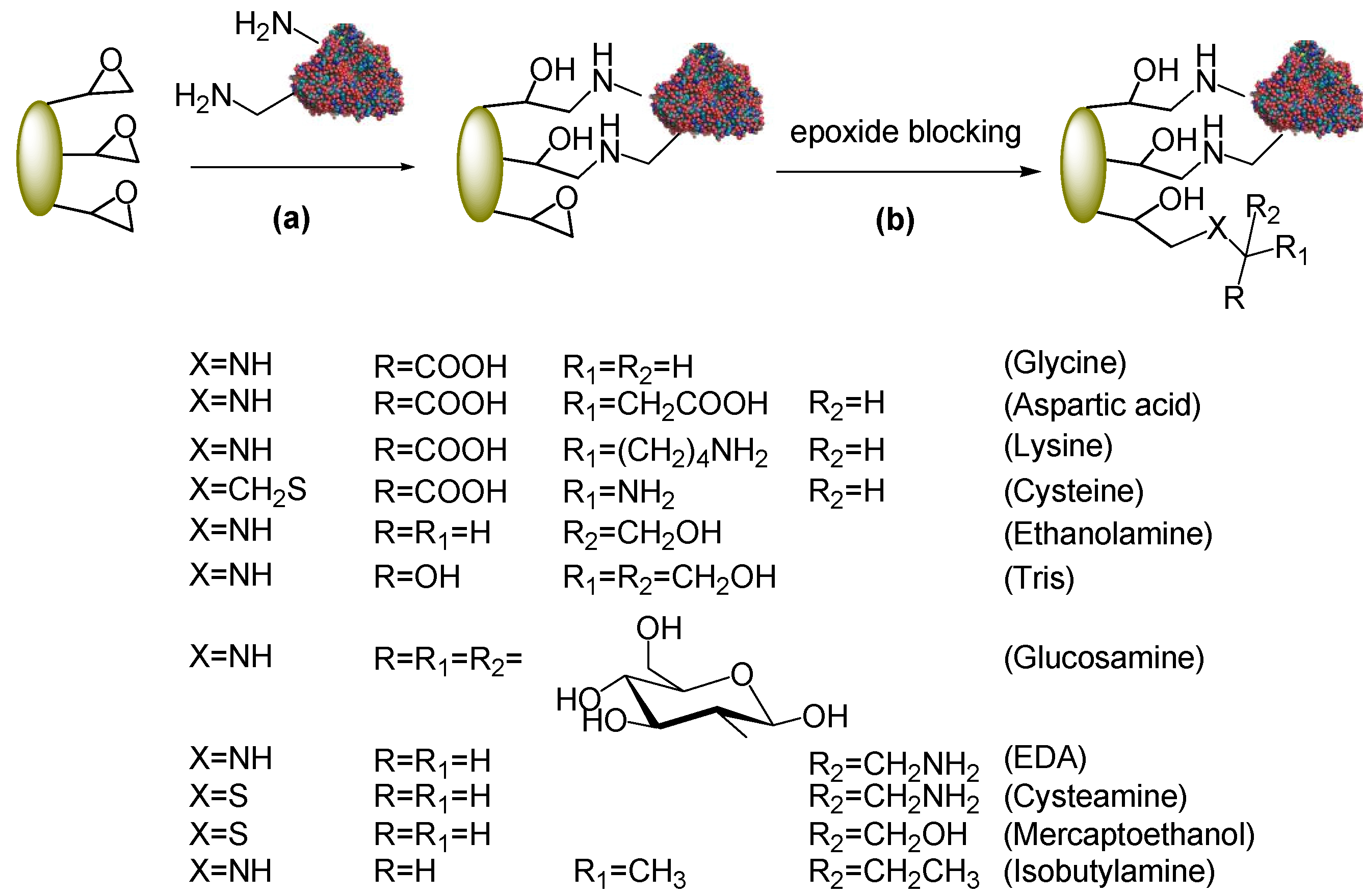
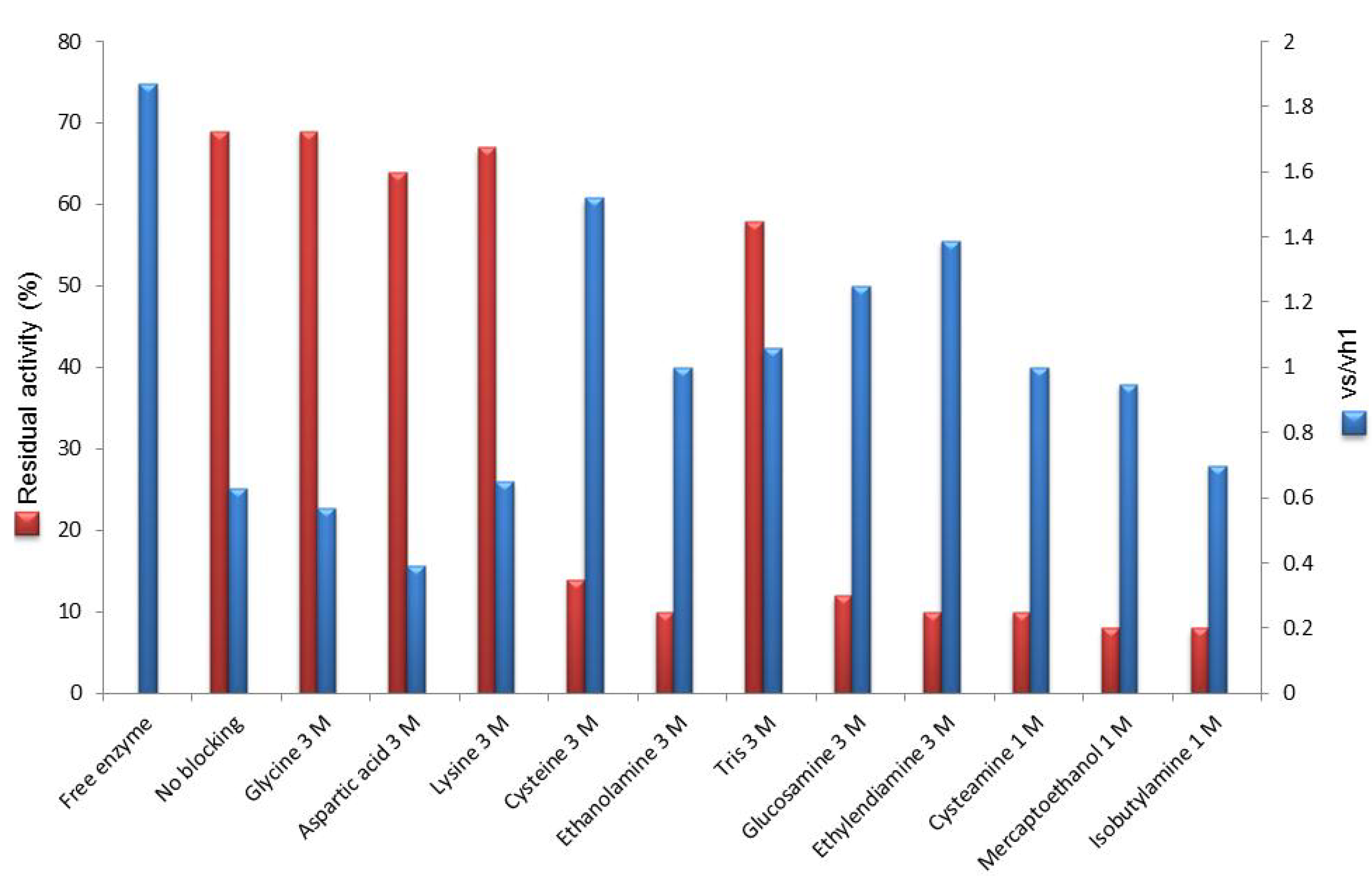
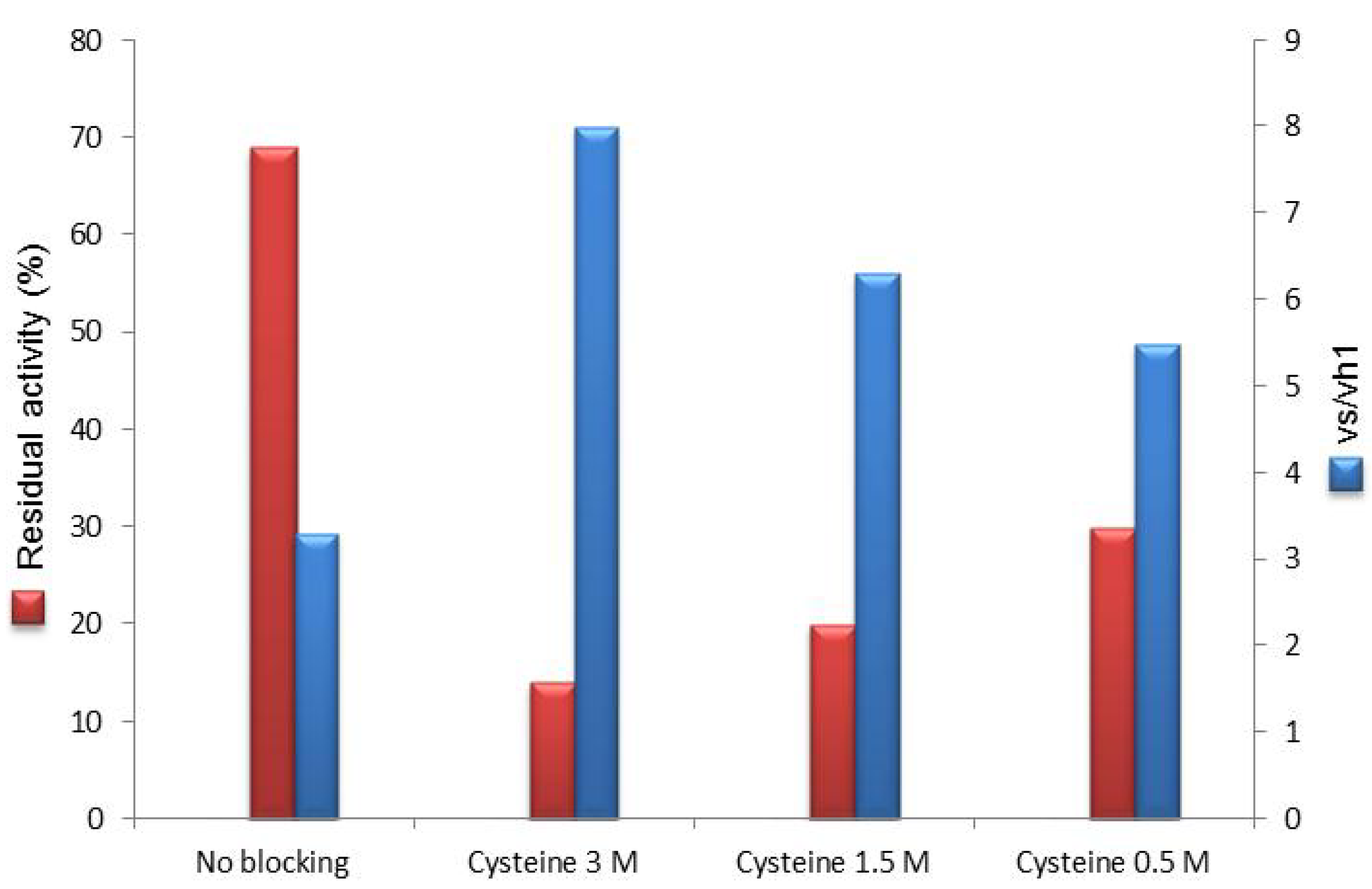

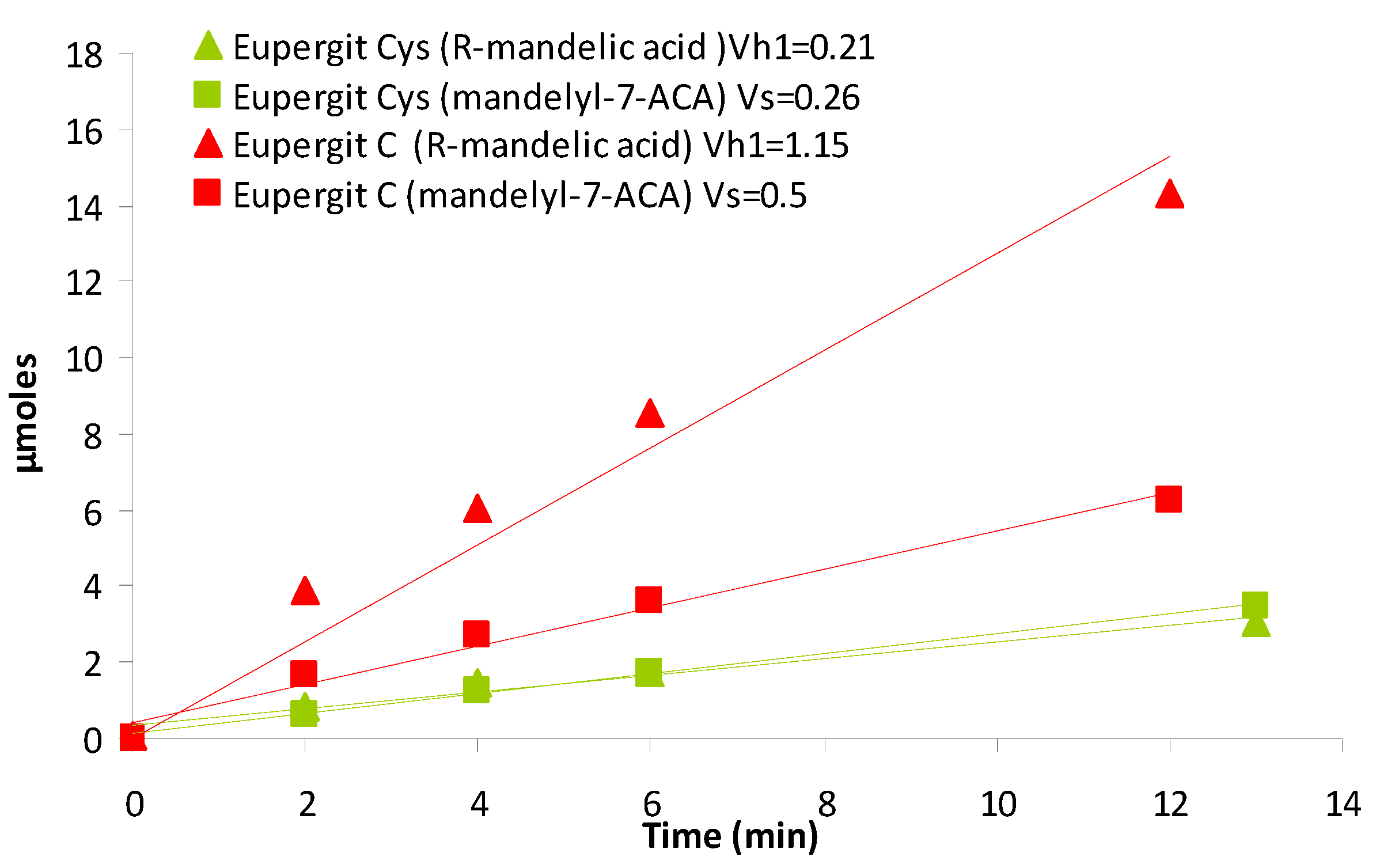
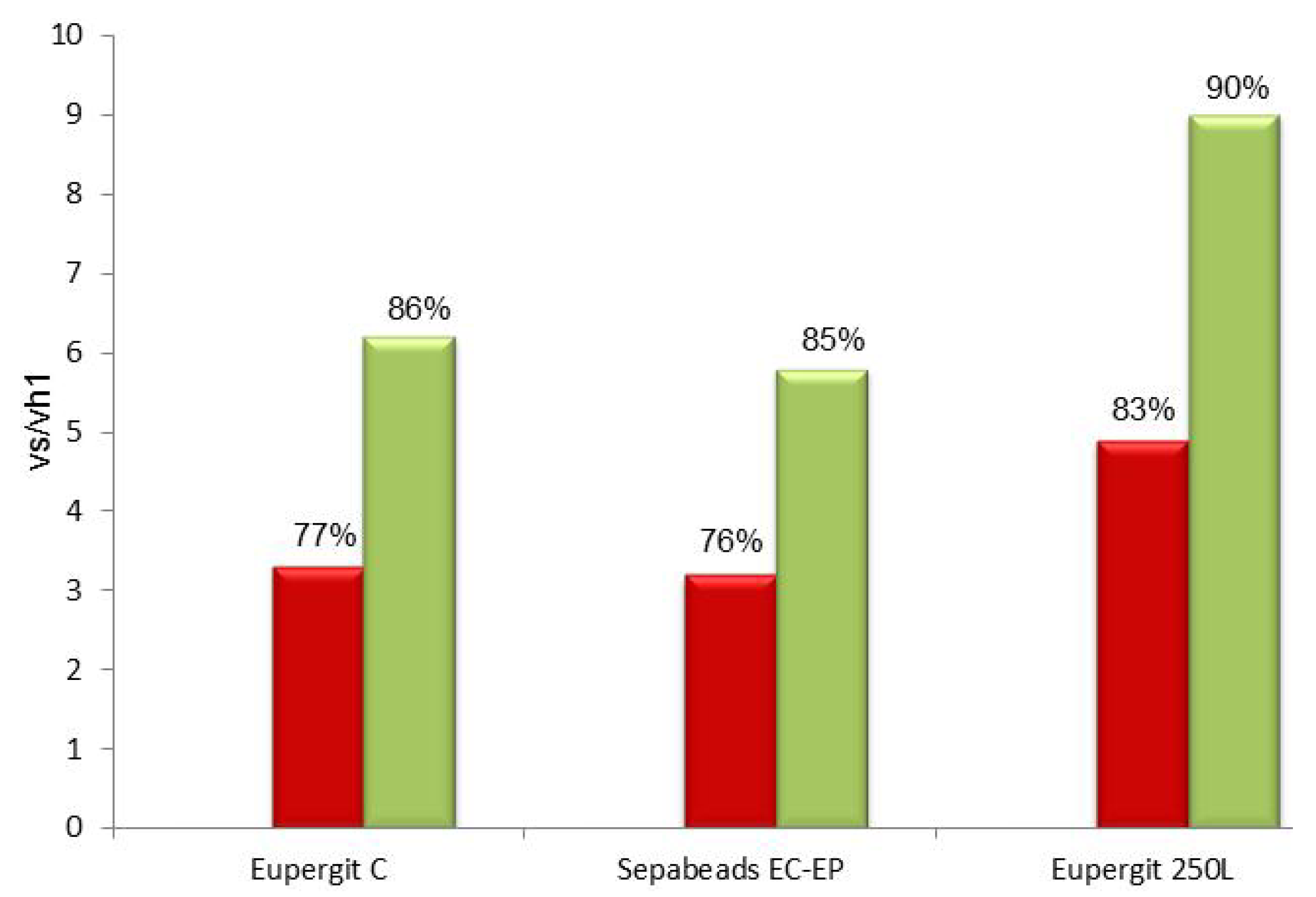
2.3. Synthesis of Cefazolin
| Support | Activation | Time (min) | Conversion (SD%) |
|---|---|---|---|
| Eupergit® C | Epoxy a | 180 | 79% (1.2) |
| Eupergit® C | Glutaraldehyde | 330 | 88% (0.9) |
| Eupergit® C | “Epoxy-Cysteine” | 270 | 87% (1.6) |
| Agarose | Aldehyde a | 330 | 90% (1.8) |
3. Experimental
3.1. General
3.2. Enzymatic Activity Assay
3.3. Enzyme Immobilization
3.3.1. Immobilization of PGA on Epoxy Acrylic Supports
3.3.2. Immobilization of PGA on Aldehyde Supports
3.3.3. Immobilization of PGA on Glutaraldehyde-Activated Acrylic Resins
3.4. Modification of Epoxy-Activated Acrylic Carriers
3.5. Enzymatic Reactions
3.6. Stability of the Immobilized Preparations
4. Conclusions
| Abbreviations | |
|---|---|
| 7-ACA | 7-amino-cephalosporanic acid |
| EDA | ethylendiamine |
| GA | glutaraldehyde |
| PGK | penicillin G potassium salt |
| 7-ZACA | 7-amino-3-(5-methyl-1,3,4-thiadiazol-2-yl)thiomethyl-3-cephem-4-carboxylic acid |
Supplementary Materials
Acknowledgments
Conflicts of Interest
References
- Chandel, A.K.; Rao, L.V.; Narasu, M.L.; Singh, O.V. The realm of penicillin G acylase in β-lactam antibiotics. Enzyme Microb. Technol. 2008, 42, 199–207. [Google Scholar] [CrossRef]
- Fernandez-Lafuente, R. Stabilization of multimeric enzymes: Strategies to prevent subunit dissociation. Enzyme Microb. Technol. 2009, 45, 405–418. [Google Scholar] [CrossRef]
