Gold Nanoparticles on Mesoporous SiO2-Coated Magnetic Fe3O4 Spheres: A Magnetically Separatable Catalyst with Good Thermal Stability
Abstract
:1. Introduction

2. Results and Discussion
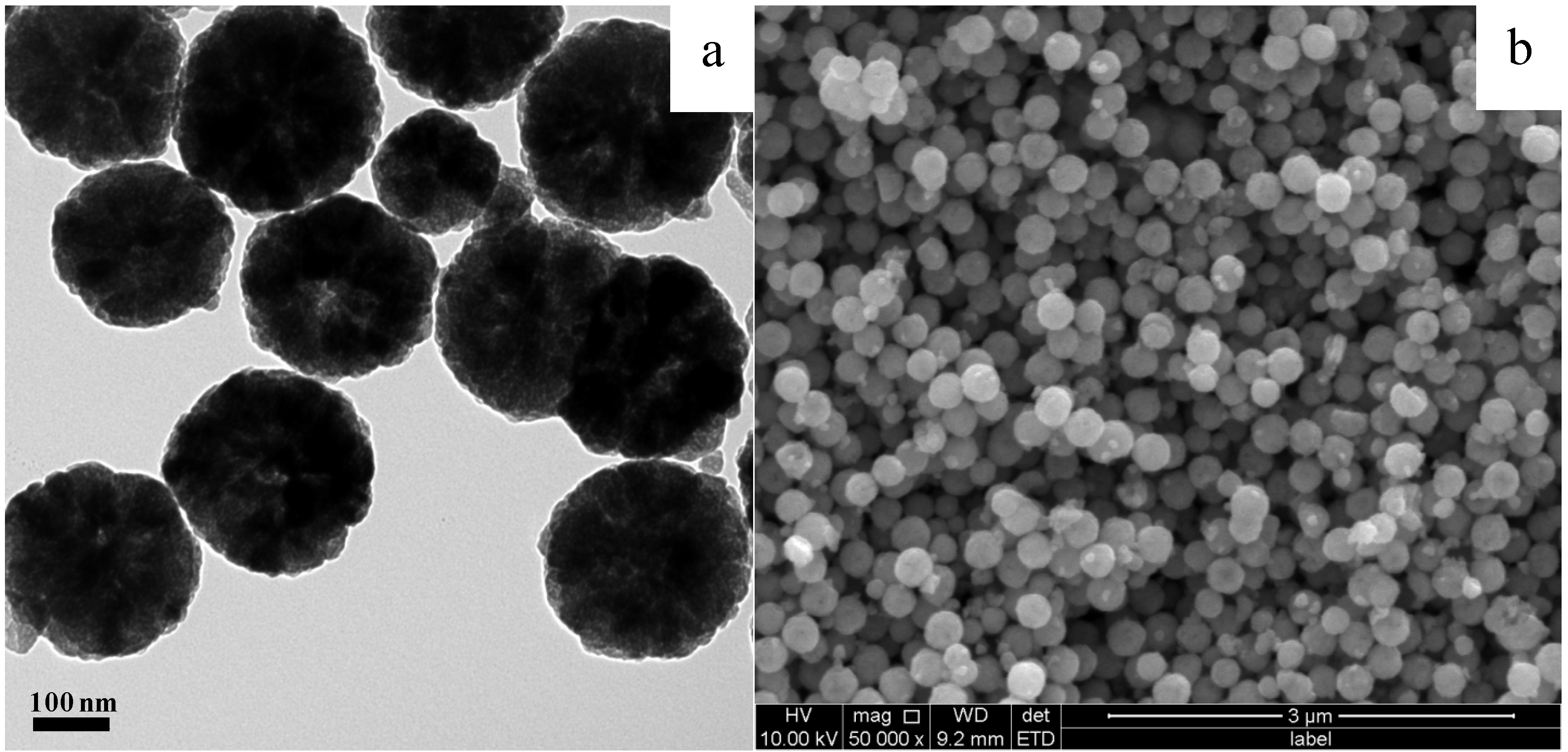
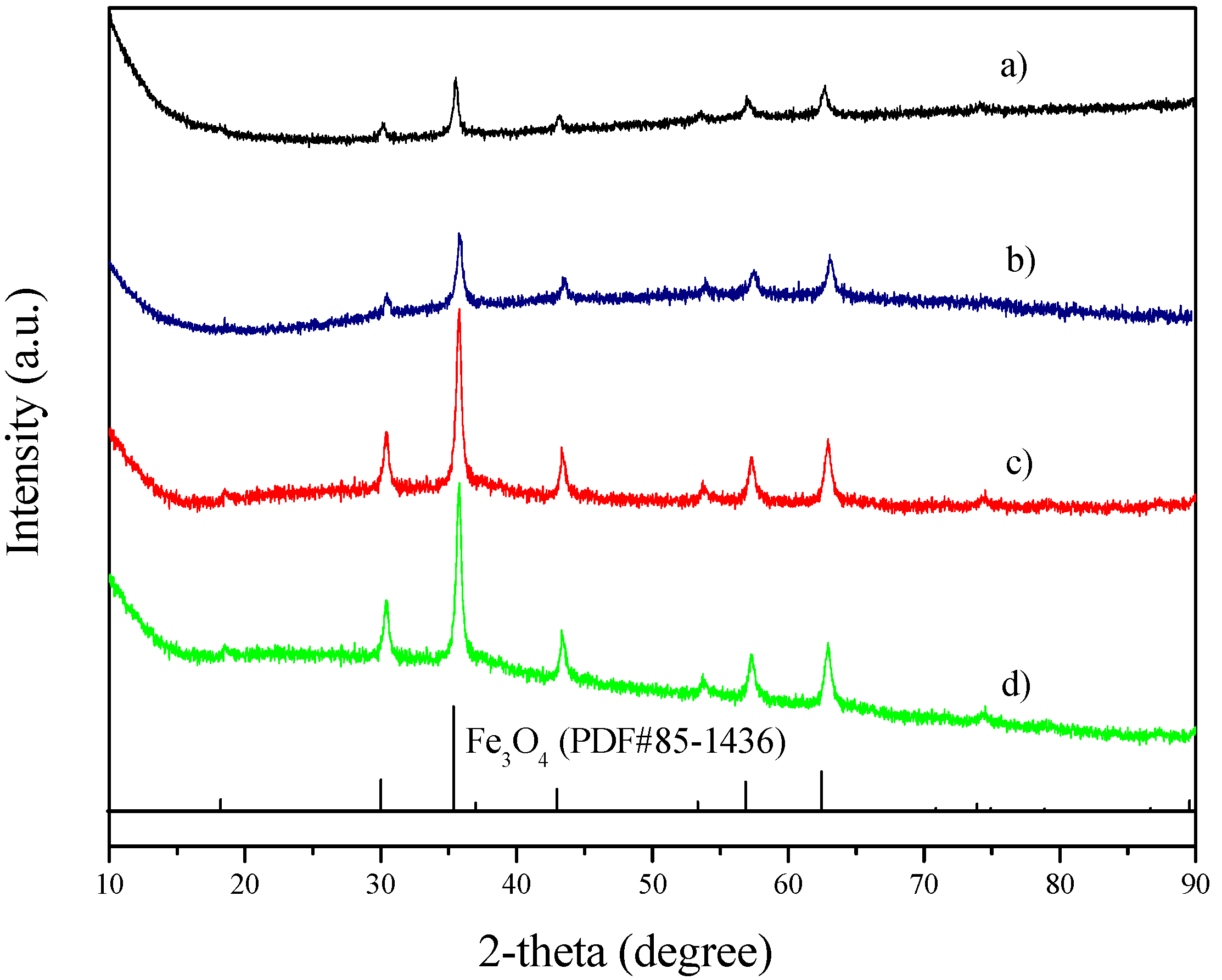
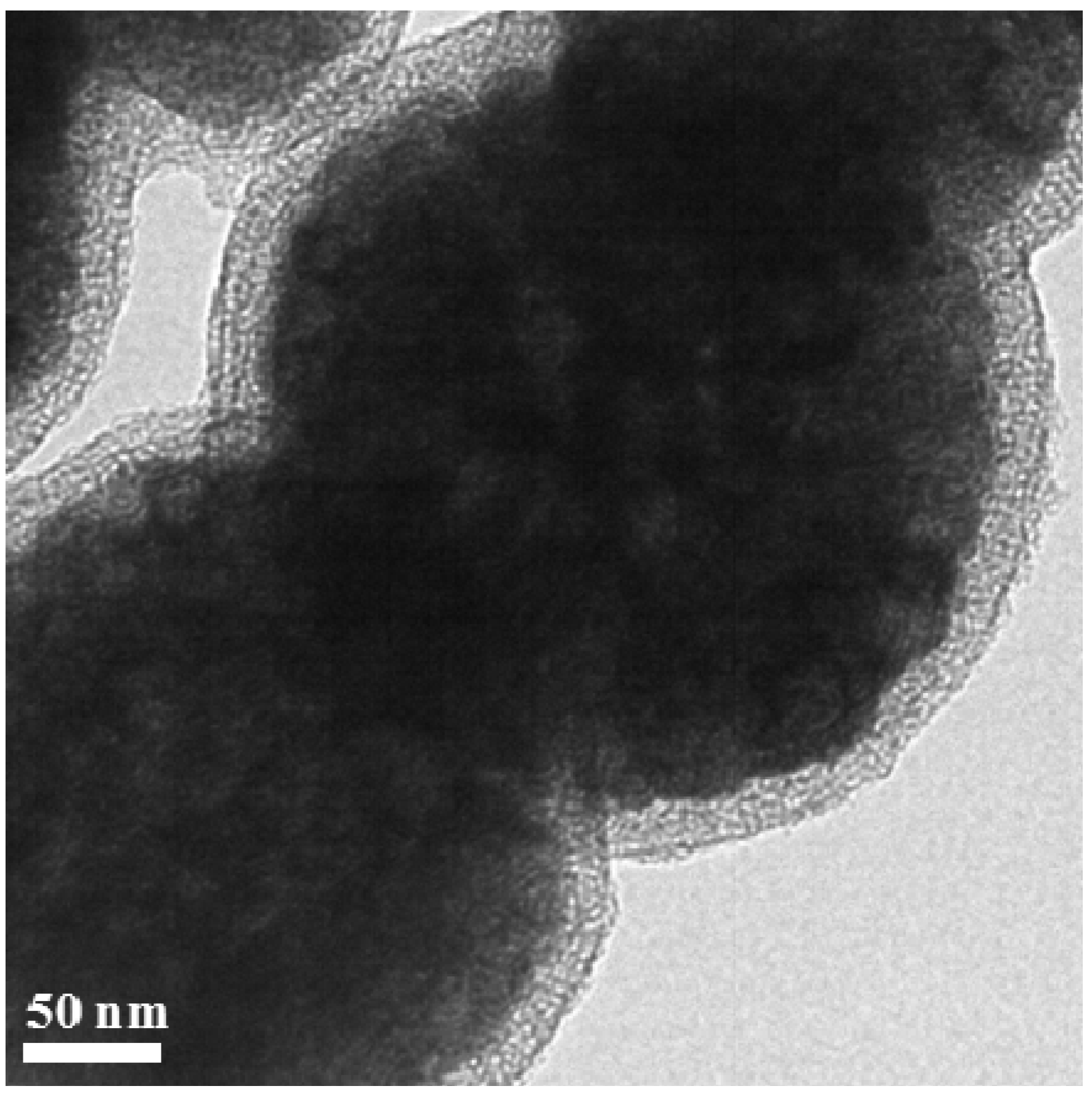
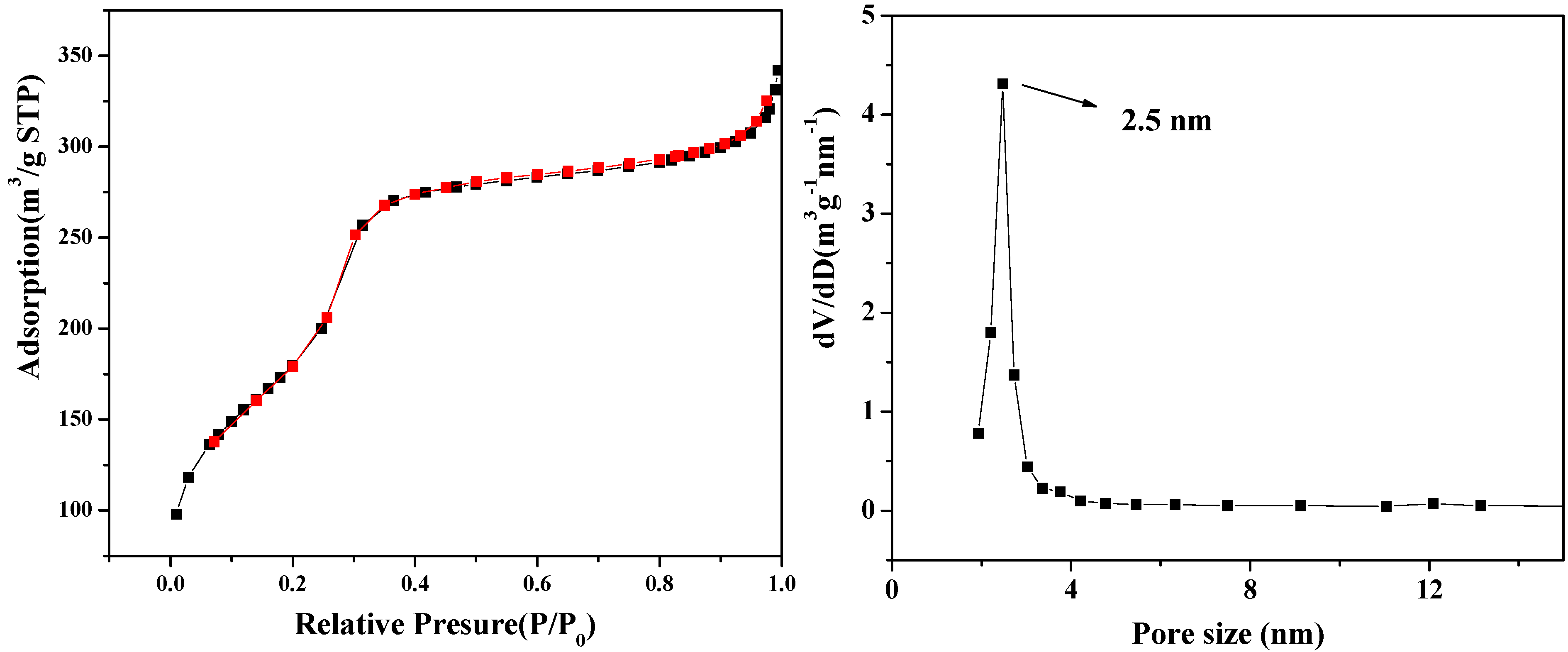

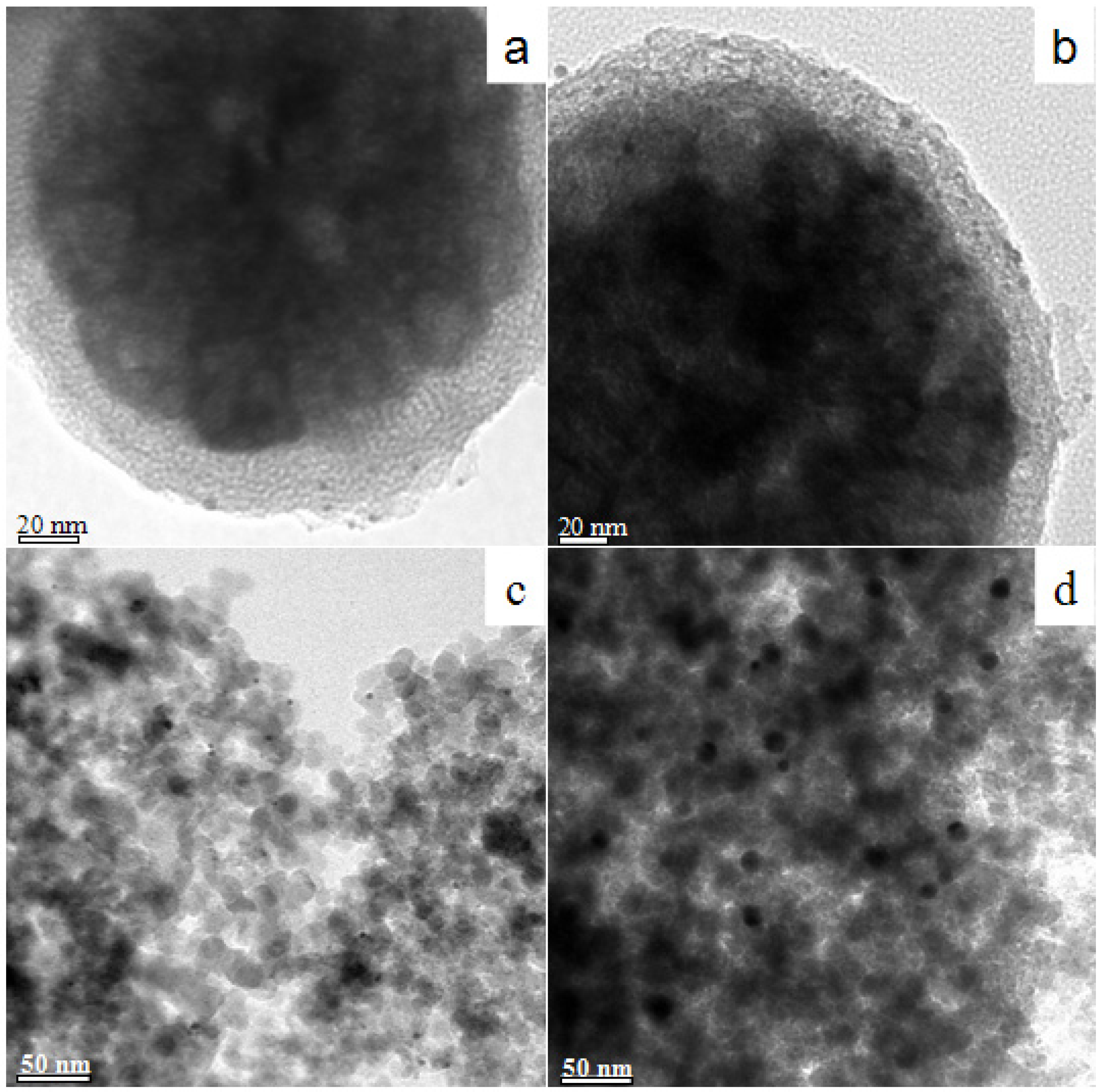

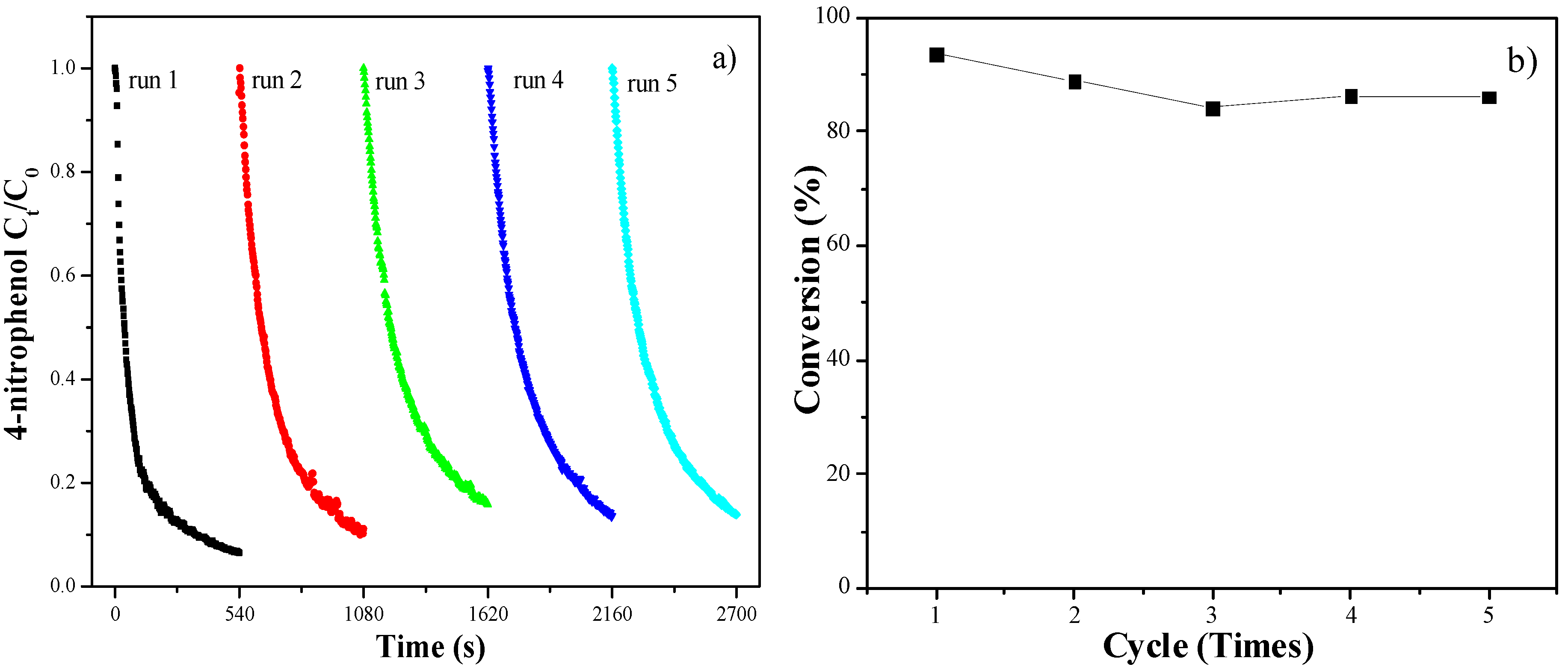
3. Experimental
3.1. Chemicals
3.2. Catalyst Preparation
3.2.1. Preparation of Fe3O4 Spheres
3.2.2. Preparation of m-SiO2/Fe3O4
3.2.3. Preparation of Au/m-SiO2/Fe3O4 and Au/SiO2 Catalysts
3.3. Characterization
3.4. Catalytic Reduction of p-Nitrophenol with NaBH4
4. Conclusions
Supplementary Materials
Acknowledgments
Conflicts of Interest
References
- Haruta, M.; Kobayashi, T.; Sano, H.; Yamada, N. Novel gold catalysts for the oxidation of carbon-monoxide at a temperature far below 0 °C. Chem. Lett. 1987, 16, 405–408. [Google Scholar]