- Iyer, P.V.; Ananthanarayan, L. Enzyme stability and stabilization-Aqueous and non-aqueous environment. Process Biochem. 2008, 43, 1019–1032. [Google Scholar] [CrossRef]
- Kallenberg, A.I.; Van Rantwijk, F.; Sheldon, R.A. Immobilization of penicillin G acylase: the key to optimum performance. Adv. Synth. Catal. 2005, 347, 905–926. [Google Scholar] [CrossRef]
- Guisàn, J.M.; Alvaro, G.; Fernàndez-Lafuente, R.; Rosell, C.M.; Garcia, J.L.; Tagliani, A. Stabilization of heterodimeric enzyme by multipoint covalent immobilization: Penicillin G acylase from Kluyvera citrophila. Biotechnol. Bioeng. 1993, 42, 455–464. [Google Scholar] [CrossRef]
- Mateo, C.; Abiàn, O.; Fernàndez-Lorente, G.; Pedroche, J.; Fernàndez-Lafuente, R.; Guisàn, J.M.; Tam, A.; Daminati, M. Epoxy Sepabeads: A novel epoxy support for stabilization of industrial enzymes via very intense multipoint covalent attachment. Biotechnol. Prog. 2002, 18, 629–634. [Google Scholar] [CrossRef]
- Mateo, C.; Fernàndez-Lorente, G.; Abiàn, O.; Fernàndez-Lafuente, R.; Guisàn, J.M. Multifunctional epoxy supports: a new tool to improve the covalent immobilization of proteins. The promotion of physical adsorptions of proteins on the supports before their covalent linkage. Biomacromolecules 2000, 1, 739–745. [Google Scholar] [CrossRef]
- Guisàn, J.M. Aldehyde-agarose gels as activated supports for immobilization-stabilization of enzymes. Enzyme Microb. Technol. 1988, 10, 375–382. [Google Scholar]