- Haruta, M.; Yamada, N.; Kobayashi, T.; Iijima, S. Gold catalysts prepared by coprecipitation for low-temperature oxidation of hydrogen and of carbon monoxide. J. Catal. 1989, 115, 301–309. [Google Scholar] [CrossRef]
- Haruta, M.; Tsubota, S.; Kobayashi, T.; Kageyama, H.; Genet, M.J.; Delmon, B. Low-temperature oxidation of CO over gold supported on TiO2, α-Fe2O3, and Co3O4. J. Catal. 1993, 144, 175–192. [Google Scholar] [CrossRef]
- Hashmi, A.S.K. The catalysis gold rush: New claims. Angew. Chem. Int. Ed. 2005, 44, 6990–6993. [Google Scholar] [CrossRef]
- Bond, G.C.; Louis, C.; Thompson, D.T. Catalysis by Gold; Imperial College Press: London, UK, 2006. [Google Scholar]
- Takei, T.; Akita, K.; Nakamura, I.; Fujitani, T.; Okumura, M.; Okazaki, K.; Huang, J.H.; Ishida, T.; Haruta, M. Heterogeneous catalysis by gold. Adv. Catal. 2012, 55, 1–126. [Google Scholar] [CrossRef]
- Della Pina, C.; Falletta, E.; Prati, L.; Rossi, M. Selective oxidation using gold. Chem. Soc. Rev. 2008, 37, 2077–2095. [Google Scholar] [CrossRef]
- Corma, A.; Garcia, H. Supported gold nanoparticles for organic reactions. Chem. Soc. Rev. 2008, 37, 2096–2126. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, X.J.; Shi, F.; Deng, Y.Q. Nano-gold catalysis in fine chemical synthesis. Chem. Rev. 2012, 112, 2467–2505. [Google Scholar] [CrossRef]
- Stratakis, M.; Garcia, H. Catalysis by supported gold nanoparticles: Beyond aerobic oxidative processes. Chem. Rev. 2012, 112, 4469–4506. [Google Scholar] [CrossRef]
- Davis, S.E.; Ide, M.S.; Davis, R.J. Selective oxidation of alcohols and aldehydes over supported metal nanoparticles. Green Chem. 2013, 15, 17–45. [Google Scholar] [CrossRef]
- Deng, Y.H.; Qi, D.W.; Deng, C.H.; Zhang, X.M.; Zhao, D.Y. Superparamagnetic high-magnetization microspheres with an Fe3O4@SiO2 core and perpendicularly aligned mesoporous SiO2 shell for removal of microcystins. J. Am. Chem. Soc. 2007, 130, 28–29. [Google Scholar]