- Alvaro, G.; Fernàndez-Lafuente, R.; Blanco, R.M.; Guisàn, J.M. Immobilization-stabilization of penicillin G acylase from Escherichia coli. Appl. Biochem. Biotechnol. 1990, 26, 181–195. [Google Scholar] [CrossRef]
- Mateo, C.; Abiàn, O.; Bernedo, M.; Cuenca, E.; Fuentes, M.; Fernàndez-Lorente, G.; Palomo, J.M.; Grazù, V.; Pessela, B.C.C.; Giacomini, C.; et al. Some special features of glyoxyl supports to immobilize proteins. Enzyme Microb. Technol. 2005, 37, 456–462. [Google Scholar] [CrossRef]
- Temporini, C.; Bonomi, P.; Serra, I.; Tagliani, A.; Bavaro, T.; Ubiali, D.; Massolini, G.; Terreni, M. Characterization and study of the orientation of immobilized enzymes by tryptic digestion and HPLC-MS: design of an efficient catalyst for the synthesis of cephalosporins. Biomacromolecules 2010, 11, 1623–1632. [Google Scholar] [CrossRef]
- Mateo, C.; Palomo, J.M.; Fuentes, M.; Betancor, L.; Grazù, V.; Lòpez-Gallego, F.; Pessela, B.C.C.; Hidalgo, A.; Fernàndez-Lorente, G.; Fernàndez-Lafuente, R.; et al. Glyoxyl agarose: a fully inert and hydrophilic support for immobilization and high stabilization of proteins. Enzyme Microb. Technol. 2006, 39, 274–280. [Google Scholar] [CrossRef]
- Mateo, C.; Grazù, V.; Palomo, J.M.; Lopez-Gallego, F.; Fernàndez-Lafuente, R.; Guisàn, J.M. Immobilization of enzymes on heterofunctional epoxy supports. Nat. Protoc. 2007, 2, 1022–1033. [Google Scholar] [CrossRef]