- Xuan, S.H.; Jiang, W.Q.; Gong, X.L.; Hu, Y.; Chen, Z.Y. Magnetically separable Fe3O4/TiO2 hollow spheres: Fabrication and photocatalytic activity. J. Phys. Chem. C 2008, 113, 553–558. [Google Scholar]
- Stjerndahl, M.; Andersson, M.; Hall, H.E.; Pajerowski, D.M.; Meisel, M.W.; Duran, R.S. Superparamagnetic Fe3O4/SiO2 nanocomposites: Enabling the tuning of both the iron oxide load and the size of the nanoparticles. Langmuir 2008, 24, 3532–3536. [Google Scholar] [CrossRef]
- Wang, P.; Shi, Q.H.; Shi, Y.F.; Clark, K.K.; Stucky, G.D.; Keller, A.A. Magnetic permanently confined micelle arrays for treating hydrophobic organic compound contamination. J. Am. Chem. Soc. 2008, 131, 182–188. [Google Scholar]
- Xu, Z.H.; Li, C.X.; Kang, X.J.; Yang, D.M.; Yang, P.P.; Hou, Z.Y.; Lin, J. Synthesis of a multifunctional nanocomposite with magnetic, mesoporous, and near-IR absorption properties. J. Phys. Chem. C 2010, 114, 16343–16350. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Li, B.; Zhang, L.M.; Li, P.; Wang, L.L.; Zhang, J. Multifunctional magnetic mesoporous silica nanocomposites with improved sensing performance and effective removal ability toward Hg(II). Langmuir 2011, 28, 1657–1662. [Google Scholar]
- Ma, W.-F.; Zhang, Y.; Li, L.-L.; You, L.-J.; Zhang, P.; Zhang, Y.-T.; Li, J.-M.; Yu, M.; Guo, J.; Lu, H.-J.; Wang, C.-C. Tailor-made magnetic Fe3O4@mTiO2 microspheres with a tunable mesoporous anatase shell for highly selective and effective enrichment of phosphopeptides. ACS Nano 2012, 6, 3179–3188. [Google Scholar] [CrossRef]
- Ding, H.L.; Zhang, Y.X.; Wang, S.; Xu, J.M.; Xu, S.C.; Li, G.H. Fe3O4@SiO2 core/shell nanoparticles: The silica coating regulations with a single core for different core sizes and shell thicknesses. Chem. Mater. 2012, 24, 4572–4580. [Google Scholar] [CrossRef]