- Serra, I.; Ubiali, D.; Cecchini, D.A.; Calleri, E.; Albertini, A.M.; Terreni, M.; Temporini, C. Assessment of immobilized PGA orientation via the LC-MS analysis of tryptic digests of the wild type and its 3K-PGA mutant assists in the rational design of a high-performance biocatalyst. Anal. Bioanal. Chem. 2013, 405, 745–753. [Google Scholar] [CrossRef]
- Penzol, G.; Armisen, P.; Fernàndez-Lafuente, R.; Rodes, L.; Guisàn, J.M. Use of dextrans as long and hydrophilic spacer arms to improve the performance of immobilized proteins acting on macromolecules. Biotechnol. Bioeng. 1998, 60, 518–523. [Google Scholar] [CrossRef]
- Fernàndez-Lafuente, R.; Rosell, C.M.; Rodriguez, V.; Guisàn, J.M. Strategies for enzyme stabilization by intramolecular crosslinking with bifunctional reagents. Enzyme Microb. Technol. 1995, 17, 517–523. [Google Scholar] [CrossRef]
- Adriano, W.S.; Filho, E.H.C.; Silva, J.A.; Giordano, R.L.C.; Goncalves, L.R.B. Stabilization of penicillin G acylase by immobilization on glutaraldehyde-activated chitosan. Brazil. J. Chem. Eng. 2005, 22, 529–538. [Google Scholar] [CrossRef]
- Fernàndez-Lorente, G.; Terreni, M.; Mateo, C.; Bastida, A.; Fernàndez-Lafuente, R.; Dalmases, P.; Huguet, J.; Guisàn, J.M. Modulation of lipase properties in macro-aqueous systems by controlled enzyme immobilization: Enantioselective hydrolysis of a chiral ester by immobilized Pseudomonas lipase. Enzyme Microb. Technol. 2001, 28, 389–396. [Google Scholar] [CrossRef]
- Fernàndez-Lafuente, R.; Rosell, C.M.; Caanan-Haden, L.; Rodes, L.; Guisàn, J.M. Facile synthesis of artificial enzyme nano-environments via solid-phase chemistry of immobilized derivatives: dramatic stabilization of penicillin acylase versus organic solvents. Enzyme Microb. Technol. 1999, 24, 96–103. [Google Scholar] [CrossRef]
- Jin, X.; Wu, Q.; Chen, Q.; Chen, C.X.; Lin, X.F. Immobilization of penicillin G acylase on a composite carrier with a biocompatible microenvironment of chitosan. J. Chem. Technol. Biotechnol. 2008, 83, 1710–1716. [Google Scholar] [CrossRef]
- Montes, T.; Grazù, V.; Manso, I.; Galan, B.; Lòpez-Gallego, F.; Gonzàlez, R.; Hermoso, J.A.; Garcia, J.L.; Guisàn, J.M.; Fernàndez-Lafuente, R. Improved stabilization of genetically modified penicillin G acylase in the presence of organic cosolvents by co-immobilization of the enzyme with polyethyleneimine. Adv. Synth. Catal. 2007, 349, 459–464. [Google Scholar] [CrossRef]
- Zhou, C.; Zhu, S.; Wu, X.; Jiang, B.; Cen, T.; Shen, S. Post-immobilization of modified macromolecular reagents using assembled penicillin acylase for microenvironmental regulation of nanopores and enhancement of enzyme stability. Biotechnol. Bioproc. Eng. 2010, 15, 376–382. [Google Scholar] [CrossRef]
- Fuentes, M.; Pessela, B.C.C.; Maquiese, J.V.; Ortiz, C.; Segura, R.L.; Palomo, J.M.; Abiàn, O.; Torres, R.; Mateo, C.; Fernàndez-Lafuente, R.; et al. Reversible and strong immobilization of proteinsby ionic exchange on supports coated with sulfate-dextran. Biotechnol. Prog. 2004, 20, 1134–1139. [Google Scholar] [CrossRef]