- Zhu, G.-T.; Li, X.-S.; Gao, Q.; Zhao, N.-W.; Yuan, B.-F.; Feng, Y.-Q. Pseudomorphic synthesis of monodisperse magnetic mesoporous silica microspheres for selective enrichment of endogenous peptides. J. Chromatogr. A 2012, 1224, 11–18. [Google Scholar]
- Ge, J.P.; Zhang, Q.; Zhang, T.R.; Yin, Y.D. Core-satellite nanocomposite catalysts protected by a porous silica shell: Controllable reactivity, high stability, and magnetic recyclability. Angew. Chem. Int. Ed. 2008, 47, 8924–8928. [Google Scholar] [CrossRef]
- Deng, Y.H.; Cai, Y.; Sun, Z.K.; Liu, J.; Liu, C.; Wei, J.; Li, W.; Liu, C.; Wang, Y.; Zhao, D.Y. Multifunctional mesoporous composite microspheres with well-designed nanostructure: A highly integrated catalyst system. J. Am. Chem. Soc. 2010, 132, 8466–8473. [Google Scholar] [CrossRef]
- Xuan, S.H.; Wang, F.; Lai, J.M.Y.; Sham, W.Y.; Wang, Y.-X.; Lee, S.-F.; Yu, J.C.; Cheng, C.H.K.; Leung, K.C. Synthesis of biocompatible, mesoporous Fe3O4 nano/microspheres with large surface area for magnetic resonance imaging and therapeutic applications. ACS Appl. Mater. Interfaces 2011, 3, 237–244. [Google Scholar] [CrossRef]
- Zhu, M.Y.; Diao, G.W. Synthesis of porous Fe3O4 nanospheres and its application for the catalytic degradation of xylenol orange. J. Phys. Chem. C 2011, 115, 18923–18934. [Google Scholar] [CrossRef]
- Liu, H.F.; Ji, S.F.; Zheng, Y.Y.; Li, M.; Yang, H. Modified solvothermal synthesis of magnetic microspheres with multifunctional surfactant cetyltrimethyl ammonium bromide and directly coated mesoporous shell. Power Technol. 2013, 246, 520–529. [Google Scholar] [CrossRef]
- Lin, C.; Tao, K.; Hua, D.Y.; Ma, Z.; Zhou, S.H. Size effect of gold nanoparticles in catalytic reduction of p-nitrophenol with NaBH4. Molecules 2013, 18, 12609–12620. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the catalyst samples are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Liu, H.; Lin, C.; Ma, Z.; Yu, H.; Zhou, S. Gold Nanoparticles on Mesoporous SiO2-Coated Magnetic Fe3O4 Spheres: A Magnetically Separatable Catalyst with Good Thermal Stability. Molecules 2013, 18, 14258-14267. https://doi.org/10.3390/molecules181114258
Liu H, Lin C, Ma Z, Yu H, Zhou S. Gold Nanoparticles on Mesoporous SiO2-Coated Magnetic Fe3O4 Spheres: A Magnetically Separatable Catalyst with Good Thermal Stability. Molecules. 2013; 18(11):14258-14267. https://doi.org/10.3390/molecules181114258
Chicago/Turabian StyleLiu, Huan, Chao Lin, Zhen Ma, Hongbo Yu, and Shenghu Zhou. 2013. "Gold Nanoparticles on Mesoporous SiO2-Coated Magnetic Fe3O4 Spheres: A Magnetically Separatable Catalyst with Good Thermal Stability" Molecules 18, no. 11: 14258-14267. https://doi.org/10.3390/molecules181114258