- Wilson, L.; Illanes, A.; Abiàn, O.; Pessela, B.C.C.; Fernàndez-Lafuente, R.; Guisàn, J.M. Co-aggregation of penicillin G acylase and polyionic polymers: An easy methodology to prepare enzyme biocatalysts stable in organic media. Biomacromolecules 2004, 5, 852–857. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, A.; Torres, R.; Fernández-Lafuente, R. Modifying enzyme activity and selectivity by immobilization. Chem. Soc. Rev. 2013, 42, 6290–6307. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Berenguer-Murcia, A.; Fernandez-Lafuente, R. Coupling chemical modification and immobilization to improve the catalytic performance of enzymes. Adv. Synth. Catal. 2011, 353, 2216–2238. [Google Scholar] [CrossRef]
- Kasche, V. Mechanism and yields in enzyme catalysed equilibrium and kinetically controlled synthesis of β-lactam antibiotics, peptides and other condensation products. Enzyme Microb. Technol. 1986, 8, 4–16. [Google Scholar] [CrossRef]
- Kasche, V.; Haufler, U.; Riechmann, L. Equilibrium and kinetically controlled synthesis with enzymes: Semisynthesis of penicillins and peptides. Meth. Enzymol. 1987, 136, 280–292. [Google Scholar] [CrossRef]
- Estruch, I.; Tagliani, A.R.; Guisàn, J.M.; Fernàndez-Lafuente, R.; Alcàntara, A.R.; Toma, L.; Terreni, M. Immobilization of the acylase from Escherichia coli on glyoxyl-agarose gives efficient catalyst for the synthesis of cephalosporins. Enzyme Microb. Technol. 2008, 42, 121–129. [Google Scholar] [CrossRef]
- Terreni, M.; Ubiali, D.; Bavaro, T.; Pregnolato, M.; Fernàndez-Lafuente, R.; Guisàn, J.M. Enzymatic synthesis of cephalosporins. The immobilized acylase from Arthrobacter viscosus: A new useful biocatalyst. Appl. Microbiol. Biotechnol. 2007, 77, 579–587. [Google Scholar] [CrossRef]
- Terreni, M.; Tchamkam, J.G.; Sarnataro, U.; Rocchietti, S.; Fernàndez-Lafuente, R.; Guisàn, J.M. Influence of substrate structure on PGA-catalyzed acylations. Evaluation of different approaches for the enzymatic synthesis of cefonicid. Adv. Synth. Catal. 2005, 347, 121–128. [Google Scholar] [CrossRef]
- Guisàn, J.M.; Penzol, G.; Armise, P.; Bastida, A.; Blanco, R.M.; Fernàndez-Lafuente, R.; Garcia-Giunceda, E. Immobilization of Enzymes and Cells; The Humana Press Inc: New York, NY, USA, 1997; pp. 261–275. [Google Scholar]
- Barbosa, O.; Torres, R.; Ortiz, C.; Berenguer-Murcia, A.; Rodrigues, R.C.; Fernandez-Lafuente, R. Heterofunctional supports in enzyme immobilization: from traditional immobilization protocols to opportunities in tuning enzyme properties. Biomacromolecules 2013, 14, 2433–2462. [Google Scholar] [CrossRef] [Green Version]
- Serra, I.; Cecchini, D.A.; UbiaIi, D.; Manazza, E.M.; Albertini, A.M.; Terreni, M. Coupling of site-directed mutagenesis and immobilization for the rational design of more efficient biocatalysts: The case of immobilized 3G3K PGA from E. coli. Eur. J. Org. Chem. 2009, 1384–1389. [Google Scholar]
- Scaramozzino, F.; Estruch, I.; Rossolillo, P.; Terreni, M.; Albertini, A.M. Improvement of catalytic properties of Escherichia coli penicillin G acylase immobilized on glyoxyl agarose by addition of a six-amino-acid tag. Appl. Environ. Microbiol. 2005, 71, 8937–8940. [Google Scholar] [CrossRef]
- Migneault, I.; Dartiguenave, C.; Bertrand, M.J.; Waldron, K.C. Glutaraldehyde: behaviour in aqueous solution, reaction with proteins, and application to enzyme crosslinking. Biotechniques 2004, 37, 790–802. [Google Scholar]
- Boller, T.; Meier, C.; Menzler, S. EUPERGIT oxirane acrylic beads: How to make enzymes fit for biocatalysis. Org. Process Res. Dev. 2002, 6, 509–519. [Google Scholar] [CrossRef]
- Sample Availability: Samples of immobilized PGA on glyoxyl agarose and on epoxy acrylic carriers are available from the Authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bonomi, P.; Bavaro, T.; Serra, I.; Tagliani, A.; Terreni, M.; Ubiali, D. Modulation of the Microenvironment Surrounding the Active Site of Penicillin G Acylase Immobilized on Acrylic Carriers Improves the Enzymatic Synthesis of Cephalosporins. Molecules 2013, 18, 14349-14365. https://doi.org/10.3390/molecules181114349
Bonomi P, Bavaro T, Serra I, Tagliani A, Terreni M, Ubiali D. Modulation of the Microenvironment Surrounding the Active Site of Penicillin G Acylase Immobilized on Acrylic Carriers Improves the Enzymatic Synthesis of Cephalosporins. Molecules. 2013; 18(11):14349-14365. https://doi.org/10.3390/molecules181114349
Chicago/Turabian StyleBonomi, Paolo, Teodora Bavaro, Immacolata Serra, Auro Tagliani, Marco Terreni, and Daniela Ubiali. 2013. "Modulation of the Microenvironment Surrounding the Active Site of Penicillin G Acylase Immobilized on Acrylic Carriers Improves the Enzymatic Synthesis of Cephalosporins" Molecules 18, no. 11: 14349-14365. https://doi.org/10.3390/molecules181114349






